Predictive Factors for Bone Cement Displacement following Percutaneous Vertebral Augmentation in Kümmell’s Disease
Abstract
1. Introduction
2. Data and Methods
2.1. General Data
2.2. Bone Cement Displacement Diagnostic Criteria
2.3. Treatment Method
2.4. Evaluation Index
2.5. Index Definition
2.6. Statistical Analysis
3. Results
3.1. General Information
3.2. Univariate Analysis
3.3. Binary Logistic Regression Analysis
4. Discussion
5. Limitation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Benedek, T.G.; Nicholas, J.J. Delayed traumatic vertebral body compression fracture; part II: Pathologic features. Semin. Arthritis Rheum. 1981, 10, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.; Choi, S.W.; Youm, J.Y.; Kwon, H.J.; Kim, S.H.; Koh, H.S. Posttraumatic Delayed Vertebral Collapse: Kummell’s Disease. J. Korean Neurosurg. Soc. 2018, 61, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Adamska, O.; Modzelewski, K.; Stolarczyk, A.; Kseniuk, J. Is Kummell’s Disease a Misdiagnosed and/or an Underreported Complication of Osteoporotic Vertebral Compression Fractures? A Pattern of the Condition and Available Treatment Modalities. J. Clin. Med. 2021, 10, 2584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, B.; Sun, Z.; Zhang, Y.; Su, J. Comparative Efficacy of Three Minimally Invasive Procedures for Kümmell’s Disease: A Systematic Review and Network Meta-Analysis. Front. Surg. 2022, 9, 893404. [Google Scholar] [CrossRef]
- Yang, H.; Pan, J.; Wang, G. A review of osteoporotic vertebral fracture nonunion management. Spine (Phila Pa 1976) 2014, 39, B4–B6. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Lee, C.J.; Yeon, J.T.; Bae, J.; Choi, E.; Lee, P.B.; Nahm, F.S. Insufficient Penetration of Bone Cement into the Trabecular Bone: A Potential Risk for Delayed Bone Cement Displacement After Kyphoplasty? Reg. Anesth. Pain Med. 2016, 41, 616–618. [Google Scholar] [CrossRef] [PubMed]
