Spontaneous Breathing and Pendelluft in Patients with Acute Lung Injury: A Narrative Review
Abstract
:1. Introduction
2. The Beneficial Effects of Spontaneous Breathing
2.1. Prevention of Diaphragm Dysfunction
2.2. Increased Ventilation in the Dependent Lung
3. The Harmful Effects of Spontaneous Breathing—Patient Self-Inflicted Lung Injury (P-SILI)
3.1. Patient-Ventilator Asynchronies
3.2. Excessive Lung Perfusion
3.3. Increased Lung Stress and Pendelluft
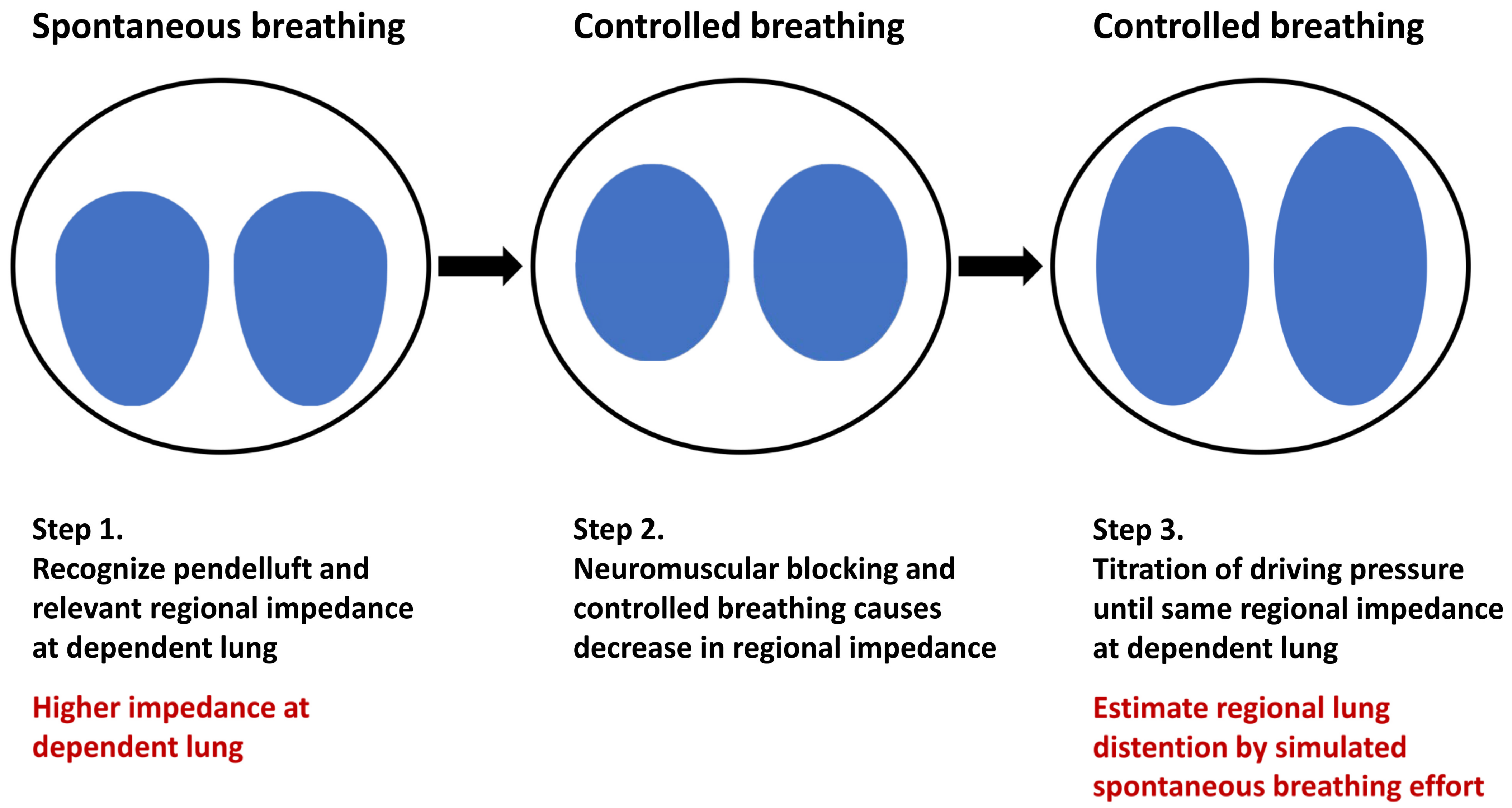
4. Monitoring Pendelluft in Patients with Lung Injury
4.1. Estimation of Regional Lung Inflation during Spontaneous Breathing by Controlled Ventilation
4.2. Estimation of Intrapulmonary Gas Flow
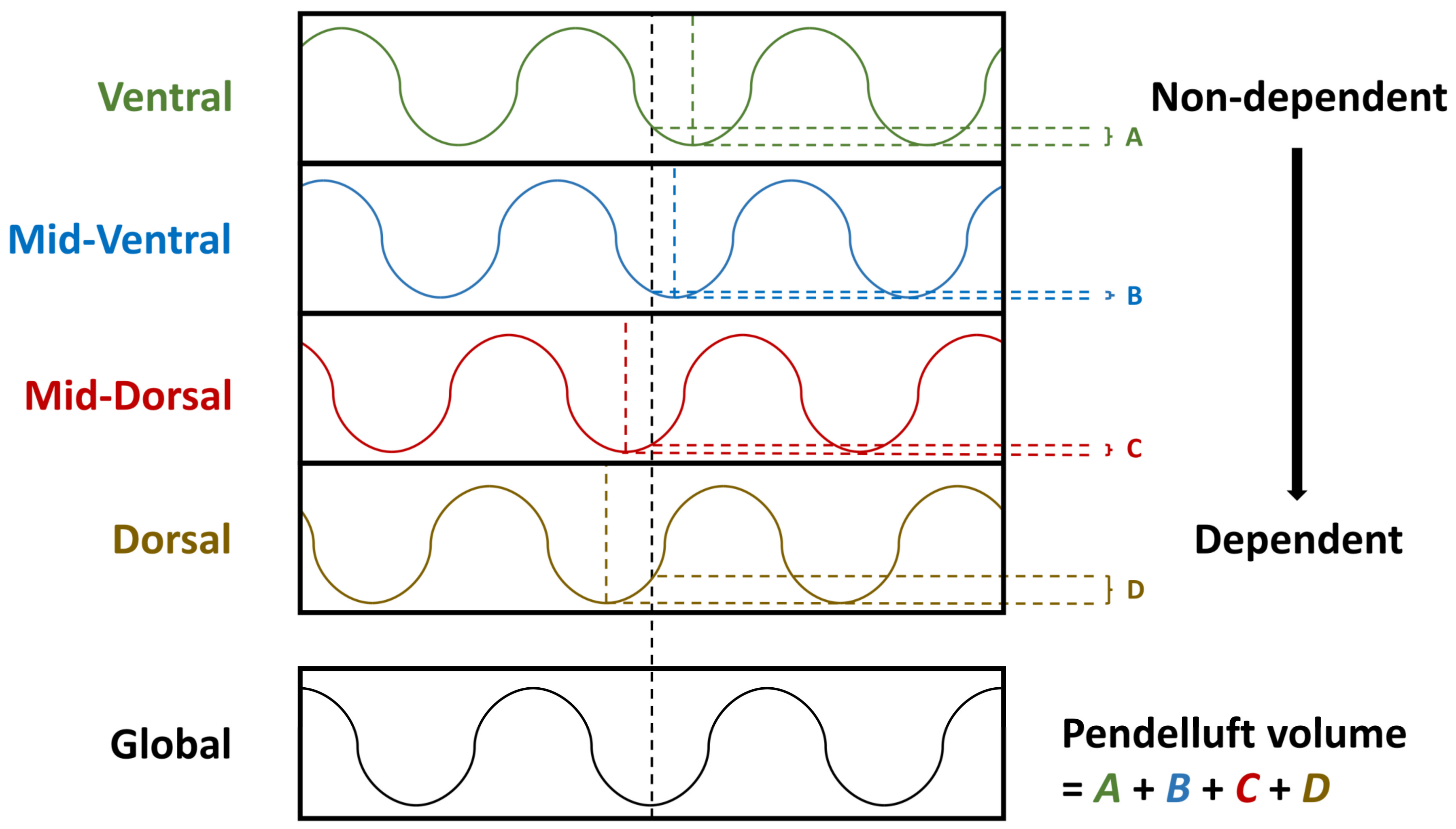
4.3. Measurement of Pendelluft Amplitude
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bellani, G.; Laffey, J.G.; Pham, T.; Fan, E.; Brochard, L.; Esteban, A.; Gattinoni, L.; van Haren, F.; Larsson, A.; McAuley, D.F.; et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA 2016, 315, 788–800. [Google Scholar] [CrossRef] [PubMed]
- Fan, E.; Brodie, D.; Slutsky, A.S. Acute respiratory distress syndrome: Advances in diagnosis and treatment. JAMA 2018, 319, 698–710. [Google Scholar] [CrossRef] [PubMed]
- Fredericks, A.S.; Bunker, M.P.; Gliga, L.A.; Ebeling, C.G.; Ringqvist, J.R.; Heravi, H.; Manley, J.; Valladares, J.; Romito, B.T. Airway pressure release ventilation: A review of the evidence, theoretical benefits, and alternative titration strategies. Clin. Med. Insights Circ. Respir. Pulm. Med. 2020, 14, 1179548420903297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, A.R.; Spieth, P.M.; Pelosi, P.; Beda, A.; Lopes, A.J.; Neykova, B.; Heller, A.R.; Koch, T.; Gama de Abreu, M. Pressure support ventilation and biphasic positive airway pressure improve oxygenation by redistribution of pulmonary blood flow. Anesth. Analg. 2009, 109, 856–865. [Google Scholar] [CrossRef] [PubMed]
- Levine, S.; Nguyen, T.; Taylor, N.; Friscia, M.E.; Budak, M.T.; Rothenberg, P.; Zhu, J.; Sachdeva, R.; Sonnad, S.; Kaiser, L.R.; et al. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N. Engl. J. Med. 2008, 358, 1327–1335. [Google Scholar] [CrossRef]
- Goligher, E.C.; Fan, E.; Herridge, M.S.; Murray, A.; Vorona, S.; Brace, D.; Rittayamai, N.; Lanys, A.; Tomlinson, G.; Singh, J.M.; et al. Evolution of diaphragm thickness during mechanical ventilation. Impact of inspiratory effort. Am. J. Respir. Crit. Care Med. 2015, 192, 1080–1088. [Google Scholar] [CrossRef]
