RETRACTED: Cardiovascular Disease and Exercise: From Molecular Mechanisms to Clinical Applications
Abstract
:1. Introduction
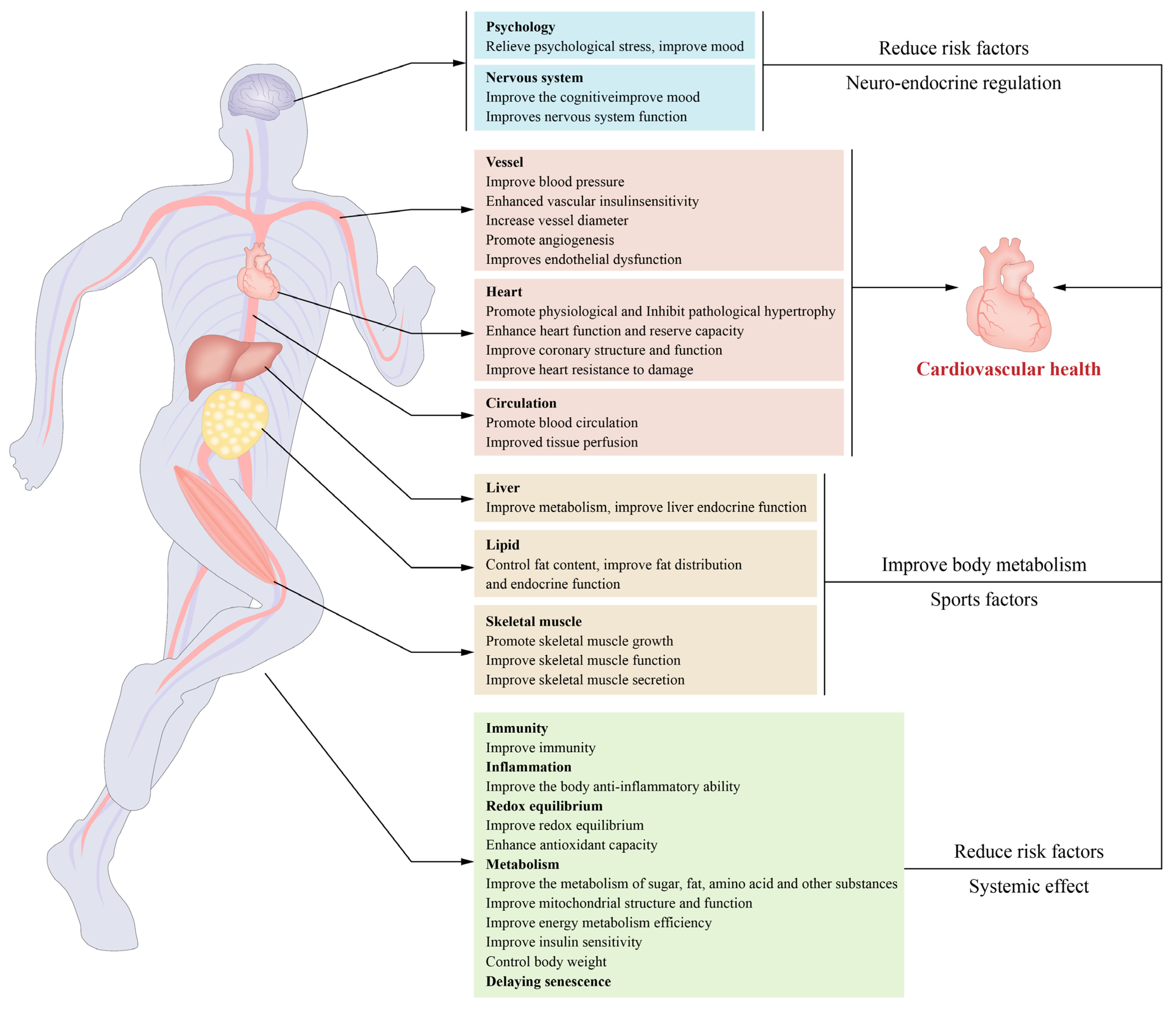
2. Exercise Reduces the Risk Factors for Cardiovascular Disease and Its Main Mechanism
2.1. Exercise Improves the Body’s Metabolism
2.2. Exercise Improves REDOX Balance and Chronic Inflammatory State
2.3. Exercise Slows Ageing
- ✓
- Senile heart β adrenoceptor desensitization makes the heart less sensitive to adrenergic stimulation. Exercise can enhance the sensitivity of the myocardium to adrenergic stimulation and thus increases the cardiac functional reserve [28].
- ✓
- The heart loses its Ca2+ processing capacity as it ages. The same stimulation reduces the intracellular Ca2+ increase and the cell contractility. Exercise can enhance intracellular Ca2+ processing capacity and cell contractility [29].
2.4. Exercise Prevention and Treatment of Hypertension
- ✓
- Reducing the resting systolic.
- ✓
- Improving the oxidative stress levels and regulating the renin–angiotensin system to affect vascular remodelling and blood vessels.
- ✓
- Increasing insulin sensitivity and nitric oxide (NO) bioavailability and promoting vasodilation and tissue perfusion.