- Tsai, T.T.; Chen, W.J.; Lai, P.L.; Chen, L.H.; Niu, C.C.; Fu, T.S.; Wong, C.B. Polymethylmethacrylate cement dislodgment following percutaneous vertebroplasty: A case report. Spine (Phila Pa 1976) 2003, 28, E457–E460. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.S.; Kim, H.S.; Ju, C.I.; Kim, S.W. Delayed bone cement displacement following balloon kyphoplasty. J. Korean Neurosurg. Soc. 2008, 43, 212–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Choi, S.S.; Lee, M.K.; Lee, D.K.; Cho, S.I. Failed Percutaneous Vertebroplasty Due to Insufficient Correction of Intravertebral Instability in Kummell’s Disease: A Case Report. Pain Pract. 2017, 17, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Nagad, P.; Rawall, S.; Kundnani, V.; Mohan, K.; Patil, S.S.; Nene, A. Postvertebroplasty instability. J. Neurosurg. Spine 2012, 16, 387–393. [Google Scholar] [CrossRef]
- Li, K.; Wong, T.; Kung, F.; Li, A.; Hsieh, C. Staging of Kümmell’s disease. J. Musculoskelet. Res. 2004, 8, 43–55. [Google Scholar] [CrossRef]
- Gao, X.; Du, J.; Gao, L.; Hao, D.; Hui, H.; He, B.; Yan, L. Risk factors for bone cement displacement after percutaneous vertebral augmentation for osteoporotic vertebral compression fractures. Front. Surg. 2022, 9, 947212. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.X.; Guo, D.Q.; Zhang, S.C.; Liang, D.; Yuan, K.; Mo, G.Y.; Li, D.X.; Guo, H.Z.; Tang, Y.; Luo, P.J. Risk factor analysis for re-collapse of cemented vertebrae after percutaneous vertebroplasty (PVP) or percutaneous kyphoplasty (PKP). Int. Orthop. 2018, 42, 2131–2139. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Cheng, X.; Wu, H. Risk factors of new vertebral compression fracture after percutaneous vertebroplasty or percutaneous kyphoplasty. Front. Endocrinol. (Lausanne) 2022, 13, 964578. [Google Scholar] [CrossRef]
- Fu, Z.; Hu, X.; Wu, Y.; Zhou, Z. Is There a Dose-Response Relationship of Cement Volume with Cement Leakage and Pain Relief after Vertebroplasty? Dose Response 2016, 14, 1559325816682867. [Google Scholar] [CrossRef]