- Vassilakopoulos, T.; Petrof, B.J. Ventilator-induced diaphragmatic dysfunction. Am. J. Respir. Crit. Care Med. 2004, 169, 336–341. [Google Scholar] [CrossRef] [Green Version]
- Dres, M.; Goligher, E.C.; Heunks, L.M.A.; Brochard, L.J. Critical illness-associated diaphragm weakness. Intensive Care Med. 2017, 43, 1441–1452. [Google Scholar] [CrossRef]
- Van Haren, F.; Pham, T.; Brochard, L.; Bellani, G.; Laffey, J.; Dres, M.; Fan, E.; Goligher, E.C.; Heunks, L.; Lynch, J.; et al. Spontaneous breathing in early acute respiratory distress syndrome: Insights from the large observational study to UNderstand the Global Impact of Severe Acute Respiratory FailurE Study. Crit. Care Med. 2019, 47, 229–238. [Google Scholar] [CrossRef]
- Yoshida, T.; Amato, M.B.P.; Kavanagh, B.P.; Yuji Fujino, Y. Impact of spontaneous breathing during mechanical ventilation in acute respiratory distress syndrome. Curr. Opin. Crit. Care 2019, 25, 192–198. [Google Scholar] [CrossRef]
- Güldner, A.; Pelosi, P.; Gama de Abreu, M. Spontaneous breathing in mild and moderate versus severe acute respiratory distress syndrome. Curr. Opin. Crit. Care 2014, 20, 69–76. [Google Scholar] [CrossRef]
- Mauri, T.; Bellani, G.; Confalonieri, A.; Tagliabue, P.; Turella, M.; Coppadoro, A.; Citerio, G.; Patroniti, N.; Pesenti, A. Topographic distribution of tidal ventilation in acute respiratory distress syndrome: Effects of positive end-expiratory pressure and pressure support. Crit. Care Med. 2013, 41, 1664–1673. [Google Scholar] [CrossRef]
- Radke, O.C.; Schneider, T.; Heller, A.R.; Koch, T. Spontaneous breathing during general anesthesia prevents the ventral redistribution of ventilation as detected by electrical impedance tomography: A randomized trial. Anesthesiology 2012, 116, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Mauri, T.; Langer, T.; Zanella, A.; Grasselli, G.; Pesenti, A. Extremely high transpulmonary pressure in a spontaneously breathing patient with early severe ARDS on ECMO. Intensive Care Med. 2016, 42, 2101–2103. [Google Scholar] [CrossRef]
- Acute Respiratory Distress Syndrome Network; Brower, R.G.; Matthay, M.A.; Morris, A.; Schoenfeld, D.; Thompson, B.T.; Wheeler, A. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1301–1308. [Google Scholar]
