Assessing the Efficacy of Early Therapies against SARS-CoV-2 in Hematological Patients: A Real-Life Study from a COVID-19 Referral Centre in Northern Italy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Study Setting
2.3. Patients’ Characteristics
2.4. SARS-CoV-2 RNA Detection
2.5. Outcomes
2.6. Statistical Analysis
3. Results
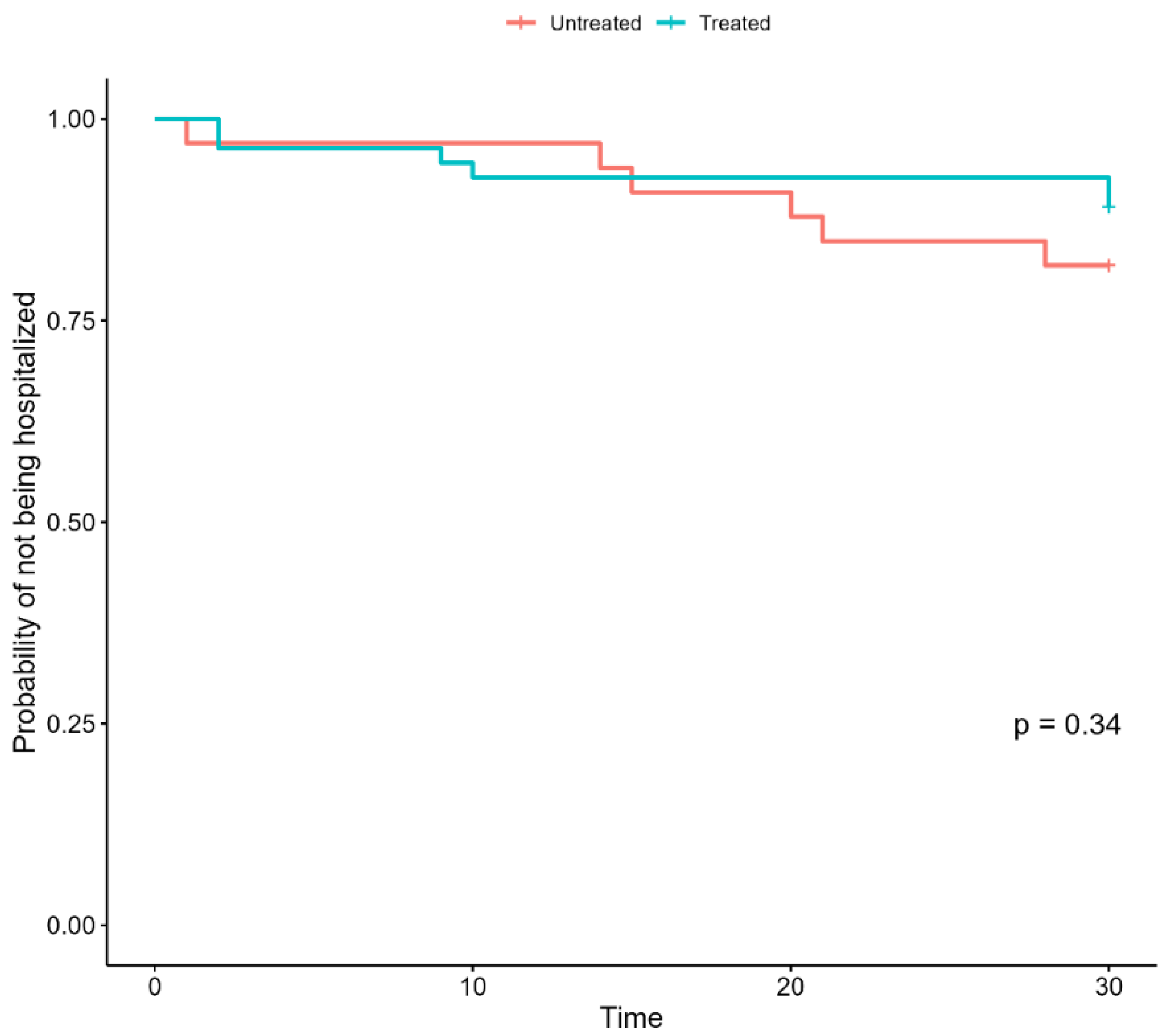
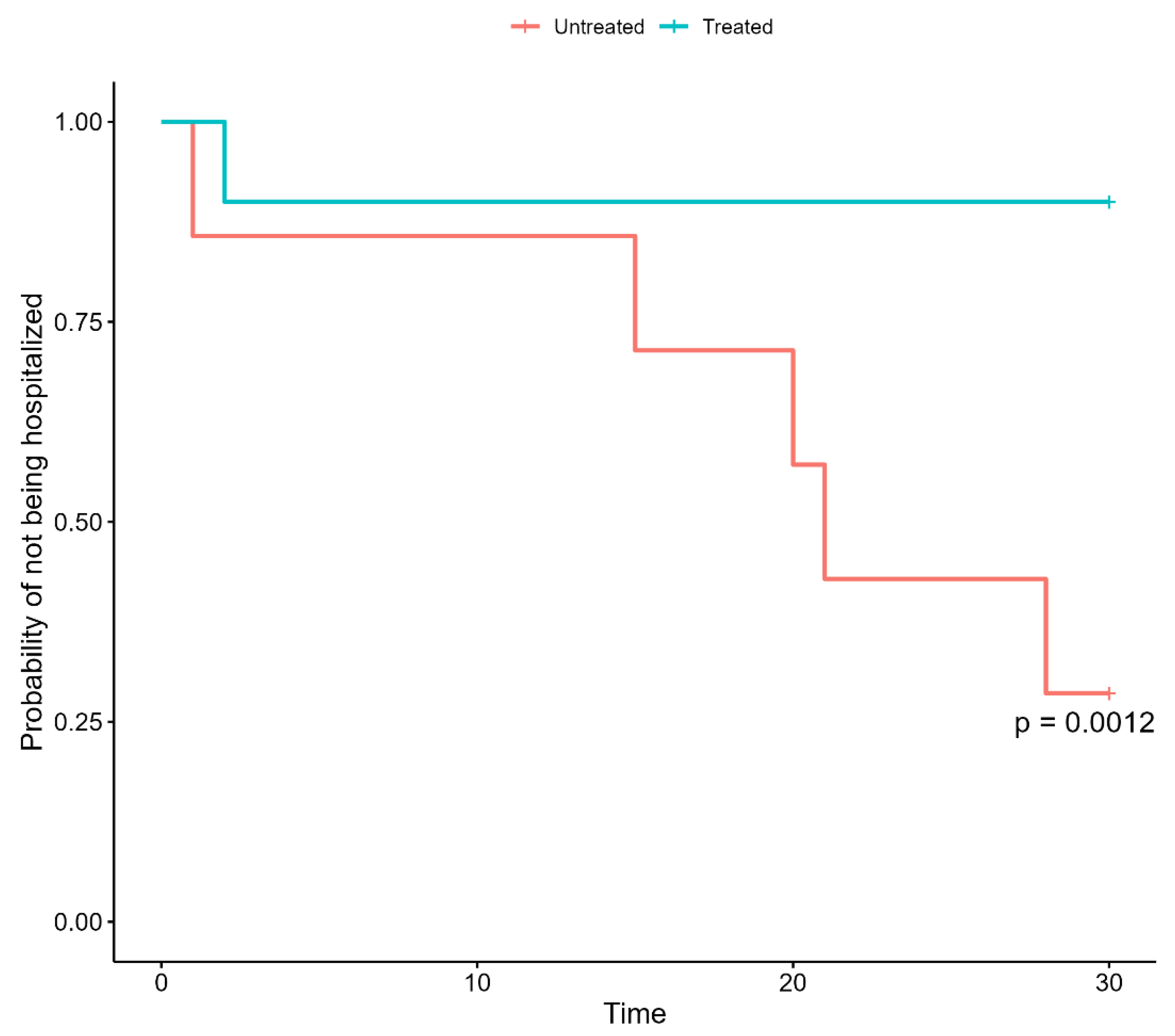
3.1. Impact of Early Therapies on the Outcomes
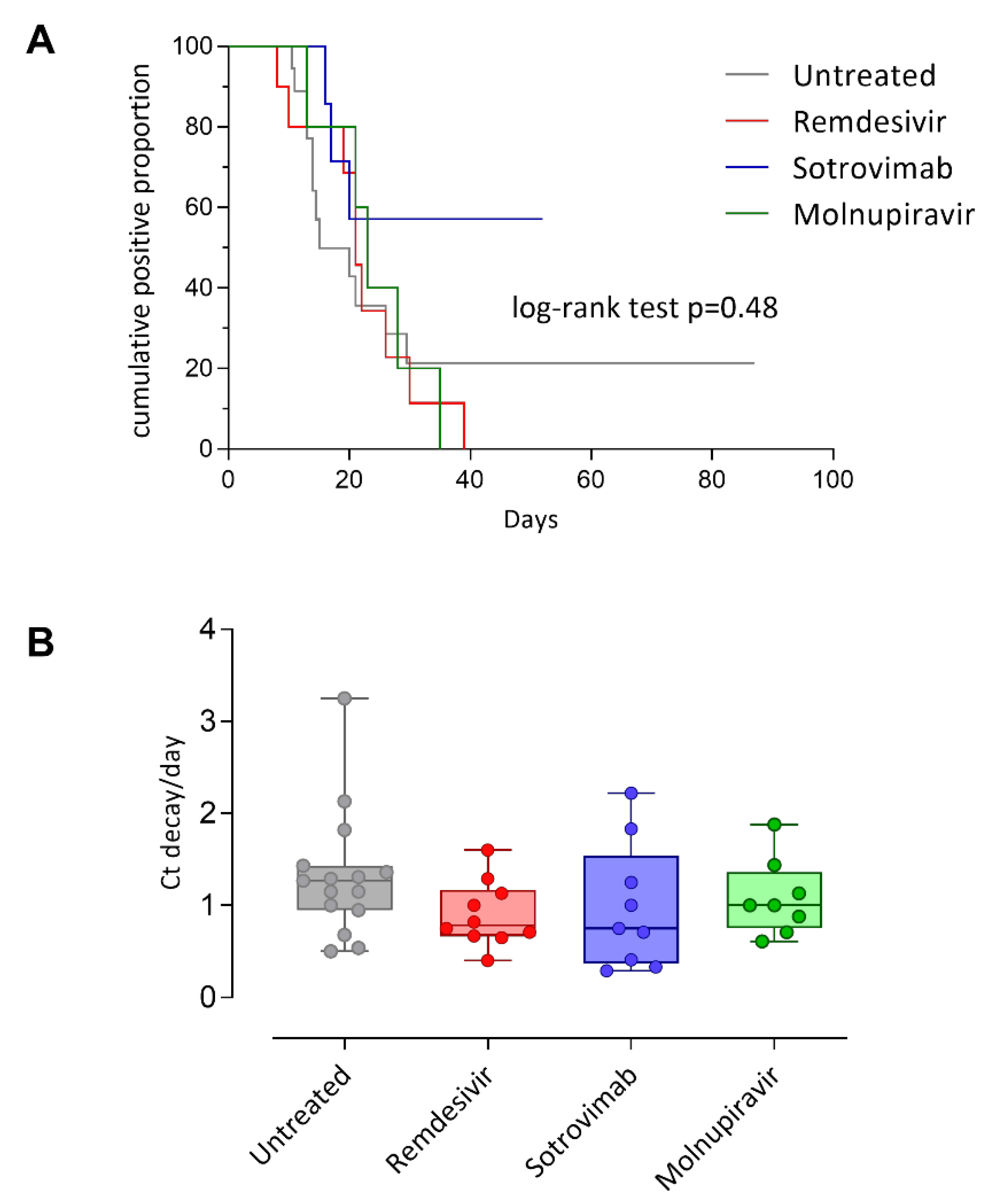
3.2. SARS-CoV-2 RNA Load Kinetics
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Pagano, L.; Salmanton-García, J.; Marchesi, F.; Busca, A.; Corradini, P.; Hoenigl, M.; Klimko, N.; Koehler, P.; Pagliuca, A.; Passamonti, F.; et al. COVID-19 infection in adult patients with hematological malignancies: A European Hematology Association Survey (EPICOVIDEHA). J. Hematol. Oncol. 2021, 14, 168. [Google Scholar] [CrossRef] [PubMed]
- Vijenthira, A.; Gong, I.Y.; Fox, T.A.; Booth, S.; Cook, G.; Fattizzo, B.; Martín-Moro, F.; Razanamahery, J.; Riches, J.C.; Zwicker, J.; et al. Outcomes of patients with hematologic malignancies and COVID-19: A systematic review and meta-analysis of 3377 patients. Blood 2020, 136, 2881–2892. [Google Scholar] [CrossRef] [PubMed]
