A Review of Ophthalmic Complications in Inflammatory Bowel Diseases
Abstract
:1. Introduction
2. Ocular Extraintestinal Manifestation
3. Episcleritis
4. Scleritis
5. Keratopathy
6. Rare Ocular Findings in Patients with IBD
7. Ocular Disorders Related to IBD Treatment
8. Treatment
9. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bertani, L. Treatment and Management of Chronic Inflammatory Bowel Diseases: Optimizing Present and Future Therapeutic Choices. J. Clin. Med. 2022, 11, 5267. [Google Scholar] [CrossRef] [PubMed]
- M’Koma, A.E. Inflammatory Bowel Disease: Clinical Diagnosis and Surgical Treatment-Overview. Medicina 2022, 58, 567. [Google Scholar] [CrossRef] [PubMed]
- Elhag, D.A.; Kumar, M.; Saadaoui, M.; Akobeng, A.K.; Al-Mudahka, F.; Elawad, M.; Al Khodor, S. Inflammatory Bowel Disease Treatments and Predictive Biomarkers of Therapeutic Response. Int. J. Mol. Sci. 2022, 23, 6966. [Google Scholar] [CrossRef]
- Younis, N.; Zarif, R.; Mahfouz, R. Inflammatory bowel disease: Between genetics and microbiota. Mol. Biol. Rep. 2020, 47, 3053–3063. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wu, G.D.; Albenberg, L.; Tomov, V.T. Gut microbiota and IBD: Causation or correlation? Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 573–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, A.; Inoue, R.; Inatomi, O.; Bamba, S.; Naito, Y.; Andoh, A. Gut microbiota in the pathogenesis of inflammatory bowel disease. Clin. J. Gastroenterol. 2018, 11, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kofla-Dłubacz, A.; Pytrus, T.; Akutko, K.; Sputa-Grzegrzółka, P.; Piotrowska, A.; Dzięgiel, P. Etiology of IBD—Is It Still a Mystery? Int. J. Mol. Sci. 2022, 23, 12445. [Google Scholar] [CrossRef]
- Kumar, M.; Garand, M.; Al Khodor, S. Integrating omics for a better understanding of Inflammatory Bowel Disease: A step towards personalized medicine. J. Transl. Med. 2019, 17, 419. [Google Scholar] [CrossRef] [Green Version]
- Silva, J.; Leite, D.; Fernandes, M.; Mena, C.; Gibbs, P.A.; Teixeira, P. Campylobacter spp. as a Foodborne Pathogen: A Review. Front. Microbiol. 2011, 2, 200. [Google Scholar] [CrossRef] [Green Version]