- Pohlman, M.C.; McCallister, K.E.; Schweickert, W.D.; Pohlman, A.S.; Nigos, C.P.; Krishnan, J.A.; Charbeneau, J.T. Excessive tidal volume from breath stacking during lung-protective ventilation for acute lung injury. Crit. Care Med. 2008, 36, 3019–3023. [Google Scholar] [CrossRef]
- Magrans, R.; Ferreira, F.; Sarlabous, L.; López-Aguilar, J.; Gomà, G.; Fernandez-Gonzalo, S.; Navarra-Ventura, G.; Fernández, R.; Montanyà, J.; Kacmarek, R.; et al. The effect of clusters of double triggering and ineffective efforts in critically ill patients. Crit. Care Med. 2022, 50, e619–e629. [Google Scholar] [CrossRef]
- Papazian, L.; Forel, J.-M.; Gacouin, A.; Penot-Ragon, C.; Perrin, G.; Loundou, A.; Jaber, S.; Arnal, J.-M.; Perez, D.; Seghboyen, J.M.; et al. Neuromuscular blockers in early acute respiratory distress syndrome. N. Engl. J. Med. 2010, 363, 1107–1116. [Google Scholar] [CrossRef] [Green Version]
- National Heart, Lung, and Blood Institute PETAL Clinical Trials Network; Moss, M.; Huang, D.T.; Brower, R.G.; Ferguson, N.D.; Ginde, A.A.; Gong, M.N.; Grissom, C.K.; Gundel, S.; Hayden, D.; et al. Early neuromuscular blockade in the acute respiratory distress syndrome. N. Engl. J. Med. 2019, 380, 1997–2008. [Google Scholar]
- Slutsky, A.S.; Villar, J. Early Paralytic Agents for ARDS? Yes, no, and sometimes. N. Engl. J. Med. 2019, 380, 2061–2063. [Google Scholar] [CrossRef]
- Bhattacharya, M.; Kallet, R.H.; Ware, L.B.; Matthay, M.A. Negative-pressure pulmonary edema. Chest 2016, 150, 927–933. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshida, T.; Fujino, Y.; Amato, M.B.P.; Kavanah, B.P. Fifty Years of Research in ARDS. Spontaneous breathing during mechanical ventilation. Risks, mechanisms, and management. Am. J. Respir. Crit. Care Med. 2017, 195, 985–992. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, T.; Roldan, R.; Beraldo, M.A.; Torsani, V.; Gomes, S.; De Santis, R.R.; Costa, E.L.V.; Tucci, M.R.; Lima, R.G.; Kavanagh, B.P.; et al. Spontaneous effort during mechanical ventilation: Maximal injury with less positive end-expiratory pressure. Crit. Care Med. 2016, 44, e678–e688. [Google Scholar] [CrossRef] [PubMed]