2.5. Muscle-Building Effects of Exercise and Cardiovascular Health
2.6. Other
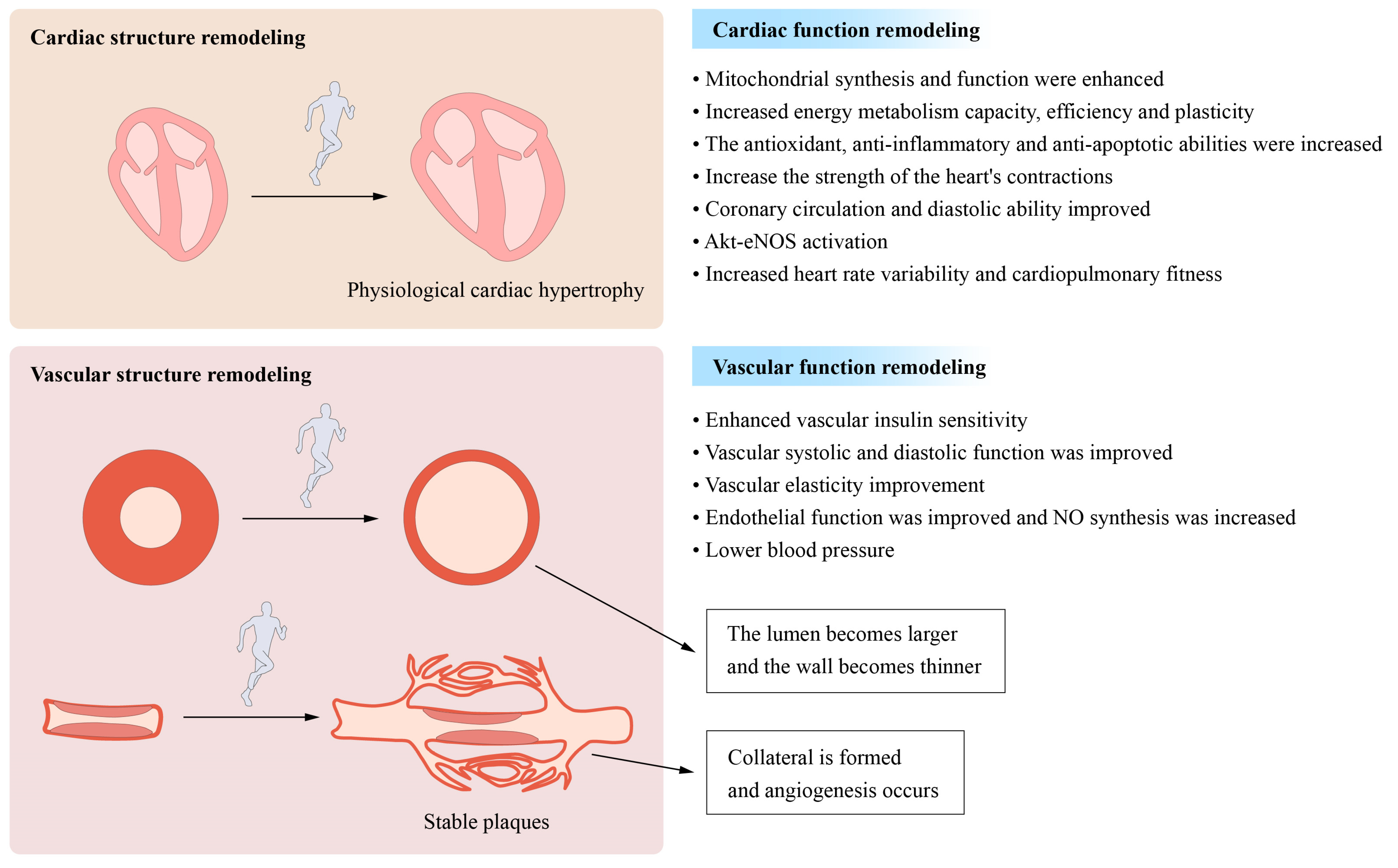
3. Exercise Improves the Cardiovascular Structure and Function
3.1. Remodeling of the Cardiovascular Structure and Function
3.2. Improvements in Myocardial Mitochondrial Function and Metabolism
3.3. Other
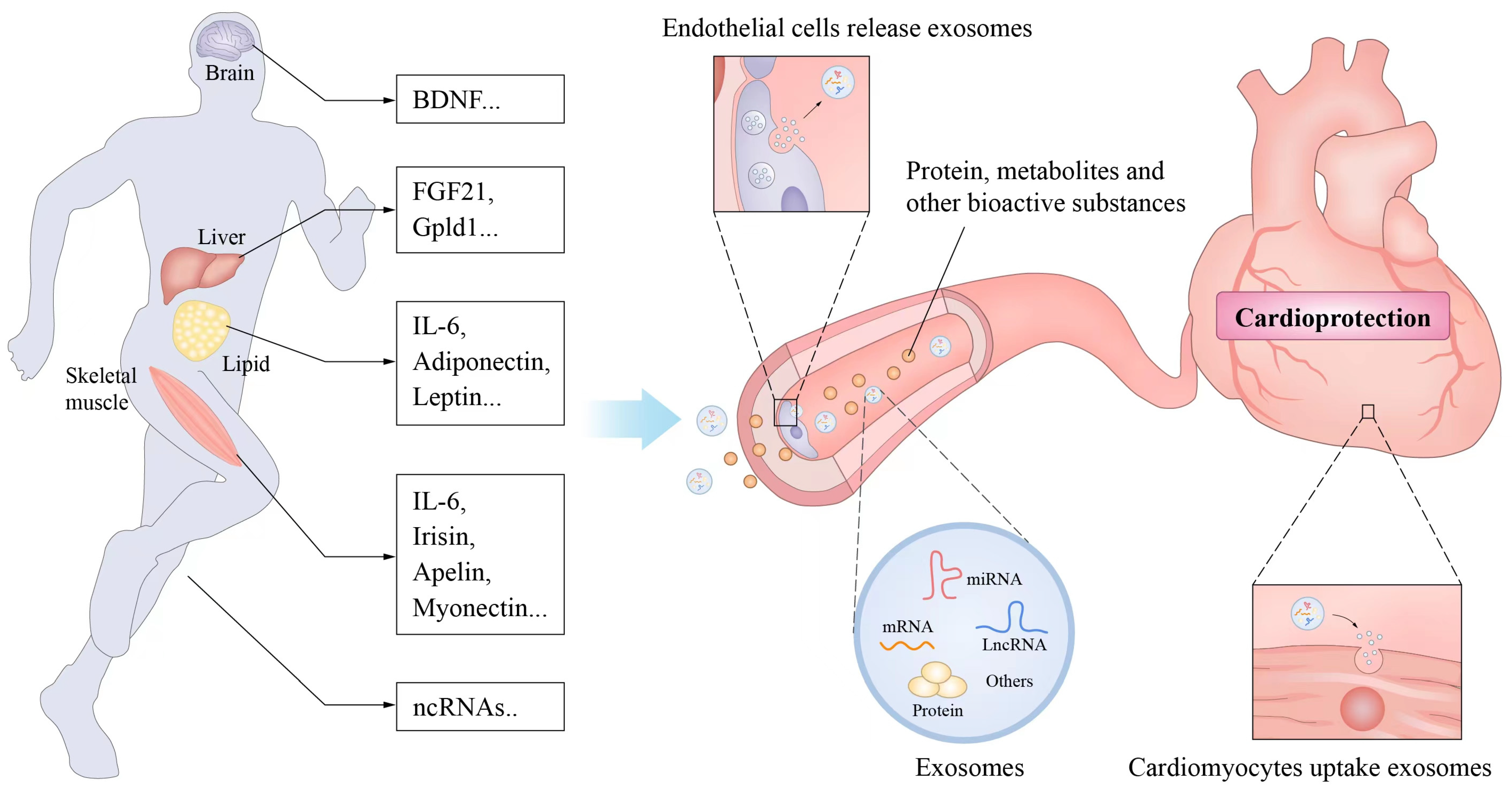
4. Exercise-Related Factors and Cardiovascular Protection
- ✓
- Bioactive substances produced during exercise or promoted by exercise.