- Naser, S.A.; Arce, M.; Khaja, A.; Fernandez, M.; Naser, N.; Elwasila, S.; Thanigachalam, S. Role of ATG16L, NOD2 and IL23R in Crohn’s disease pathogenesis. World J. Gastroenterol. 2012, 18, 412–424. [Google Scholar] [CrossRef]
- Nambu, R.; Muise, A.M. Advanced Understanding of Monogenic Inflammatory Bowel Disease. Front. Pediatr. 2020, 8, 618918. [Google Scholar] [CrossRef] [PubMed]
- Cheema, H.A.; Waheed, N.; Saeed, A.; Fayyaz, Z.; Anjum, M.N.; Alvi, M.A.; Batool, S.S. Very early onset inflammatory bowel disease: Spectrum of clinical presentation, diagnostic tools and outcome in children. J. Pak. Med. Assoc. 2021, 71, 2350–2354. [Google Scholar] [CrossRef] [PubMed]
- Ananthakrishnan, A.N.; Kaplan, G.G.; Ng, S.C. Changing Global Epidemiology of Inflammatory Bowel Diseases: Sustaining Health Care Delivery Into the 21st Century. Clin. Gastroenterol. Hepatol. 2020, 18, 1252–1260. [Google Scholar] [CrossRef]
- Burisch, J. Crohn’s disease and ulcerative colitis. Occurrence, course and prognosis during the first year of disease in a European population-based inception cohort. Dan. Med. J. 2014, 61, B4778. [Google Scholar] [PubMed]
- Mak, W.Y.; Zhao, M.; Ng, S.C.; Burisch, J. The epidemiology of inflammatory bowel disease: East meets west. J. Gastroenterol. Hepatol. 2020, 35, 380–389. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhou, R.; Bai, X.; Guo, M.; Ruan, G.; Wang, L.; Qian, J. Trend and Geographic Variation in Incidence and Prevalence of Inflammatory Bowel Disease in Regions Across China: A Nationwide Employee Study Between 2013 and 2016. Front. Med. 2022, 9. [Google Scholar] [CrossRef]
- Lirhus, S.S.; Høivik, M.L.; Moum, B.; Anisdahl, K.; Melberg, H.O. Incidence and Prevalence of Inflammatory Bowel Disease in Norway and the Impact of Different Case Definitions: A Nationwide Registry Study. Clin. Epidemiol. 2021, 13, 287–294. [Google Scholar] [CrossRef]
- Perler, B.K.; Ungaro, R.; Baird, G.; Mallette, M.; Bright, R.; Shah, S.; Shapiro, J.; Sands, B.E. Presenting symptoms in inflammatory bowel disease: Descriptive analysis of a community-based inception cohort. BMC Gastroenterol. 2019, 19, 47. [Google Scholar] [CrossRef] [Green Version]
- McDowell, C.; Farooq, U.; Haseeb, M. Inflammatory Bowel Disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. [Google Scholar]