- Lv, N.N.; Hou, M.Z.; Zhou, Z.Z.; Feng, X.X.; Liu, H.J.; Shan, F.R.; Li, E.H.; Guan, B.Y.; He, S.J.; Liu, M.M. Does the Relationship Between Bone Cement and the Intravertebral Cleft of Kummell Disease Affect the Efficacy of PKP? World Neurosurg. 2022, 160, e430–e435. [Google Scholar] [CrossRef]
- Liang, D.; Ye, L.Q.; Jiang, X.B.; Yang, P.; Zhou, G.Q.; Yao, Z.S.; Zhang, S.C.; Yang, Z.D. Biomechanical effects of cement distribution in the fractured area on osteoporotic vertebral compression fractures: A three-dimensional finite element analysis. J. Surg. Res. 2015, 195, 246–256. [Google Scholar] [CrossRef]
- Li, W.; Wang, J.; Liu, W.; Xu, C.; Li, W.; Zhang, K.; Su, S.; Li, R.; Hu, Z.; Liu, Q.; et al. Machine Learning Applications for the Prediction of Bone Cement Leakage in Percutaneous Vertebroplasty. Front. Public Health 2021, 9, 812023. [Google Scholar] [CrossRef]
- Kawaguchi, S.; Horigome, K.; Yajima, H.; Oda, T.; Kii, Y.; Yoshimoto, M.; Takebayashi, T.; Yamashita, T. Conversion to hypertrophic vertebral pseudarthrosis following percutaneous vertebroplasty. Eur. Spine J. 2010, 19, 901–906. [Google Scholar] [CrossRef]
- Mao, W.; Dong, F.; Huang, G.; He, P.; Chen, H.; Qin, S.; Li, A. Risk factors for secondary fractures to percutaneous vertebroplasty for osteoporotic vertebral compression fractures: A systematic review. J. Orthop. Surg. Res. 2021, 16, 644. [Google Scholar] [CrossRef]
- Dai, C.; Liang, G.; Zhang, Y.; Dong, Y.; Zhou, X. Risk factors of vertebral re-fracture after PVP or PKP for osteoporotic vertebral compression fractures, especially in Eastern Asia: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2022, 17, 161. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.K.; Lee, C.W.; Park, N.K.; Kang, T.W.; Lim, J.W.; Cha, K.Y.; Kim, J.H. Predictive risk factors for refracture after percutaneous vertebroplasty. Ann. Rehabil. Med. 2011, 35, 844–851. [Google Scholar] [CrossRef] [PubMed]
- Ensrud, K.E.; Crandall, C.J. Osteoporosis. Ann. Intern. Med. 2017, 167, Itc17–Itc32. [Google Scholar] [CrossRef] [PubMed]