- ✓
- Release from cells to adjacent cells or into the blood circulation to act on other organs or tissue cells.
- ✓
- Affect the growth or functional activity of recipient cells and thus usually mediate the health effects of exercise.
4.1. Muscle Factors
4.2. Adipose Factor
4.3. Noncoding RNA
5. Exercise Improves Insulin Sensitivity and Loss Resistance in the Cardiovascular System
6. Application of Disease Treatment and Rehabilitation in Heart Vessel Disease
- ✓
- Patients with exercise contraindications, such as hypotension or hypertension during quiet or exercise, unstable or worsening disease, significant myocardial ischaemia or concomitant serious lung disease, etc., were excluded (not recommended to practice long-term exercise).
- ✓
- The basic functional assessment includes comprehensive detection of cardiac function, complications, maximum exercise capacity, and risk assessment. Optimized treatment plans and standard treatment should be given to patients with heart failure [109].
- ✓
- The cardiopulmonary exercise test is essential for determining a patient’s maximal exercise capacity to determine an appropriate training intensity and modality [110].
7. Discussion and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Lavie, C.J.; Ozemek, C.; Carbone, S.; Katzmarzyk, P.T.; Blair, S.N. Sedentary Behavior, Exercise, and Cardiovascular Health. Circ. Res. 2019, 124, 799–815. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A. An Overview of the Beneficial Effects of Exercise on Health and Performance. Adv. Exp. Med. Biol. 2020, 1228, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Coats, A.J.S.; Forman, D.E.; Haykowsky, M.; Kitzman, D.W.; McNeil, A.; Campbell, T.S.; Arena, R. Physical function and exercise training in older patients with heart failure. Nat. Rev. Cardiol. 2017, 14, 550–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.L.; Sun, M.L.; Zhang, X.A. Exercise-Induced Adult Cardiomyocyte Proliferation in Mammals. Front. Physiol. 2021, 12, 729364. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Tian, Z.; Zang, W.; Jiang, H.; Li, Y.; Wang, S.; Chen, S. Exercise training reduces insulin resistance in postmyocardial infarction rats. Physiol. Rep. 2015, 3, e12339. [Google Scholar] [CrossRef] [PubMed]
- Jia, D.; Hou, L.; Lv, Y.; Xi, L.; Tian, Z. Postinfarction exercise training alleviates cardiac dysfunction and adverse remodeling via mitochondrial biogenesis and SIRT1/PGC-1alpha/PI3K/Akt signaling. J. Cell. Physiol. 2019, 234, 23705–23718. [Google Scholar] [CrossRef] [PubMed]
- Neri Serneri, G.G.; Boddi, M.; Modesti, P.A.; Cecioni, I.; Coppo, M.; Padeletti, L.; Michelucci, A.; Colella, A.; Galanti, G. Increased cardiac sympathetic activity and insulin-like growth factor-I formation are associated with physiological hypertrophy in athletes. Circ. Res. 2001, 89, 977–982. [Google Scholar] [CrossRef]
- Lavie, C.J.; Arena, R.; Swift, D.L.; Johannsen, N.M.; Sui, X.; Lee, D.C.; Earnest, C.P.; Church, T.S.; O’Keefe, J.H.; Milani, R.V.; et al. Exercise and the cardiovascular system: Clinical science and cardiovascular outcomes. Circ. Res. 2015, 117, 207–219. [Google Scholar] [CrossRef]
- Guo, Y.; Wang, S.; Liu, Y.; Fan, L.; Booz, G.W.; Roman, R.J.; Chen, Z.; Fan, F. Accelerated cerebral vascular injury in diabetes is associated with vascular smooth muscle cell dysfunction. Geroscience 2020, 42, 547–561. [Google Scholar] [CrossRef]
- Short, K.R.; Chadwick, J.Q.; Teague, A.M.; Tullier, M.A.; Wolbert, L.; Coleman, C.; Copeland, K.C. Effect of Obesity and Exercise Training on Plasma Amino Acids and Amino Metabolites in American Indian Adolescents. J. Clin. Endocrinol. Metab. 2019, 104, 3249–3261. [Google Scholar] [CrossRef]
- Umpierre, D.; Ribeiro, P.A.; Kramer, C.K.; Leitao, C.B.; Zucatti, A.T.; Azevedo, M.J.; Gross, J.L.; Ribeiro, J.P.; Schaan, B.D. Physical activity advice only or structured exercise training and association with HbA1c levels in type 2 diabetes: A systematic review and meta-analysis. JAMA 2011, 305, 1790–1799. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xiong, Z.; Yan, W.; Gao, E.; Cheng, H.; Wu, G.; Liu, Y.; Zhang, L.; Li, C.; Wang, S.; et al. Branched chain amino acids exacerbate myocardial ischemia/reperfusion vulnerability via enhancing GCN2/ATF6/PPAR-alpha pathway-dependent fatty acid oxidation. Theranostics 2020, 10, 5623–5640. [Google Scholar] [CrossRef] [PubMed]