- Nóbrega, V.G.; Silva, I.N.N.; Brito, B.S.; Silva, J.; Silva, M.; Santana, G.O. The onset of clinical manifestations in inflammatory bowel disease patients. Arq. Gastroenterol. 2018, 55, 290–295. [Google Scholar] [CrossRef]
- Troncoso, L.L.; Biancardi, A.L.; de Moraes, H.V., Jr.; Zaltman, C. Ophthalmic manifestations in patients with inflammatory bowel disease: A review. World J. Gastroenterol. 2017, 23, 5836–5848. [Google Scholar] [CrossRef]
- Annese, V. A Review of Extraintestinal Manifestations and Complications of Inflammatory Bowel Disease. Saudi. J. Med. Med. Sci. 2019, 7, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Olpin, J.D.; Sjoberg, B.P.; Stilwill, S.E.; Jensen, L.E.; Rezvani, M.; Shaaban, A.M. Beyond the Bowel: Extraintestinal Manifestations of Inflammatory Bowel Disease. Radiographics 2017, 37, 1135–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.M.; Cheon, J.H. Pathogenesis and clinical perspectives of extraintestinal manifestations in inflammatory bowel diseases. Intest. Res. 2020, 18, 249–264. [Google Scholar] [CrossRef] [Green Version]
- Roberts, H.; Rai, S.N.; Pan, J.; Rao, J.M.; Keskey, R.C.; Kanaan, Z.; Short, E.P.; Mottern, E.; Galandiuk, S. Extraintestinal manifestations of inflammatory bowel disease and the influence of smoking. Digestion 2014, 90, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Mady, R.; Grover, W.; Butrus, S. Ocular Complications of Inflammatory Bowel Disease. Sci. World J. 2015, 2015, 438402. [Google Scholar] [CrossRef] [Green Version]
- King, D.; Chandan, J.S.; Thomas, T.; Denniston, A.K.; Braithwaite, T.; Niranthrankumar, K.; Reulen, R.; Adderley, N.; Trudgill, N.J. Risk of a subsequent diagnosis of inflammatory bowel disease in subjects with ophthalmic disorders associated with inflammatory bowel disease: A retrospective cohort analysis of UK primary care data. BMJ Open. 2022, 12, e052833. [Google Scholar] [CrossRef]
- Thomas, A.S.; Lin, P. Ocular manifestations of inflammatory bowel disease. Curr. Opin. Ophthalmol. 2016, 27, 552–560. [Google Scholar] [CrossRef]
- Ottaviano, G.; Salvatore, S.; Salvatoni, A.; Martelossi, S.; Ventura, A.; Naviglio, S. Ocular Manifestations of Paediatric Inflammatory Bowel Disease: A Systematic Review and Meta-analysis. J. Crohns. Colitis 2018, 12, 870–879. [Google Scholar] [CrossRef]