- Cotts, K.G.; Cifu, A.S. Treatment of Osteoporosis. Jama 2018, 319, 1040–1041. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Xia, H.; Wang, J.; Zhu, X.; Huang, F.; Lu, L.; He, L. Re-fracture and correlated risk factors in patients with osteoporotic vertebral fractures. J. Bone Miner. Metab. 2019, 37, 722–728. [Google Scholar] [CrossRef]
- Svejme, O.; Ahlborg, H.G.; Nilsson, J.; Karlsson, M.K. Low BMD is an independent predictor of fracture and early menopause of mortality in post-menopausal women—A 34-year prospective study. Maturitas 2013, 74, 341–345. [Google Scholar] [CrossRef]

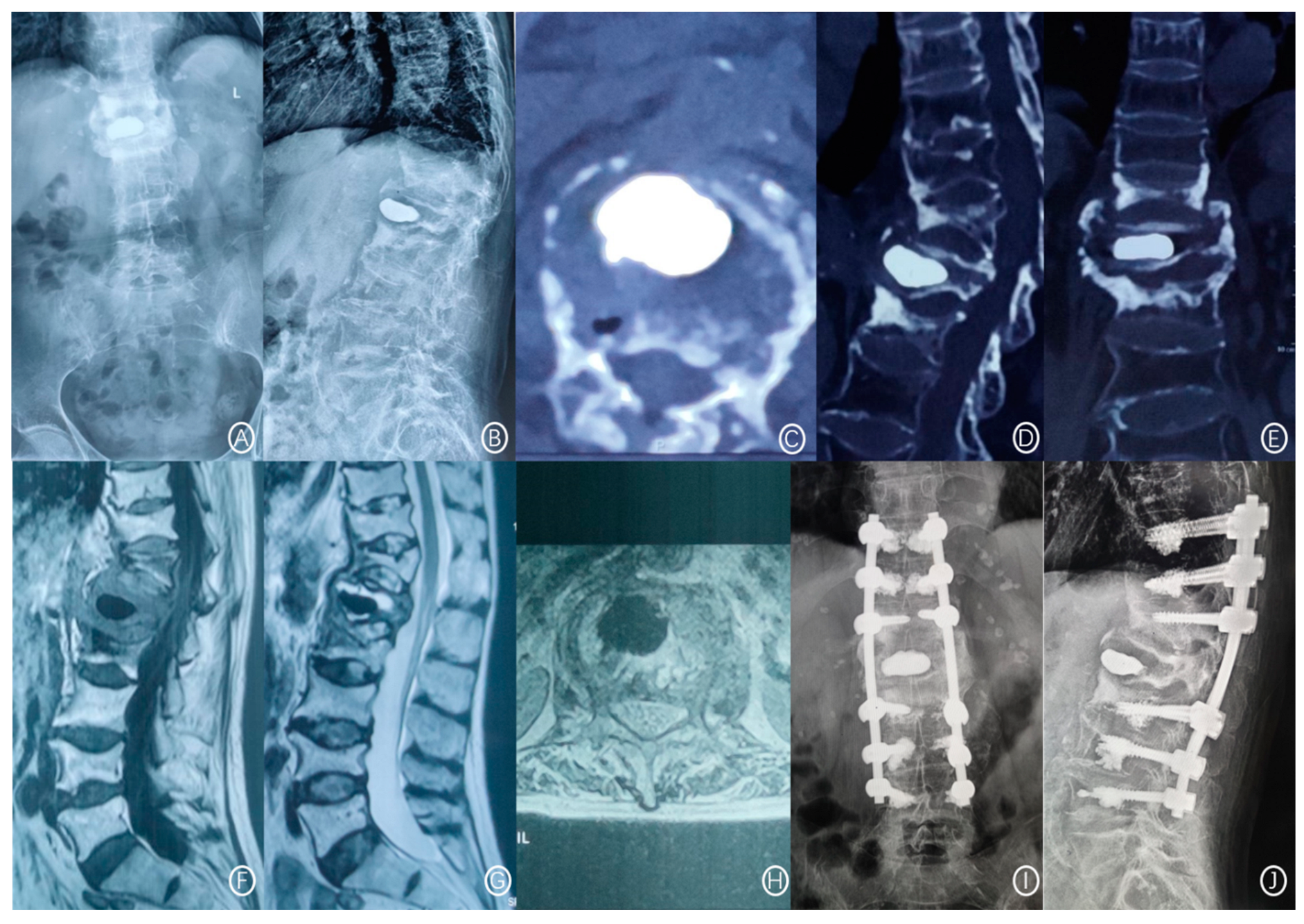
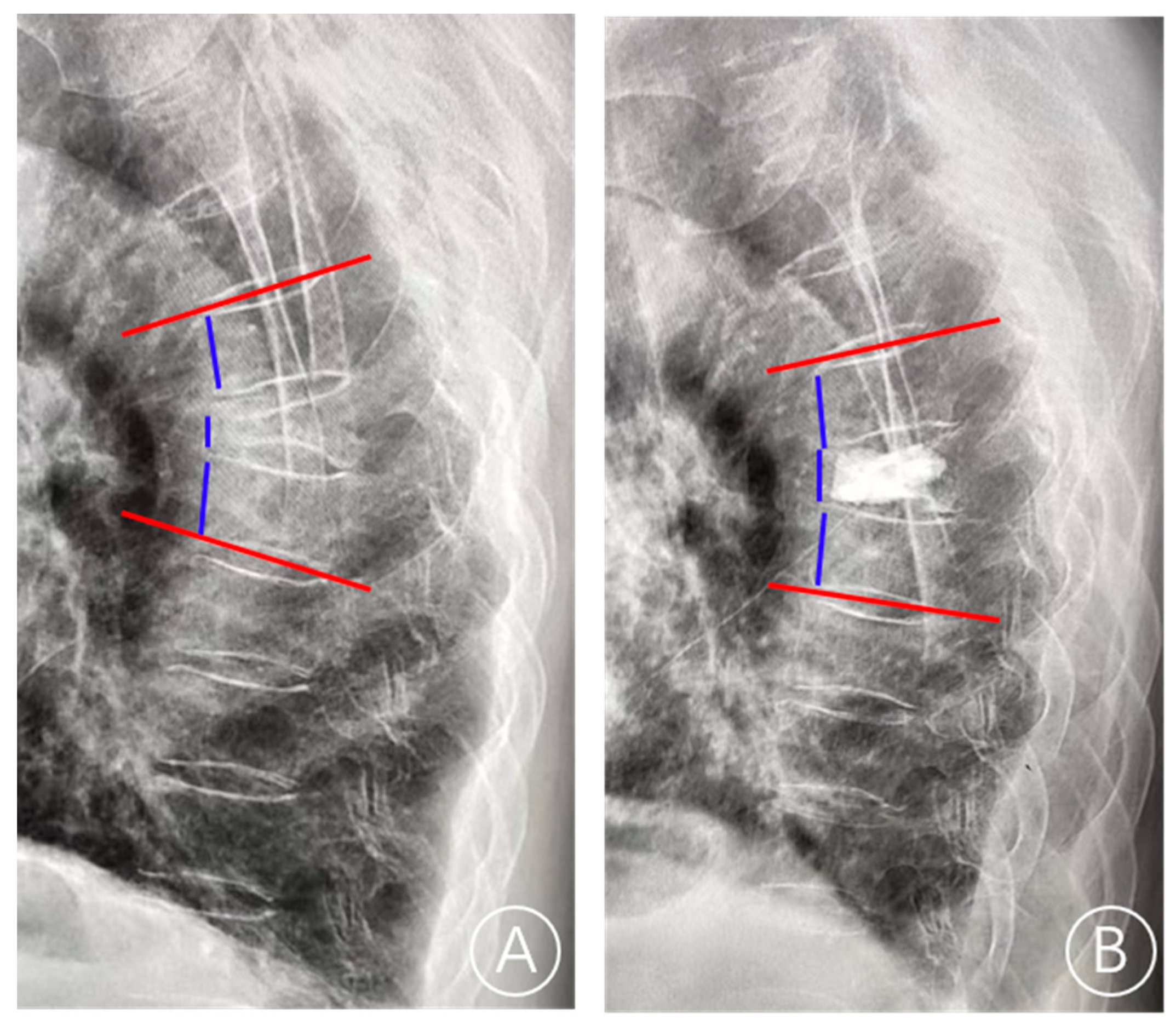


| Number of Patients, n | 824 |
|---|---|
| Gender | |
| Male/Female | 248/576 |
| Mean age (range, years) | 70 (55–95) |
| Mean BMI (range, kg/m²) | 24.0 (19.1–28.0) |
| Underlying diseases | |
| Hypertension/Diabetes/Heart disease/Others | 121/119/70/514 |
| Mean BMD (range) | −3.6 (−2.5–−5.4) |
| Injured vertebral segment | |
| Thoracolumbar junction/thoracic junction/lumbar junction | 619/121/84 |
| Kümmell’s disease staging | |
| I/II | 219/605 |
| Median cement displacement (range, months) | 15 (1.8–38.2) |
| Mean preoperative anterior vertebral height (range, mm) | 25.0 (16.3–29.5) |
| Mean postoperative anterior vertebral height (range, mm) | 28.0 (23.1–31.6) |
| Mean preoperative local Cobb angles (range, °) | 22.8 (11.8–28.3) |
| Mean postoperative local Cobb angles (range, °) | 13.1 (9.1–16.7) |
| Integrity of anterior vertebral cortex | |
| No/Yes | 406/418 |
| Integrity of endplate in surgical vertebrae | |
| No/Yes | 150/674 |
| Surgical method | |
| PVP/PKP | 189/635 |
| Surgical approach | |
| Unilateral/Bilateral | 678/146 |
| Mean volume of cement (range, mL) | 4.5 (2.4–6.7) |
| Even cement distribution | |
| No/Yes | 365/459 |
| Viscosity of cement | |
| High/Low | 531/293 |
| Cement leakage | |
| No/Yes | 405/419 |
| Postoperative anti-osteoporosis treatment | |
| No/Yes | 511/313 |
| Postoperative bracing treatment | |
| No/Yes | 239/585 |