- Murthy, V.L.; Yu, B.; Wang, W.; Zhang, X.; Alkis, T.; Pico, A.R.; Yeri, A.; Bhupathiraju, S.N.; Bressler, J.; Ballantyne, C.M.; et al. Molecular Signature of Multisystem Cardiometabolic Stress and Its Association with Prognosis. JAMA Cardiol. 2020, 5, 1144–1153. [Google Scholar] [CrossRef]
- Shaw, E.; Leung, G.K.W.; Jong, J.; Coates, A.M.; Davis, R.; Blair, M.; Huggins, C.E.; Dorrian, J.; Banks, S.; Kellow, N.J.; et al. The Impact of Time of Day on Energy Expenditure: Implications for Long-Term Energy Balance. Nutrients 2019, 11, 2383. [Google Scholar] [CrossRef] [PubMed]
- Powers, S.K.; Deminice, R.; Ozdemir, M.; Yoshihara, T.; Bomkamp, M.P.; Hyatt, H. Exercise-induced oxidative stress: Friend or foe? J. Sport Health Sci. 2020, 9, 415–425. [Google Scholar] [CrossRef]
- Jackson, M.J. Control of reactive oxygen species production in contracting skeletal muscle. Antioxid. Redox Signal. 2011, 15, 2477–2486. [Google Scholar] [CrossRef]
- Taherkhani, S.; Suzuki, K.; Castell, L. A Short Overview of Changes in Inflammatory Cytokines and Oxidative Stress in Response to Physical Activity and Antioxidant Supplementation. Antioxidants 2020, 9, 886. [Google Scholar] [CrossRef]
- Ristow, M.; Zarse, K.; Oberbach, A.; Kloting, N.; Birringer, M.; Kiehntopf, M.; Stumvoll, M.; Kahn, C.R.; Bluher, M. Antioxidants prevent health-promoting effects of physical exercise in humans. Proc. Natl. Acad. Sci. USA 2009, 106, 8665–8670. [Google Scholar] [CrossRef]
- Ghahremani, R.; Damirchi, A.; Salehi, I.; Komaki, A.; Esposito, F. Mitochondrial dynamics as an underlying mechanism involved in aerobic exercise training-induced cardioprotection against ischemia-reperfusion injury. Life Sci. 2018, 213, 102–108. [Google Scholar] [CrossRef]
- Powers, S.K.; Sollanek, K.J.; Wiggs, M.P.; Demirel, H.A.; Smuder, A.J. Exercise-induced improvements in myocardial antioxidant capacity: The antioxidant players and cardioprotection. Free Radic. Res. 2014, 48, 43–51. [Google Scholar] [CrossRef]
- Done, A.J.; Traustadottir, T. Nrf2 mediates redox adaptations to exercise. Redox Biol. 2016, 10, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Milani, R.V.; Lavie, C.J.; Mehra, M.R. Reduction in C-reactive protein through cardiac rehabilitation and exercise training. J. Am. Coll. Cardiol. 2004, 43, 1056–1061. [Google Scholar] [CrossRef] [PubMed]
- Starkie, R.; Ostrowski, S.R.; Jauffred, S.; Febbraio, M.; Pedersen, B.K. Exercise and IL-6 infusion inhibit endotoxin-induced TNF-alpha production in humans. FASEB J. 2003, 17, 884–886. [Google Scholar] [CrossRef] [PubMed]
- Mathur, N.; Pedersen, B.K. Exercise as a mean to control low-grade systemic inflammation. Mediat. Inflamm. 2008, 2008, 109502. [Google Scholar] [CrossRef]
- Rysz, J.; Franczyk, B.; Rysz-Gorzynska, M.; Gluba-Brzozka, A. Ageing, Age-Related Cardiovascular Risk and the Beneficial Role of Natural Components Intake. Int. J. Mol. Sci. 2021, 23, 183. [Google Scholar] [CrossRef]
- Szalewska, D.; Radkowski, M.; Demkow, U.; Winklewski, P.J. Exercise Strategies to Counteract Brain Aging Effects. Adv. Exp. Med. Biol. 2017, 1020, 69–79. [Google Scholar] [CrossRef]
- Janssen, J.A. Impact of Physical Exercise on Endocrine Aging. Front. Horm. Res. 2016, 47, 68–81. [Google Scholar] [CrossRef]
- Leosco, D.; Parisi, V.; Femminella, G.D.; Formisano, R.; Petraglia, L.; Allocca, E.; Bonaduce, D. Effects of exercise training on cardiovascular adrenergic system. Front. Physiol. 2013, 4, 348. [Google Scholar] [CrossRef]
- Baekkerud, F.H.; Salerno, S.; Ceriotti, P.; Morland, C.; Storm-Mathisen, J.; Bergersen, L.H.; Hoydal, M.A.; Catalucci, D.; Stolen, T.O. High Intensity Interval Training Ameliorates Mitochondrial Dysfunction in the Left Ventricle of Mice with Type 2 Diabetes. Cardiovasc. Toxicol. 2019, 19, 422–431. [Google Scholar] [CrossRef]
- Roh, J.; Rhee, J.; Chaudhari, V.; Rosenzweig, A. The Role of Exercise in Cardiac Aging: From Physiology to Molecular Mechanisms. Circ. Res. 2016, 118, 279–295. [Google Scholar] [CrossRef]
- Gliemann, L.; Nyberg, M.; Hellsten, Y. Effects of exercise training and resveratrol on vascular health in aging. Free Radic. Biol. Med. 2016, 98, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Torma, F.; Berkes, I.; Goto, S.; Mimura, T.; Posa, A.; Balogh, L.; Boldogh, I.; Suzuki, K.; Higuchi, M.; et al. Exercise effects on physiological function during aging. Free Radic. Biol. Med. 2019, 132, 33–41. [Google Scholar] [CrossRef] [PubMed]