- Orchard, T.R.; Chua, C.N.; Ahmad, T.; Cheng, H.; Welsh, K.I.; Jewell, D.P. Uveitis and erythema nodosum in inflammatory bowel disease: Clinical features and the role of HLA genes. Gastroenterology 2002, 123, 714–718. [Google Scholar] [CrossRef]
- Taylor, S.R.; McCluskey, P.; Lightman, S. The ocular manifestations of inflammatory bowel disease. Curr. Opin. Ophthalmol. 2006, 17, 538–544. [Google Scholar] [CrossRef]
- Taleban, S.; Li, D.; Targan, S.R.; Ippoliti, A.; Brant, S.R.; Cho, J.H.; Duerr, R.H.; Rioux, J.D.; Silverberg, M.S.; Vasiliauskas, E.A.; et al. Ocular Manifestations in Inflammatory Bowel Disease Are Associated with Other Extra-intestinal Manifestations, Gender, and Genes Implicated in Other Immune-related Traits. J. Crohns. Colitis 2016, 10, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Denniston, A.; Murray, P. Oxford Handbook of Ophthalmology, 4th ed.; Oxford University Press: Oxford, UK, 2018; pp. 443–464. [Google Scholar]
- Cuny, A.; Guillo, L.; Baumann, C.; Netter, P.; Danese, S.; Caron, B.; Peyrin-Biroulet, L.; Angioi, K. Ocular Manifestations in Patients with Inflammatory Bowel Disease in the Biologics Era. J. Clin. Med. 2022, 11, 4538. [Google Scholar] [CrossRef] [PubMed]
- Vavricka, S.R.; Schoepfer, A.; Scharl, M.; Lakatos, P.L.; Navarini, A.; Rogler, G. Extraintestinal Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2015, 21, 1982–1992. [Google Scholar] [CrossRef] [Green Version]
- Shah, J.; Shah, A.; Hassman, L.; Gutierrez, A. Ocular Manifestations of Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2021, 27, 1832–1838. [Google Scholar] [CrossRef]
- Jansen, F.M.; Vavricka, S.R.; den Broeder, A.A.; de Jong, E.M.; Hoentjen, F.; van Dop, W.A. Clinical management of the most common extra-intestinal manifestations in patients with inflammatory bowel disease focused on the joints, skin and eyes. United Eur. Gastroenterol. J. 2020, 8, 1031–1044. [Google Scholar] [CrossRef] [PubMed]
- Calvo, P.; Pablo, L. Managing IBD Outside the Gut: Ocular Manifestations. Dig. Dis. 2013, 31, 229–232. [Google Scholar] [CrossRef] [PubMed]
- Manganelli, C.; Turco, S.; Balestrazzi, E. Ophthalmological aspects of IBD. Eur. Rev. Med. Pharm. Sci. 2009, 13 (Suppl. 1), 11–13. [Google Scholar]