| Characteristic | Displacement Group (n = 150) | Non-Displacement Group (n = 674) | χ2/t Value | p-Value |
|---|---|---|---|---|
| Gender | 3.04 | 0.081 | ||
| Male | 54 | 194 | ||
| Female | 96 | 480 | ||
| Age (years, ± s) | 73.9 ± 7.1 | 75.1 ± 6.9 | 1.92 | 0.06 |
| BMI (kg/m2, ± s) | 24.2 ± 2.1 | 23.9 ± 2.9 | 1.47 | 0.144 |
| Underlying diseases | 6.55 | 0.088 | ||
| Hypertension | 26 | 95 | ||
| Diabetes | 30 | 89 | ||
| Heart disease | 11 | 59 | ||
| Others | 83 | 431 | ||
| BMD ( ± s) | −3.8 ± 0.9 | −3.5 ± 1.9 | 2.89 | 0.004 |
| Thoracolumbar junction | 10.72 | 0.001 | ||
| No | 53 | 152 | ||
| Yes | 97 | 522 | ||
| Kümmell’s disease staging | 8.34 | 0.004 | ||
| I | 54 | 165 | ||
| II | 96 | 509 | ||
| Preoperative anterior vertebral height (mm) | 25.30 ± 3.51 | 25.62 ± 4.11 | 0.98 | 0.329 |
| Postoperative anterior vertebral height (mm) | 26.84 ± 3.08 | 27.02 ± 4.02 | 0.61 | 0.543 |
| Restoration of anterior height of vertebra (%) | 2.50 ± 0.11 | 2.52 ± 0.23 | 1.59 | 0.114 |
| Preoperative local Cobb angles (°) | 22.96 ± 4.72 | 23.79 ± 4.91 | 1.89 | 0.060 |
| Postoperative local Cobb angles (°) | 12.87 ± 3.89 | 13.11 ± 3.33 | 0.70 | 0.484 |
| Restoration of local Cobb angle (%) | 41.04 ± 7.81 | 32.67 ± 8,52 | 11.38 | <0.001 |
| Integrity of anterior vertebral cortex | 14.51 | <0.001 | ||
| No | 95 | 311 | ||
| Yes | 55 | 363 | ||
| Integrity of endplate in surgical vertebrae | 9.66 | 0.002 | ||
| No | 62 | 373 | ||
| Yes | 88 | 301 | ||
| Surgical method | 4.99 | 0.026 | ||
| PVP | 24 | 165 | ||
| PKP | 126 | 509 | ||
| Surgical approach | 2.31 | 0.129 | ||
| Unilateral | 117 | 561 | ||
| Bilateral | 33 | 113 | ||
| Volume of cement (mL) | 4.54 ± 1.12 | 4.72 ± 1.56 | 1.65 | 0.101 |
| Even cement distribution | 16.81 | <0.001 | ||
| No | 89 | 276 | ||
| Yes | 61 | 398 | ||
| Viscosity of cement | 4.57 | 0.033 | ||
| High | 108 | 423 | ||
| Low | 42 | 251 | ||
| Cement leakage | 7.07 | 0.008 | ||
| No | 59 | 346 | ||
| Yes | 91 | 328 | ||
| Postoperative anti-osteoporosis treatment | 11.18 | <0.001 | ||