- Naci, H.; Salcher-Konrad, M.; Dias, S.; Blum, M.R.; Sahoo, S.A.; Nunan, D.; Ioannidis, J.P.A. How does exercise treatment compare with antihypertensive medications? A network meta-analysis of 391 randomised controlled trials assessing exercise and medication effects on systolic blood pressure. Br. J. Sports Med. 2019, 53, 859–869. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Hay, J.L.; Kehler, D.S.; Boreskie, K.F.; Arora, R.C.; Umpierre, D.; Szwajcer, A.; Duhamel, T.A. Effects of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training on Blood Pressure in Adults with Pre- to Established Hypertension: A Systematic Review and Meta-Analysis of Randomized Trials. Sports Med. 2018, 48, 2127–2142. [Google Scholar] [CrossRef]
- Ciolac, E.G.; Bocchi, E.A.; Bortolotto, L.A.; Carvalho, V.O.; Greve, J.M.; Guimaraes, G.V. Effects of high-intensity aerobic interval training vs. moderate exercise on hemodynamic, metabolic and neuro-humoral abnormalities of young normotensive women at high familial risk for hypertension. Hypertens. Res. 2010, 33, 836–843. [Google Scholar] [CrossRef]
- Feraco, A.; Gorini, S.; Armani, A.; Camajani, E.; Rizzo, M.; Caprio, M. Exploring the Role of Skeletal Muscle in Insulin Resistance: Lessons from Cultured Cells to Animal Models. Int. J. Mol. Sci. 2021, 22, 9327. [Google Scholar] [CrossRef]
- Powers, S.K.; Morton, A.B.; Ahn, B.; Smuder, A.J. Redox control of skeletal muscle atrophy. Free Radic. Biol. Med. 2016, 98, 208–217. [Google Scholar] [CrossRef]
- Kokkinidis, D.G.; Arfaras-Melainis, A.; Giannakoulas, G. Sarcopenia in heart failure: ‘waste’ the appropriate time and resources, not the muscles. Eur. J. Prev. Cardiol. 2021, 28, 1019–1021. [Google Scholar] [CrossRef]
- Konishi, M.; Kagiyama, N.; Kamiya, K.; Saito, H.; Saito, K.; Ogasahara, Y.; Maekawa, E.; Misumi, T.; Kitai, T.; Iwata, K.; et al. Impact of sarcopenia on prognosis in patients with heart failure with reduced and preserved ejection fraction. Eur. J. Prev. Cardiol. 2021, 28, 1022–1029. [Google Scholar] [CrossRef]
- Son, J.; Jang, L.G.; Kim, B.Y.; Lee, S.; Park, H. The Effect of Athletes’ Probiotic Intake May Depend on Protein and Dietary Fiber Intake. Nutrients 2020, 12, 2947. [Google Scholar] [CrossRef]
- Allen, J.M.; Mailing, L.J.; Niemiro, G.M.; Moore, R.; Cook, M.D.; White, B.A.; Holscher, H.D.; Woods, J.A. Exercise Alters Gut Microbiota Composition and Function in Lean and Obese Humans. Med. Sci. Sports Exerc. 2018, 50, 747–757. [Google Scholar] [CrossRef] [PubMed]
- Barton, W.; Penney, N.C.; Cronin, O.; Garcia-Perez, I.; Molloy, M.G.; Holmes, E.; Shanahan, F.; Cotter, P.D.; O’Sullivan, O. The microbiome of professional athletes differs from that of more sedentary subjects in composition and particularly at the functional metabolic level. Gut 2018, 67, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Ni, Y.; Cheung, C.K.Y.; Lam, K.S.L.; Wang, Y.; Xia, Z.; Ye, D.; Guo, J.; Tse, M.A.; et al. Gut Microbiome Fermentation Determines the Efficacy of Exercise for Diabetes Prevention. Cell Metab. 2020, 31, 77–91.e5. [Google Scholar] [CrossRef]
- Lavie, C.J.; Milani, R.V. Prevalence of anxiety in coronary patients with improvement following cardiac rehabilitation and exercise training. Am. J. Cardiol. 2004, 93, 336–339. [Google Scholar] [CrossRef]
- Hambrecht, R.; Walther, C.; Mobius-Winkler, S.; Gielen, S.; Linke, A.; Conradi, K.; Erbs, S.; Kluge, R.; Kendziorra, K.; Sabri, O.; et al. Percutaneous coronary angioplasty compared with exercise training in patients with stable coronary artery disease: A randomized trial. Circulation 2004, 109, 1371–1378. [Google Scholar] [CrossRef]
- Thijssen, D.H.; Cable, N.T.; Green, D.J. Impact of exercise training on arterial wall thickness in humans. Clin. Sci. 2012, 122, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Shimada, K.; Mikami, Y.; Murayama, T.; Yokode, M.; Fujita, M.; Kita, T.; Kishimoto, C. Atherosclerotic plaques induced by marble-burying behavior are stabilized by exercise training in experimental atherosclerosis. Int. J. Cardiol. 2011, 151, 284–289. [Google Scholar] [CrossRef]
- Seals, D.R.; Desouza, C.A.; Donato, A.J.; Tanaka, H. Habitual exercise and arterial aging. J. Appl. Physiol. 2008, 105, 1323–1332. [Google Scholar] [CrossRef]
- Schuttler, D.; Clauss, S.; Weckbach, L.T.; Brunner, S. Molecular Mechanisms of Cardiac Remodeling and Regeneration in Physical Exercise. Cells 2019, 8, 1128. [Google Scholar] [CrossRef]