- Rogler, G.; Singh, A.; Kavanaugh, A.; Rubin, D.T. Extraintestinal Manifestations of Inflammatory Bowel Disease: Current Concepts, Treatment, and Implications for Disease Management. Gastroenterology 2021, 161, 1118–1132. [Google Scholar] [CrossRef]
- Barberio, J.; Kim, S.C.; Roh, M.; Lewis, J.D.; Desai, R.J. Risk of Uveitis in Patients With Inflammatory Bowel Disease on Immunosuppressive Drug Therapy. Crohn’s Colitis 360 2020, 2, otaa041. [Google Scholar] [CrossRef]
- Biedermann, L.; Renz, L.; Fournier, N.; Rossel, J.B.; Butter, M.; Bluemel, S.; Vavricka, S.R.; Rogler, G.; Scharl, M. Uveitis manifestations in patients of the Swiss Inflammatory Bowel Disease Cohort Study. Ther. Adv. Gastroenterol. 2019, 12, 1756284819865142. [Google Scholar] [CrossRef] [Green Version]
- Imam, L.; Haboubi, H.N. G-Eye: Ocular manifestations of gastrointestinal disease. Frontline Gastroenterol. 2020, 11, 162. [Google Scholar] [CrossRef] [PubMed]
- Czompa, L.; Barta, Z.; Ziad, H.; Nemeth, G.; Rentka, A.; Aszalos, Z.; Zold, E.; Gesztelyi, R.; Zsuga, J.; Szodoray, P.; et al. Corneal Manifestations of Inflammatory Bowel Disease. Semin. Ophthalmol. 2019, 34, 543–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sainz de la Maza, M.; Foster, C.S.; Jabbur, N.S.; Baltatzis, S. Ocular Characteristics and Disease Associations in Scleritis-Associated Peripheral Keratopathy. Arch. Ophthalmol. 2002, 120, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Angioi, K.; Kaminsky, P.; Peyrin-Biroulet, L. Infliximab for severe peripheral ulcerative keratopathy revealing Crohn’s disease. Inflamm. Bowel Dis. 2011, 17, 866–867. [Google Scholar] [CrossRef] [PubMed]
- Ceresara, G.; Fogagnolo, P.; De Cillà, S.; Panizzo, V.; Danelli, P.G.; Orzalesi, N.; Rossetti, L. Corneal involvement in Crohn’s disease: An in vivo confocal microscopy study. Cornea 2011, 30, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Barta, Z.; Czompa, L.; Rentka, A.; Zold, E.; Remenyik, J.; Biro, A.; Gesztelyi, R.; Zsuga, J.; Szodoray, P.; Kemeny-Beke, A. Evaluation of Objective Signs and Subjective Symptoms of Dry Eye Disease in Patients with Inflammatory Bowel Disease. Biomed. Res. Int. 2019, 2019, 8310583. [Google Scholar] [CrossRef] [Green Version]