- El-Sharkawi, D.; Iyengar, S. Haematological cancers and the risk of severe COVID-19: Exploration and critical evaluation of the evidence to date. Br. J. Haematol. 2020, 190, 336–345. [Google Scholar] [CrossRef] [PubMed]
- García-Suárez, J.; de la Cruz, J.; De La Cruz, J.; Cedillo, Á.; Llamas, P.; Duarte, R.; Jiménez-Yuste, V.; Hernández-Rivas, J.Á.; Gil-Manso, R.; Kwon, M.; et al. Impact of hematologic malignancy and type of cancer therapy on COVID-19 severity and mortality: Lessons from a large population-based registry study. J. Hematol. Oncol. 2020, 13, 133. [Google Scholar] [CrossRef] [PubMed]
- Mittelman, M.; Magen, O.; Barda, N.; Dagan, N.; Oster, H.S.; Leader, A.; Balicer, R. Effectiveness of the BNT162b2mRNA COVID-19 vaccine in patients with hematological neoplasms in a nationwide mass vaccination setting. Blood 2022, 139, 1439–1451. [Google Scholar] [CrossRef]
- Rotshild, V.; Hirsh-Raccah, B.; Miskin, I.; Muszkat, M.; Matok, I. Comparing the clinical efficacy of COVID-19 vaccines: A systematic review and network meta-analysis. Sci. Rep. 2021, 11, 22777. Available online: https://www.nature.com/articles/s41598-021-02321-z (accessed on 11 July 2022). [CrossRef]
- Garcia-Vidal, C.; Puerta-Alcalde, P.; Mateu, A.; Cuesta-Chasco, G.; Meira, F.; Lopera, C.; Monzo, P.; Santos-Bravo, M.; Duenas, G.; Chumbita, M.; et al. Prolonged viral replication in patients with hematologic malignancies hospitalized with COVID-19. Haematologica 2022, 107, 1731–1735. [Google Scholar] [CrossRef]
- Tarabay, J.; Babiker, A.; Adelman, M.W.; Stittleburg, V.D.; Varkey, J.; Pouch, S.M.; Waggoner, J.; Piantadosi, A. 278. Immunocompromised Patients with Prolonged Viral Shedding of SARS-COV-2. Open Forum Infect. Dis. 2021, 8 (Suppl. S1), S244–S245. [Google Scholar] [CrossRef]
- Nakajima, Y.; Ogai, A.; Furukawa, K.; Arai, R.; Anan, R.; Nakano, Y.; Kurihara, Y.; Shimizu, H.; Misaki, T.; Okabe, N. Prolonged viral shedding of SARS-CoV-2 in an immunocompromised patient. J. Infect. Chemother. 2021, 27, 387–389. [Google Scholar] [CrossRef]