- Golbidi, S.; Laher, I. Molecular mechanisms in exercise-induced cardioprotection. Cardiol. Res. Pract. 2011, 2011, 972807. [Google Scholar] [CrossRef]
- Gu, J.W.; Gadonski, G.; Wang, J.; Makey, I.; Adair, T.H. Exercise increases endostatin in circulation of healthy volunteers. BMC Physiol. 2004, 4, 2. [Google Scholar] [CrossRef] [PubMed]
- Heaps, C.L.; Robles, J.C.; Sarin, V.; Mattox, M.L.; Parker, J.L. Exercise training-induced adaptations in mediators of sustained endothelium-dependent coronary artery relaxation in a porcine model of ischemic heart disease. Microcirculation 2014, 21, 388–400. [Google Scholar] [CrossRef] [PubMed]
- Green, D.J.; O’Driscoll, G.; Joyner, M.J.; Cable, N.T. Exercise and cardiovascular risk reduction: Time to update the rationale for exercise? J. Appl. Physiol. 2008, 105, 766–768. [Google Scholar] [CrossRef] [PubMed]
- Hambrecht, R.; Adams, V.; Erbs, S.; Linke, A.; Krankel, N.; Shu, Y.; Baither, Y.; Gielen, S.; Thiele, H.; Gummert, J.F.; et al. Regular physical activity improves endothelial function in patients with coronary artery disease by increasing phosphorylation of endothelial nitric oxide synthase. Circulation 2003, 107, 3152–3158. [Google Scholar] [CrossRef]
- Hou, Z.; Qin, X.; Hu, Y.; Zhang, X.; Li, G.; Wu, J.; Li, J.; Sha, J.; Chen, J.; Xia, J.; et al. Longterm Exercise-Derived Exosomal miR-342-5p: A Novel Exerkine for Cardioprotection. Circ. Res. 2019, 124, 1386–1400. [Google Scholar] [CrossRef]
- Ko, J.; Deprez, D.; Shaw, K.; Alcorn, J.; Hadjistavropoulos, T.; Tomczak, C.; Foulds, H.; Chilibeck, P.D. Stretching is Superior to Brisk Walking for Reducing Blood Pressure in People with High-Normal Blood Pressure or Stage I Hypertension. J. Phys. Act. Health 2021, 18, 21–28. [Google Scholar] [CrossRef]
- Beech, D.J.; Kalli, A.C. Force Sensing by Piezo Channels in Cardiovascular Health and Disease. Arterioscler. Thromb. Vasc. Biol. 2019, 39, 2228–2239. [Google Scholar] [CrossRef]
- Shah, V.; Patel, S.; Shah, J. Emerging role of Piezo ion channels in cardiovascular development. Dev. Dyn. 2022, 251, 276–286. [Google Scholar] [CrossRef]
- Golbidi, S.; Laher, I. Exercise and the cardiovascular system. Cardiol. Res. Pract. 2012, 2012, 210852. [Google Scholar] [CrossRef]
- Young, C.G.; Knight, C.A.; Vickers, K.C.; Westbrook, D.; Madamanchi, N.R.; Runge, M.S.; Ischiropoulos, H.; Ballinger, S.W. Differential effects of exercise on aortic mitochondria. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1683–H1689. [Google Scholar] [CrossRef]
- Fulghum, K.; Hill, B.G. Metabolic Mechanisms of Exercise-Induced Cardiac Remodeling. Front. Cardiovasc. Med. 2018, 5, 127. [Google Scholar] [CrossRef]
- Gibb, A.A.; Epstein, P.N.; Uchida, S.; Zheng, Y.; McNally, L.A.; Obal, D.; Katragadda, K.; Trainor, P.; Conklin, D.J.; Brittian, K.R.; et al. Exercise-Induced Changes in Glucose Metabolism Promote Physiological Cardiac Growth. Circulation 2017, 136, 2144–2157. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Min, K.; Talbert, E.E.; Kavazis, A.N.; Smuder, A.J.; Willis, W.T.; Powers, S.K. Exercise protects cardiac mitochondria against ischemia-reperfusion injury. Med. Sci. Sports Exerc. 2012, 44, 397–405. [Google Scholar] [CrossRef] [PubMed]
- Boulghobra, D.; Coste, F.; Geny, B.; Reboul, C. Exercise training protects the heart against ischemia-reperfusion injury: A central role for mitochondria? Free Radic. Biol. Med. 2020, 152, 395–410. [Google Scholar] [CrossRef]
- Seals, D.R.; Dinenno, F.A. Collateral damage: Cardiovascular consequences of chronic sympathetic activation with human aging. Am. J. Physiol. Heart Circ. Physiol. 2004, 287, H1895–H1905. [Google Scholar] [CrossRef] [PubMed]
- DeFina, L.F.; Haskell, W.L.; Willis, B.L.; Barlow, C.E.; Finley, C.E.; Levine, B.D.; Cooper, K.H. Physical activity versus cardiorespiratory fitness: Two (partly) distinct components of cardiovascular health? Prog. Cardiovasc. Dis. 2015, 57, 324–329. [Google Scholar] [CrossRef]
- Myers, J.; McAuley, P.; Lavie, C.J.; Despres, J.P.; Arena, R.; Kokkinos, P. Physical activity and cardiorespiratory fitness as major markers of cardiovascular risk: Their independent and interwoven importance to health status. Prog. Cardiovasc. Dis. 2015, 57, 306–314. [Google Scholar] [CrossRef]
- Safdar, A.; Saleem, A.; Tarnopolsky, M.A. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat. Rev. Endocrinol. 2016, 12, 504–517. [Google Scholar] [CrossRef]
- Heineke, J.; Auger-Messier, M.; Xu, J.; Sargent, M.; York, A.; Welle, S.; Molkentin, J.D. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation 2010, 121, 419–425. [Google Scholar] [CrossRef]