| No | 111 | 400 | ||
| Yes | 39 | 274 | ||
| Postoperative bracing treatment | 2.85 | 0.091 | ||
| No | 52 | 187 | ||
| Yes | 98 | 487 |
| Characteristic | OR Value | 95% CI | p Value |
|---|---|---|---|
| BMD | 3.56 | 0.79–4.12 | 0.109 |
| Thoracolumbar junction | 3.23 | 2.12–4.50 | 0.011 |
| Kümmell’s disease staging | 2.23 | 1.81–3.41 | <0.001 |
| Anterior cortex defect | 5.34 | 3.53–7.21 | <0.001 |
| Vertebral endplates defect | 0.54 | 0.35–0.71 | <0.001 |
| Surgical method | 1.54 | 0.84–1.79 | 0.413 |
| Cement distribution | 2.86 | 2.03–3.52 | 0.002 |
| Viscosity of cement | 1.36 | 0.78–1.77 | 0.154 |
| Cement leakage | 4.59 | 3.85–5.72 | <0.001 |
| Restoration of local Cobb angle | 3.17 | 2.40–5.73 | 0.024 |
| Postoperative anti-osteoporosis treatment | 0.48 | 0.18–0.72 | 0.025 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Du, J.; Zhang, Y.; Gong, Y.; Zhang, B.; Qu, Z.; Hao, D.; He, B.; Yan, L. Predictive Factors for Bone Cement Displacement following Percutaneous Vertebral Augmentation in Kümmell’s Disease. J. Clin. Med. 2022, 11, 7479. https://doi.org/10.3390/jcm11247479
Gao X, Du J, Zhang Y, Gong Y, Zhang B, Qu Z, Hao D, He B, Yan L. Predictive Factors for Bone Cement Displacement following Percutaneous Vertebral Augmentation in Kümmell’s Disease. Journal of Clinical Medicine. 2022; 11(24):7479. https://doi.org/10.3390/jcm11247479
Chicago/Turabian StyleGao, Xiangcheng, Jinpeng Du, Yongyuan Zhang, Yining Gong, Bo Zhang, Zechao Qu, Dingjun Hao, Baorong He, and Liang Yan. 2022. "Predictive Factors for Bone Cement Displacement following Percutaneous Vertebral Augmentation in Kümmell’s Disease" Journal of Clinical Medicine 11, no. 24: 7479. https://doi.org/10.3390/jcm11247479
APA StyleGao, X., Du, J., Zhang, Y., Gong, Y., Zhang, B., Qu, Z., Hao, D., He, B., & Yan, L. (2022). Predictive Factors for Bone Cement Displacement following Percutaneous Vertebral Augmentation in Kümmell’s Disease. Journal of Clinical Medicine, 11(24), 7479. https://doi.org/10.3390/jcm11247479