- Katira, B.H.; Engelberts, D.; Otulaowski, G.; Giesinger, R.E.; Yoshida, T.; Post, M.; Kuebler, W.M.; Connelly, K.A.; Kavanagh, B.P. Abrupt deflation after sustained inflation causes lung injury. Am. J. Respir. Crit. Care Med. 2018, 198, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Katira, B.H.; Engelberts, D.; Bouch, S.; Fliss, J.; Bastia, L.; Osada, K.; Connelly, K.A.; Amato, M.B.P.; Ferguson, N.D.; Kuebler, W.M.; et al. Repeated endo-tracheal tube disconnection generates pulmonary edema in a model of volume overload: An experimental study. Crit. Care 2022, 26, 47. [Google Scholar] [CrossRef]
- Leray, V.; Bourdin, G.; Flandreau, G.; Bayle, F.; Wallet, F.; Richard, J.C.; Guérin, C. A Case of pneumomediastinum in a patient with acute respiratory distress syndrome on pressure support ventilation. Respir. Care 2010, 55, 770–773. [Google Scholar]
- Yoshida, T.; Torsani, V.; Gomes, S.; De Santis, R.R.; Beraldo, M.A.; Costa, E.L.V.; Tucci, M.R.; Zin, W.A.; Kavanagh, B.P.; Amato, M.B.P. Spontaneous effort causes occult pendelluft during mechanical ventilation. Am. J. Respir. Crit. Care Med. 2013, 188, 1420–1427. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, T.; Grieco, D.L.; Brochard, L.; Fujino, Y. Patient self-inflicted lung injury and positive end-expiratory pressure for safe spontaneous breathing. Curr. Opin. Crit. Care 2020, 26, 59–65. [Google Scholar] [CrossRef]
- Yoshida, T.; Nakamura, M.A.M.; Morais, C.C.A.; Amato, M.B.P.; Kavanagh, B.P. Reverse triggering causes an injurious inflation pattern during mechanical ventilation. Am. J. Respir. Crit. Care Med. 2018, 198, 1096–1099. [Google Scholar] [CrossRef]
- Coppadoro, A.; Grassi, A.; Giovannoni, C.; Rabboni, F.; Eronia, N.; Bronco, A.; Foti, G.; Fumagalli, R.; Bellani, G. Occurrence of pendelluft under pressure support ventilation in patients who failed a spontaneous breathing trial: An observational study. Ann. Intensive Care 2020, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Cornejo, R.A.; Arellano, D.H.; Ruiz-Rudolph, P.; Guiñez, D.V.; Morais, C.C.A.; Gajardo, A.I.J.; Lazo, M.T.; Brito, R.E.; Cerda, M.A.; González, S.J.; et al. Inflammatory biomarkers and pendelluft magnitude in ards patients transitioning from controlled to partial support ventilation. Sci. Rep. 2022, 12, 20233. [Google Scholar] [CrossRef]
- Brochard, L.; Slutsky, A.; Pesenti, A. Mechanical ventilation to minimize progression of lung injury in acute respiratory failure. Am. J. Respir. Crit. Care Med. 2017, 195, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Guervilly, C.; Bisbal, M.; Forel, J.M.; Mechati, M.; Lehingue, S.; Bourenne, J.; Perrin, G.; Rambaud, R.; Adda, M.; Hraiech, S.; et al. Effects of neuromuscular blockers on transpulmonary pressures in moderate to severe acute respiratory distress syndrome. Intensive Care Med. 2017, 43, 408–418. [Google Scholar] [CrossRef]