- Lange, A.P.; Bahar, I.; Sansanayudh, W.; Kaisermann, I.; Slomovic, A.R. Salzmann nodules—A possible new ocular manifestation of Crohn’s disease: A case report. Cornea 2009, 28, 85–86. [Google Scholar] [CrossRef]
- Roszkowska, A.M.; Spinella, R.; Aragona, P. Recurrence of Salzmann nodular degeneration of the cornea in a Crohn’s disease patient. Int. Ophthalmol. 2013, 33, 185–187. [Google Scholar] [CrossRef]
- Aman-Ullah, M.; Gimbel, H.V.; Purba, M.K.; van Westenbrugge, J.A. Necrotizing keratitis after laser refractive surgery in patients with inactive inflammatory bowel disease. Case Rep. Ophthalmol. 2012, 3, 54–60. [Google Scholar] [CrossRef]
- Matet, A.; Daruich, A.; Beydoun, T.; Cosnes, J.; Bourges, J.-L. Systemic adalimumab induces peripheral corneal infiltrates: A case report. BMC Ophthalmol. 2015, 15, 57. [Google Scholar] [CrossRef] [Green Version]
- Drury, J.; Hickman, S.J. Internuclear ophthalmoplegia associated with anti-TNFα medication. Strabismus 2015, 23, 30–32. [Google Scholar] [CrossRef] [PubMed]
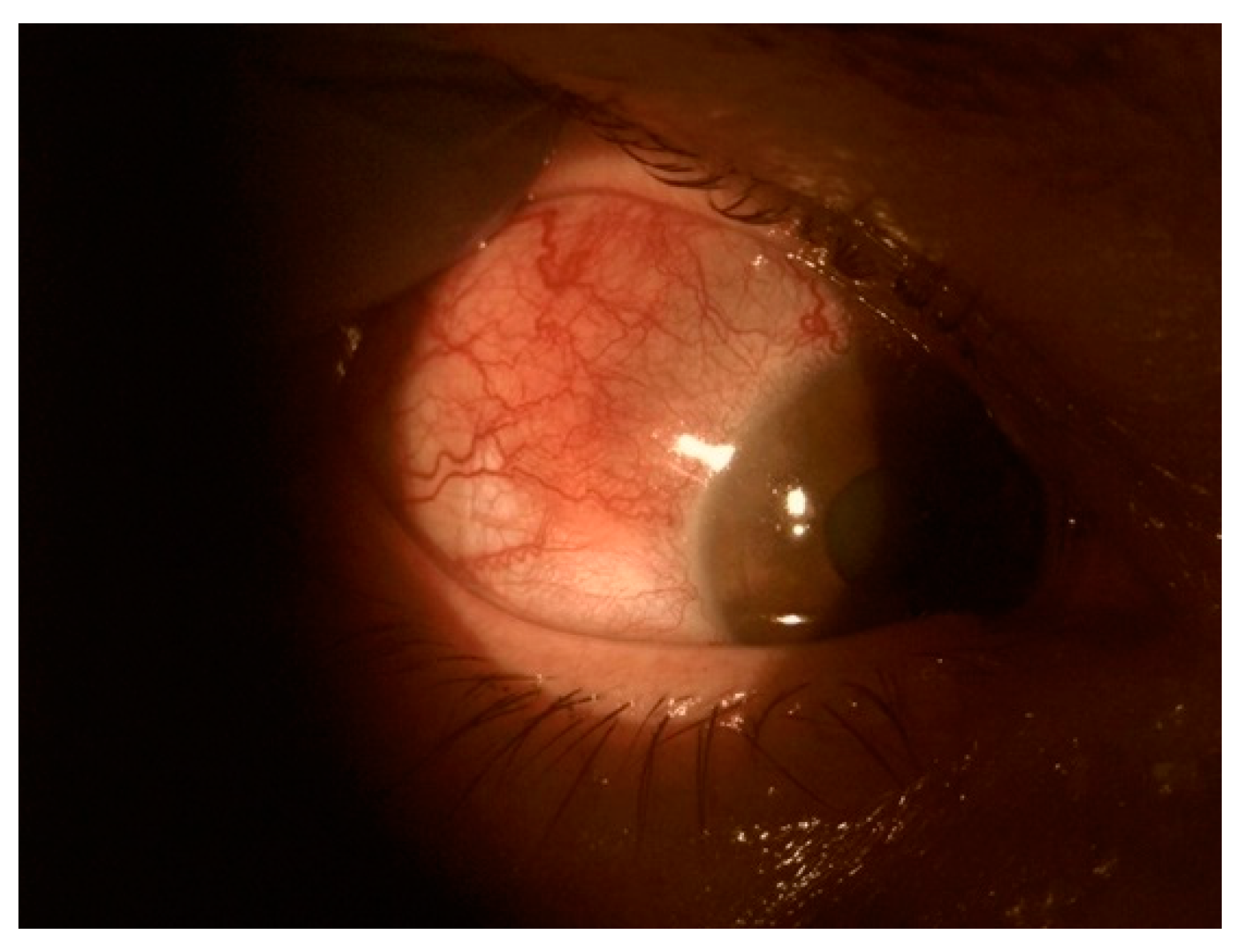

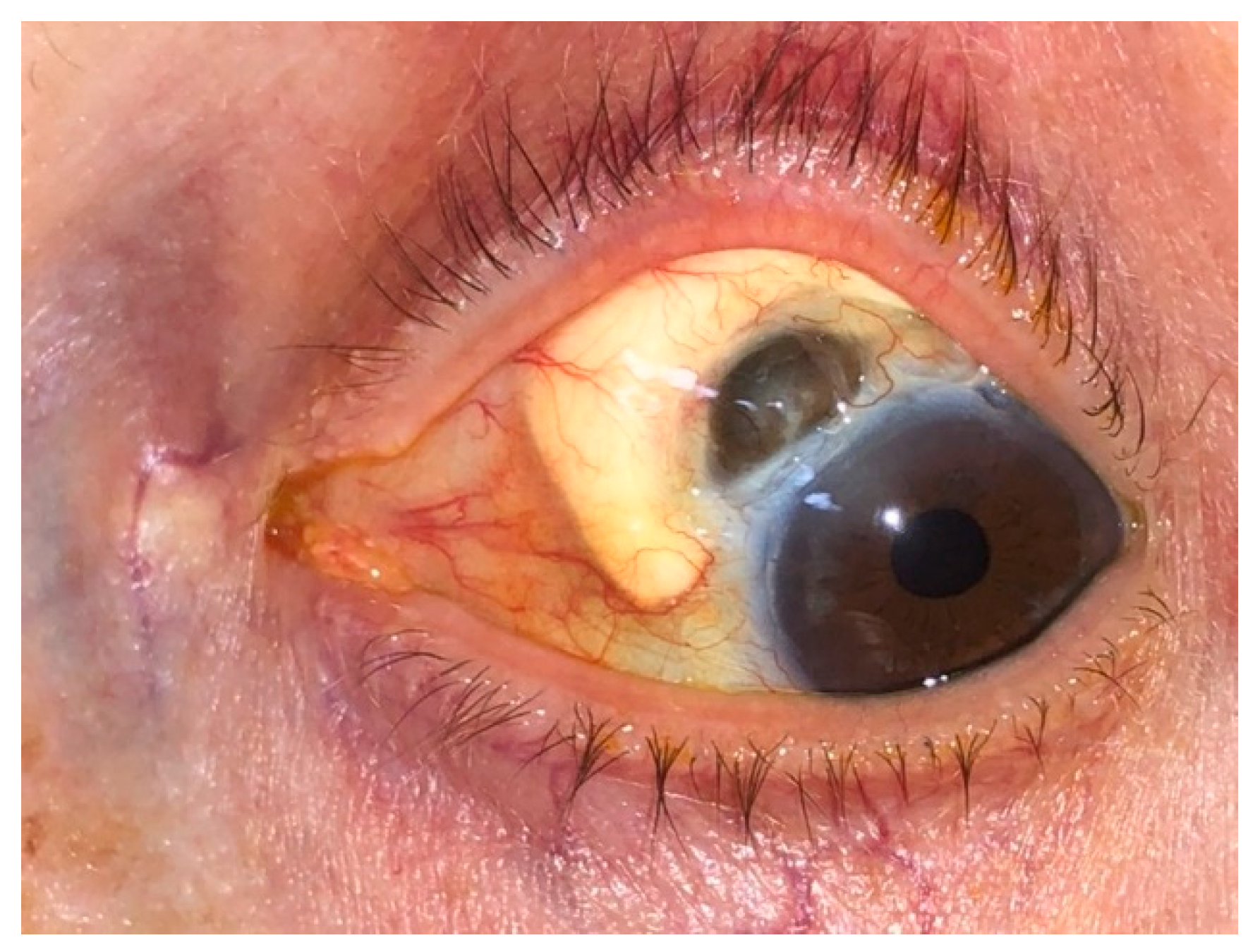

| Episcleritis | Scleritis | Uveitis |
|---|---|---|
| Indicates IBD flares. | May develop during a remission stage. | May develop during a remission stage. |
| Self-limiting condition. | Potentially blinding inflammation | May have devious and persistent course. |
| Resolves with IBD treatment. | Systemic steroids or immunosuppression. | Topical steroids and topical cycloplegics (recurrent cases require systemic steroids or immunosuppression). |
| Good prognosis. | Uncertain prognosis. | Uncertain prognosis. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pytrus, W.; Akutko, K.; Pytrus, T.; Turno-Kręcicka, A. A Review of Ophthalmic Complications in Inflammatory Bowel Diseases. J. Clin. Med. 2022, 11, 7457. https://doi.org/10.3390/jcm11247457
Pytrus W, Akutko K, Pytrus T, Turno-Kręcicka A. A Review of Ophthalmic Complications in Inflammatory Bowel Diseases. Journal of Clinical Medicine. 2022; 11(24):7457. https://doi.org/10.3390/jcm11247457
Chicago/Turabian StylePytrus, Wiktoria, Katarzyna Akutko, Tomasz Pytrus, and Anna Turno-Kręcicka. 2022. "A Review of Ophthalmic Complications in Inflammatory Bowel Diseases" Journal of Clinical Medicine 11, no. 24: 7457. https://doi.org/10.3390/jcm11247457