- Biesemann, N.; Mendler, L.; Wietelmann, A.; Hermann, S.; Schafers, M.; Kruger, M.; Boettger, T.; Borchardt, T.; Braun, T. Myostatin regulates energy homeostasis in the heart and prevents heart failure. Circ. Res. 2014, 115, 296–310. [Google Scholar] [CrossRef]
- Nara, H.; Watanabe, R. Anti-Inflammatory Effect of Muscle-Derived Interleukin-6 and Its Involvement in Lipid Metabolism. Int. J. Mol. Sci. 2021, 22, 9889. [Google Scholar] [CrossRef] [PubMed]
- Ricard, N.; Tu, L.; Le Hiress, M.; Huertas, A.; Phan, C.; Thuillet, R.; Sattler, C.; Fadel, E.; Seferian, A.; Montani, D.; et al. Increased pericyte coverage mediated by endothelial-derived fibroblast growth factor-2 and interleukin-6 is a source of smooth muscle-like cells in pulmonary hypertension. Circulation 2014, 129, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Kolak, A.; Kaminska, M.; Wysokinska, E.; Surdyka, D.; Kieszko, D.; Pakiela, M.; Burdan, F. The problem of fatigue in patients suffering from neoplastic disease. Contemp. Oncol. 2017, 21, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Xu, M.; Bryant, J.L.; Ma, J.; Xu, X. Exercise-induced myokine FNDC5/irisin functions in cardiovascular protection and intracerebral retrieval of synaptic plasticity. Cell Biosci. 2019, 9, 32. [Google Scholar] [CrossRef]
- Rao, R.R.; Long, J.Z.; White, J.P.; Svensson, K.J.; Lou, J.; Lokurkar, I.; Jedrychowski, M.P.; Ruas, J.L.; Wrann, C.D.; Lo, J.C.; et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell 2014, 157, 1279–1291. [Google Scholar] [CrossRef]
- Zhong, J.C.; Zhang, Z.Z.; Wang, W.; McKinnie, S.M.K.; Vederas, J.C.; Oudit, G.Y. Targeting the apelin pathway as a novel therapeutic approach for cardiovascular diseases. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1942–1950. [Google Scholar] [CrossRef]
- Ouchi, N.; Oshima, Y.; Ohashi, K.; Higuchi, A.; Ikegami, C.; Izumiya, Y.; Walsh, K. Follistatin-like 1, a secreted muscle protein, promotes endothelial cell function and revascularization in ischemic tissue through a nitric-oxide synthase-dependent mechanism. J. Biol. Chem. 2008, 283, 32802–32811. [Google Scholar] [CrossRef]
- Reynolds, J.C.; Lai, R.W.; Woodhead, J.S.T.; Joly, J.H.; Mitchell, C.J.; Cameron-Smith, D.; Lu, R.; Cohen, P.; Graham, N.A.; Benayoun, B.A.; et al. MOTS-c is an exercise-induced mitochondrial-encoded regulator of age-dependent physical decline and muscle homeostasis. Nat. Commun. 2021, 12, 470. [Google Scholar] [CrossRef]
- Roberts, L.D.; Bostrom, P.; O’Sullivan, J.F.; Schinzel, R.T.; Lewis, G.D.; Dejam, A.; Lee, Y.K.; Palma, M.J.; Calhoun, S.; Georgiadi, A.; et al. beta-Aminoisobutyric acid induces browning of white fat and hepatic beta-oxidation and is inversely correlated with cardiometabolic risk factors. Cell Metab. 2014, 19, 96–108. [Google Scholar] [CrossRef]
- Moschen, A.R.; Wieser, V.; Tilg, H. Adiponectin: Key player in the adipose tissue-liver crosstalk. Curr. Med. Chem. 2012, 19, 5467–5473. [Google Scholar] [CrossRef]
- Ouchi, N.; Shibata, R.; Walsh, K. Cardioprotection by adiponectin. Trends Cardiovasc. Med. 2006, 16, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Aljafary, M.A.; Al-Suhaimi, E.A. Adiponectin System (Rescue Hormone): The Missing Link between Metabolic and Cardiovascular Diseases. Pharmaceutics 2022, 14, 1430. [Google Scholar] [CrossRef] [PubMed]
- Mattu, H.S.; Randeva, H.S. Role of adipokines in cardiovascular disease. J. Endocrinol. 2013, 216, T17–T36. [Google Scholar] [CrossRef]
- Ma, S.; Liao, Y. Noncoding RNAs in exercise-induced cardio-protection for chronic heart failure. EBioMedicine 2019, 46, 532–540. [Google Scholar] [CrossRef]
- Wu, G.; Zhang, X.; Gao, F. The epigenetic landscape of exercise in cardiac health and disease. J. Sport Health Sci. 2021, 10, 648–659. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, S.; Scheele, C.; Yfanti, C.; Akerstrom, T.; Nielsen, A.R.; Pedersen, B.K.; Laye, M.J. Muscle specific microRNAs are regulated by endurance exercise in human skeletal muscle. J. Physiol. 2010, 588, 4029–4037. [Google Scholar] [CrossRef] [PubMed]
- Radom-Aizik, S.; Zaldivar, F., Jr.; Oliver, S.; Galassetti, P.; Cooper, D.M. Evidence for microRNA involvement in exercise-associated neutrophil gene expression changes. J. Appl. Physiol. 2010, 109, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Qi, J.; Meng, S.; Wen, B.; Zhang, J. Swimming exercise training-induced left ventricular hypertrophy involves microRNAs and synergistic regulation of the PI3K/AKT/mTOR signaling pathway. Eur. J. Appl. Physiol. 2013, 113, 2473–2486. [Google Scholar] [CrossRef]