- Nakamura, S.; Kanemasa, Y.; Atsuta, Y.; Fujiwara, S.; Tanaka, M.; Fukushima, K.; Kobayashi, T.; Shimoyama, T.; Omuro, Y.; Sekiya, N.; et al. Characteristics and outcomes of coronavirus disease 2019 (COVID-19) patients with cancer: A single-center retrospective observational study in Tokyo, Japan. Int. J. Clin. Oncol. 2020, 26, 485–493. [Google Scholar] [CrossRef]
- Levi, G.; Rocchetti, C.; Magri, R.; Uccelli, S.; Bottone, D.; Quadri, F.; Novali, M.; Santin, A.D.; Bezzi, M. Hyperimmune plasma infusion in an immunocompromised COVID-19 patient previously treated for follicular lymphoma. Monaldi Arch. Chest Dis. 2021, 91, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, A.; Monti, M.; Fenwick, C.; Eckerle, I.; Beigelman-Aubry, C.; Pellaton, C.; Jaton, K.; Dumas, D.; Stamm, G.-M.; Infanti, L.; et al. Case Report: Stepwise Anti-Inflammatory and Anti-SARS-CoV-2 Effects Following Convalescent Plasma Therapy with Full Clinical Recovery. Front. Immunol. 2021, 12, 613502. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2021.613502 (accessed on 11 July 2022). [CrossRef] [PubMed]
- Ritonavir-Boosted Nirmatrelvir (Paxlovid). COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/ritonavir-boosted-nirmatrelvir--paxlovid-/ (accessed on 28 June 2022).