- Frerichs, I.; Amato, M.B.P.; van Kaam, A.H.; Tingay, D.G.; Zhao, Z.; Grychtol, B.; Bodenstein, M.; Gagnon, H.; Böhm, S.H.; Teschner, E.; et al. Electrical impedance tomography examination, data analysis, terminology, clinical use and recommendations: Consensus statement of the TRanslational EIT developmeNt stuDy group. Thorax 2017, 72, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, M.C.; Morais, C.; Bugedo, G.; Bruhn, A.; Morales, A.; Borges, J.B.; Costa, E.; Retamal, J. Electrical impedance tomography in acute respiratory distress syndrome. Crit. Care 2018, 22, 263. [Google Scholar] [CrossRef] [Green Version]
- Sang, L.; Zhao, Z.; Yun, P.J.; Frerichs, I.; Möller, K.; Fu, F.; Liu, X.; Zhong, N.; Li, Y. Qualitative and quantitative assessment of pendelluft: A simple method based on electrical impedance tomography. Ann. Transl. Med. 2020, 8, 1216. [Google Scholar] [CrossRef]
- Chi, Y.; Zhao, Z.; Frerichs, I.; Lon, Y.; He, H. Prevalence and prognosis of respiratory pendelluft phenomenon in in mechanically ventilated ICU patients with acute respiratory failure: A retrospective cohort study. Ann. Intensive Care 2022, 12, 22. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, P.-L.; Zhao, Z.; Ko, Y.-F.; Chen, C.-W.; Cheng, K.-S. Spontaneous Breathing and Pendelluft in Patients with Acute Lung Injury: A Narrative Review. J. Clin. Med. 2022, 11, 7449. https://doi.org/10.3390/jcm11247449
Su P-L, Zhao Z, Ko Y-F, Chen C-W, Cheng K-S. Spontaneous Breathing and Pendelluft in Patients with Acute Lung Injury: A Narrative Review. Journal of Clinical Medicine. 2022; 11(24):7449. https://doi.org/10.3390/jcm11247449
Chicago/Turabian StyleSu, Po-Lan, Zhanqi Zhao, Yen-Fen Ko, Chang-Wen Chen, and Kuo-Sheng Cheng. 2022. "Spontaneous Breathing and Pendelluft in Patients with Acute Lung Injury: A Narrative Review" Journal of Clinical Medicine 11, no. 24: 7449. https://doi.org/10.3390/jcm11247449
APA StyleSu, P.-L., Zhao, Z., Ko, Y.-F., Chen, C.-W., & Cheng, K.-S. (2022). Spontaneous Breathing and Pendelluft in Patients with Acute Lung Injury: A Narrative Review. Journal of Clinical Medicine, 11(24), 7449. https://doi.org/10.3390/jcm11247449