- Hammond, J.; Leister-Tebbe, H.; Gardner, A.; Abreu, P.; Bao, W.; Wisemandle, W.; Baniecki, M.; Hendrick, V.M.; Damle, B.; Simón-Campos, A.; et al. Oral Nirmatrelvir for High-Risk, Nonhospitalized Adults with COVID-19. N. Engl. J. Med. 2022, 386, 1397–1408. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa2118542 (accessed on 11 July 2022). [CrossRef] [PubMed]
- Gottlieb, R.L.; Vaca, C.E.; Paredes, R.; Mera, J.; Webb, B.J.; Perez, G.; Oguchi, G.; Ryan, P.; Nielsen, B.U.; Brown, M.; et al. Early Remdesivir to Prevent Progression to Severe COVID-19 in Outpatients. N. Engl. J. Med. 2022, 386, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with COVID-19. N. Engl. J. Med. 2021, 384, 229–237. Available online: https://www.nejm.org/doi/full/10.1056/nejmoa2029849 (accessed on 11 July 2022). [CrossRef]
- Cao, Y.; Wang, J.; Jian, F.; Xiao, T.; Song, W.; Yisimayi, A.; Huang, W.; Li, Q.; Wang, P.; An, R.; et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature 2022, 602, 657–663. Available online: https://www.nature.com/articles/s41586-021-04385-3 (accessed on 11 July 2022). [CrossRef]
- Jayk Bernal, A.; Gomes da Silva, M.M.; Musungaie, D.B.; Kovalchuk, E.; Gonzalez, A.; Delos Reyes, V.; Martín-Quirós, A.; Caraco, Y.; Williams-Diaz, A.; Brown, M.L.; et al. Molnupiravir for Oral Treatment of COVID-19 in Nonhospitalized Patients. N. Engl. J. Med. 2022, 386, 509–520. Available online: https://www.nejm.org/doi/full/10.1056/NEJMoa2116044 (accessed on 11 July 2022). [CrossRef]
- Molnupiravir. COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/therapies/antiviral-therapy/molnupiravir/ (accessed on 28 June 2022).
- Kunal, S.; Sakthivel, P.; Malhotra, N.; Ish, P. Newer oral antivirals for COVID-19: Are they the real game changer? Hear. Lung 2022, 52, 200–203. [Google Scholar] [CrossRef]
- Cesaro, S.; Ljungman, P.; Mikulska, M.; Hirsch, H.H.; von Lilienfeld-Toal, M.; Cordonnier, C.; Meylan, S.; Mehra, V.; Styczynski, J.; Marchesi, F.; et al. Recommendations for the management of COVID-19 in patients with haematological malignancies or haematopoietic cell transplantation, from the 2021 European Conference on Infections in Leukaemia (ECIL 9). Leukemia 2022, 36, 1467–1480. [Google Scholar] [CrossRef] [PubMed]
- Information on COVID-19 Treatment, Prevention and Research. COVID-19 Treatment Guidelines. Available online: https://www.covid19treatmentguidelines.nih.gov/ (accessed on 11 July 2022).
- Bai, Y.; Shen, M.; Zhang, L. Antiviral Efficacy of Molnupiravir for COVID-19 Treatment. Viruses 2022, 14, 763. [Google Scholar] [CrossRef]
- Biancofiore, A.; Mirijello, A.; Puteo, M.A.; Di Viesti, M.P.; Labonia, M.; Copetti, M.; De Cosmo, S.; Lombardi, R. CSS-COVID-19 Group Remdesivir significantly reduces SARS-CoV-2 viral load on nasopharyngeal swabs in hospitalized patients with COVID-19: A retrospective case–control study. J. Med. Virol. 2022, 94, 2284–2289. [Google Scholar] [CrossRef] [PubMed]
- Grewal, R.; Kitchen, S.A.; Nguyen, L.; Buchan, S.A.; Wilson, S.E.; Costa, A.P.; Kwong, J.C. Effectiveness of a fourth dose of COVID-19 mRNA vaccine against the omicron variant among long term care residents in Ontario, Canada: Test negative design study. BMJ 2022, 378, e071502. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, F.; Myers, J.; Basu, D.; Tintinger, G.; Ueckermann, V.; Mathebula, M.; Ramlall, R.; Spoor, S.; de Villiers, T.; Van der Walt, Z.; et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, south africa. Int. J. Infect. Dis. 2021, 116, 38–42. [Google Scholar] [CrossRef] [PubMed]
- Early assessment of the clinical severity of the SARS-CoV-2 omicron variant in South Africa: A data linkage study. Lancet 2022, 399, 437–446. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(22)00017-4/fulltext (accessed on 20 October 2022). [CrossRef] [PubMed]
- Williamson, E.J.; Walker, A.J.; Bhaskaran, K.; Bacon, S.; Bates, C.; Morton, C.E.; Curtis, H.J.; Mehrkar, A.; Evans, D.; Inglesby, P.; et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature 2020, 584, 430–436. [Google Scholar] [CrossRef]
- Haeusler, G.M.; Ammann, R.A.; Carlesse, F.; Groll, A.H.; Averbuch, D.; Castagnola, E.; Agyeman, P.K.; Phillips, B.; Gilli, F.; Solopova, G.; et al. SARS-CoV-2 in children with cancer or after haematopoietic stem cell transplant: An analysis of 131 patients. Eur. J. Cancer 2021, 159, 78–86. [Google Scholar] [CrossRef]
- Zaki, A.; Soomar, S.M.; Khan, D.H.; Sheikh, H.S.; Iftikhar, R.; Mir, A.; Aziz, Z.; Bano, K.; Naseer, H.; Chaudhry, Q.U.; et al. Outcomes of COVID-19 infection in patients with hematological malignancies—A multicenter analysis from Pakistan”. PLoS ONE 2022, 17, e0267139. Available online: https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0267139 (accessed on 11 July 2022). [CrossRef]
| All Patients (88) | Treated (55) | Non-Treated (33) | p-Value | ||
|---|---|---|---|---|---|
| Sex, n (%) | Female | 47 (53) | 27 (31) | 20 (23) | |
| Male | 41 (47) | 28 (32) | 13 (15) | 0.41 | |
| Age, Median (IQR) | 63 (49.0, 71.2) | 62 (52.5, 70.0) | 63 (48, 72) | 0.89 | |
| Vaccination doses, Mean (sd) | 2.7 (0.7) | 2.6 (0.8) | 2.7 (0.5) | 0.69 | |
| Days from last vaccination, Mean (sd) | 124.1 (65) | 128.1(64.3) | 116.9 (67.1) | 0.51 | |
| Remdesivir, n (%) | - | 15 (27) | - | ||
| Ritonavir-boosted Nirmatrelvir, n (%) | - | 10 (18) | - | ||
| Sotrovimab, n (%) | - | 15 (27) | - | ||
| Molnupiravir, n (%) | - | 15 (27) | |||
| Bone marrow transplantation, n (%) | 24 (27) | 18 (75) | 6 (25) | 0.22 | |
| Days from bone marrow transplantation, Mean (sd) | 1307.4 (1793.8) | 1390.3 (1981.8) | 1009 (929.2) | 0.68 | |
| Type of Bone marrow transplantation, n (%) | Autologous | 20 (22) | 14 (25) | 6 (18) | |
| Allogenic | 4 (4) | 4 (7) | 0 (0) | 0.28 | |
| Hematological disease, n (%) | Myeloma | 26 (29) | 17 (31) | 9 (27) | |
| Hodgkin Lymphoma | 8 (9) | 3 (5) | 5 (15) | ||
| High-Grade Non-Hodgkin Lymphoma | 12 (14) | 10 (18) | 2 (6) | ||
| Acute Myeloid Leukemia | 4 (4) | 3 (5) | 1 (3) | ||
| Low-Grade Non-Hodgkin Lymphoma | 16 (18) | 7 (13) | 9 (27) | ||
| Chronic Lymphocytic Leukemia | 4 (4) | 3 (5) | 1 (3) | ||
| Chronic Myeloid Leukemia | 8 (9) | 7 (13) | 1 (3) | ||
| MDS/MPN | 3 (3) | 2 (4) | 1 (3) | ||
| Paroxysmal Nocturnal Hemoglobinuria | 1 (1) | 0 (0) | 1 (3) | ||
| Acute Lymphocytic Leukemia | 4 (4) | 2 (4) | 2 (6) | ||
| Amyloidosis AL | 1 (1) | 1 (2) | 0 (0) | 0.25 | |
| Immunosuppressive therapies, n (%) | Rituximab | 20 (23) | 13 (24) | 7 (21) | 1.00 |
| Obinutuzumab | 5 (6) | 3 (6) | 2 (6) | 1.00 | |
| Methotrexate | 10 (11) | 5 (9) | 5 (15) | 0.60 | |
| CHOP | 15 (17) | 12 (22) | 3 (9) | 0.21 | |
| CHOEP | 1 (1) | 0 (0) | 1 (3) | 0.79 | |
| ABVD | 4 (4) | 1 (2) | 3 (9) | 0.29 | |
| Poli chemotherapy (VCR, Ara-C, Ida, EDX, Cisplatin, Bendamustine) | 21 (24) | 13 (4) | 8 (24) | 1.00 | |
| VD (Bortezomib-Dexamethasone) | 12 (14) | 8 (14) | 4 (12) | 1.00 | |
| Eculizumab | 1 (1) | 0 (0) | 1 (3) | 0.80 | |
| Tyrosine kinase inhibitors (TKIs) | 13 (15) | 10 (18) | 3 (9) | 0.37 | |
| Others (Daratumumab, Isatuximab, IMIDs, Brentuximab, Ab anti-PD1-PDL1) | 30 (34) | 17 (31) | 13 (39) | 0.60 | |
| Days between last therapy and examination, mean (sd) | 3205.2 (11,379.2) | 2902.1 (10,844.7) | 3799 (12,582.5) | 0.75 | |
| Positive anti SARS-CoV-2 antibodies, n (%) | 44 (50) | 20 (36) | 24 (73) | <0.01 | |
| Viral decay (sd) | 26.3 (21.6) | 25.4 (18.0) | 27.7 (24) | 0.63 | |
| Comorbidities | NPL, n (%) | 59 (69) | 32 (63) | 25 (78) | 0.22 |
| CKD, n (%) | 8 (10) | 3 (7) | 5 (15) | ||
| CVD, n (%) | 14 (16) | 8 (15) | 6 (18) | 0.90 | |
| HTN, n (%) | 34 (39) | 21 (39) | 13 (39) | 1.00 | |
| DM, n (%) | 10 (11) | 5 (9) | 5 (15) | 0.62 | |
| LD, n (%) | 10 (11) | 7 (13) | 3 (9) | 0.83 | |
| HCV, n (%) | 2 (3) | 2 (4) | 0 (0) | 0.70 | |
| Obesity, n (%) | 1 (1) | 0 (0) | 1 (3) | 0.80 | |
| Smoke, n (%) | 10 (13) | 5 (12) | 5 (16) | 0.90 | |
| Number of comorbidities, mean (sd) | 1.5 (1.2) | 1.4 (1.1) | 1.7 (1.3) | 0.24 | |
| Mortality, n (%) | 2 (2) | 0 (0) | 2 (6) | 0.27 | |
| Hospital admission, n (%) | 12 (14) | 6 (11) | 6 (18) | 0.52 | |
| ICU admission, n (%) | 1 (1) | 0 (0) | 1 (3) | 0.79 | |
| Stay at Home, n (%) | 78 (87) | 50 (91) | 28 (85) | 0.60 | |
| Symptoms, n (%) | |||||
| Asymptomatic | 10 (12) | 1 (2) | 9 (30) | <0.01 | |
| Fever | 39 (48) | 30 (57) | 9 (32) | 0.06 | |
| Cough | 32 (39) | 24 (45) | 8 (29) | 0.2 | |
| Pharyngodinia | 25 (31) | 16 (30) | 9 (32) | 1.00 | |
| Dyspnea | 10 (13) | 3 (6) | 7 (25) | 0.04 | |
| Diarrhea | 2 (2) | 2 (4) | 0 (0) | 0.77 | |
| Asthenia | 15 (18) | 10 (19) | 5 (17) | 1.00 | |
| Pneumonia | 12 (14) | 6 (11) | 6 (21) | 0.39 | |
| Oxygen therapy | 11 (13) | 5 (9) | 6 (19) | 0.33 |
| Variable | HR | SE | p-Value |
|---|---|---|---|
| Treatment | 0.51 | 0.63 | 0.28 |
| Sex | 0.29 | 0.68 | 0.07 |
| Age | 1.01 | 0.02 | 0.73 |
| Number of vaccinations | 1.42 | 0.61 | 0.56 |
| Comorbidities | 1.63 | 0.26 | 0.06 |
| Variable | HR | SE | p-Value |
|---|---|---|---|
| Paxlovid | 0.51 | 1.10 | 0.55 |
| Remdesivir | 1.16 | 0.71 | 0.83 |
| Molnupiravir | 0.28 | 1.09 | 0.24 |
| Sotrovimab | 0.24 | 1.09 | 0.19 |
| Sex | 0.32 | 0.62 | 0.07 |
| Age | 1.03 | 1.41 | 1.41 |
| Number of vaccinations | 1.43 | 0.56 | 0.57 |
| Variable | HR | SE | p-Value |
|---|---|---|---|
| Treatment | 0.07 | 1.04 | 0.001 |
| Sex | 0.37 | 0.98 | 0.31 |
| Age | 1.00 | 0.04 | 0.91 |
| Number of vaccinations | 1.05 | 0.74 | 0.93 |
| Comorbidities | 1.63 | 0.35 | 0.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Colaneri, M.; Pieri, T.C.; Roda, S.; Ricciardi, A.; Gotti, M.; Ferrari, J.; Arcaini, L.; Rattotti, S.; Piralla, A.; Giardina, F.; et al. Assessing the Efficacy of Early Therapies against SARS-CoV-2 in Hematological Patients: A Real-Life Study from a COVID-19 Referral Centre in Northern Italy. J. Clin. Med. 2022, 11, 7452. https://doi.org/10.3390/jcm11247452
Colaneri M, Pieri TC, Roda S, Ricciardi A, Gotti M, Ferrari J, Arcaini L, Rattotti S, Piralla A, Giardina F, et al. Assessing the Efficacy of Early Therapies against SARS-CoV-2 in Hematological Patients: A Real-Life Study from a COVID-19 Referral Centre in Northern Italy. Journal of Clinical Medicine. 2022; 11(24):7452. https://doi.org/10.3390/jcm11247452
Chicago/Turabian StyleColaneri, Marta, Teresa Chiara Pieri, Silvia Roda, Alessandra Ricciardi, Manuel Gotti, Jacqueline Ferrari, Luca Arcaini, Sara Rattotti, Antonio Piralla, Federica Giardina, and et al. 2022. "Assessing the Efficacy of Early Therapies against SARS-CoV-2 in Hematological Patients: A Real-Life Study from a COVID-19 Referral Centre in Northern Italy" Journal of Clinical Medicine 11, no. 24: 7452. https://doi.org/10.3390/jcm11247452
APA StyleColaneri, M., Pieri, T. C., Roda, S., Ricciardi, A., Gotti, M., Ferrari, J., Arcaini, L., Rattotti, S., Piralla, A., Giardina, F., Ferrari, G., Sacchi, P., Zuccaro, V., Baldanti, F., & Bruno, R. (2022). Assessing the Efficacy of Early Therapies against SARS-CoV-2 in Hematological Patients: A Real-Life Study from a COVID-19 Referral Centre in Northern Italy. Journal of Clinical Medicine, 11(24), 7452. https://doi.org/10.3390/jcm11247452