- DA Silva, N.D., Jr.; Fernandes, T.; Soci, U.P.; Monteiro, A.W.; Phillips, M.I.; de Oliveira, E.M. Swimming training in rats increases cardiac MicroRNA-126 expression and angiogenesis. Med. Sci. Sports Exerc. 2012, 44, 1453–1462. [Google Scholar] [CrossRef]
- Melo, S.F.; Barauna, V.G.; Neves, V.J.; Fernandes, T.; Lara Lda, S.; Mazzotti, D.R.; Oliveira, E.M. Exercise training restores the cardiac microRNA-1 and -214 levels regulating Ca2+ handling after myocardial infarction. BMC Cardiovasc. Disord. 2015, 15, 166. [Google Scholar] [CrossRef]
- Melo, S.F.; Fernandes, T.; Barauna, V.G.; Matos, K.C.; Santos, A.A.; Tucci, P.J.; Oliveira, E.M. Expression of MicroRNA-29 and Collagen in Cardiac Muscle after Swimming Training in Myocardial-Infarcted Rats. Cell Physiol. Biochem. 2014, 33, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Zheng, F.; Xie, K.L.; Xie, M.R.; Jiang, L.J.; Cai, Y. Exercise Reduces Insulin Resistance in Type 2 Diabetes Mellitus via Mediating the lncRNA MALAT1/MicroRNA-382-3p/Resistin Axis. Mol. Ther. Nucleic Acids 2019, 18, 34–44. [Google Scholar] [CrossRef] [PubMed]
- Jia, G.; DeMarco, V.G.; Sowers, J.R. Insulin resistance and hyperinsulinaemia in diabetic cardiomyopathy. Nat. Rev. Endocrinol. 2016, 12, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Martinez-Rodriguez, J.; Gonzalez-Lucan, M.; Fernandez-Fernandez, C.; Castro-Quintela, E. Insulin resistance is a cardiovascular risk factor in humans. Diabetol. Metab. Syndr. 2019, 13, 1449–1455. [Google Scholar] [CrossRef] [PubMed]
- Iaccarino, G.; Franco, D.; Sorriento, D.; Strisciuglio, T.; Barbato, E.; Morisco, C. Modulation of Insulin Sensitivity by Exercise Training: Implications for Cardiovascular Prevention. J. Cardiovasc. Transl. Res. 2021, 14, 256–270. [Google Scholar] [CrossRef]
- Nasi, M.; Patrizi, G.; Pizzi, C.; Landolfo, M.; Boriani, G.; Dei Cas, A.; Cicero, A.F.G.; Fogacci, F.; Rapezzi, C.; Sisca, G.; et al. The role of physical activity in individuals with cardiovascular risk factors: An opinion paper from Italian Society of Cardiology-Emilia Romagna-Marche and SIC-Sport. J. Cardiovasc. Med. 2019, 20, 631–639. [Google Scholar] [CrossRef]
- Hawley, J.A.; Lessard, S.J. Exercise training-induced improvements in insulin action. Acta Physiol. 2008, 192, 127–135. [Google Scholar] [CrossRef]
- Borghouts, L.B.; Keizer, H.A. Exercise and insulin sensitivity: A review. Int. J. Sports Med. 2000, 21, 1–12. [Google Scholar] [CrossRef]
- Grunewald, Z.I.; Ramirez-Perez, F.I.; Woodford, M.L.; Morales-Quinones, M.; Mejia, S.; Manrique-Acevedo, C.; Siebenlist, U.; Martinez-Lemus, L.A.; Chandrasekar, B.; Padilla, J. TRAF3IP2 (TRAF3 Interacting Protein 2) Mediates Obesity-Associated Vascular Insulin Resistance and Dysfunction in Male Mice. Hypertension 2020, 76, 1319–1329. [Google Scholar] [CrossRef]
- Saotome, M.; Ikoma, T.; Hasan, P.; Maekawa, Y. Cardiac Insulin Resistance in Heart Failure: The Role of Mitochondrial Dynamics. Int. J. Mol. Sci. 2019, 20, 3552. [Google Scholar] [CrossRef]
- Fu, F.; Zhao, K.; Li, J.; Xu, J.; Zhang, Y.; Liu, C.; Yang, W.; Gao, C.; Li, J.; Zhang, H.; et al. Direct Evidence that Myocardial Insulin Resistance following Myocardial Ischemia Contributes to Post-Ischemic Heart Failure. Sci. Rep. 2015, 5, 17927. [Google Scholar] [CrossRef] [PubMed]
- Xing, W.; Yan, W.; Liu, P.; Ji, L.; Li, Y.; Sun, L.; Tao, L.; Zhang, H.; Gao, F. A novel mechanism for vascular insulin resistance in normotensive young SHRs: Hypoadiponectinemia and resultant APPL1 downregulation. Hypertension 2013, 61, 1028–1035. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.R.; Liu, H.T.; Zhang, H.F.; Zhang, Q.J.; Li, Q.X.; Yu, Q.J.; Guo, W.Y.; Wang, H.C.; Gao, F. Long-term aerobic exercise protects the heart against ischemia/reperfusion injury via PI3 kinase-dependent and Akt-mediated mechanism. Apoptosis 2007, 12, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.J.; Li, Q.X.; Zhang, H.F.; Zhang, K.R.; Guo, W.Y.; Wang, H.C.; Zhou, Z.; Cheng, H.P.; Ren, J.; Gao, F. Swim training sensitizes myocardial response to insulin: Role of Akt-dependent eNOS activation. Cardiovasc. Res. 2007, 75, 369–380. [Google Scholar] [CrossRef]
- Frasier, C.R.; Moore, R.L.; Brown, D.A. Exercise-induced cardiac preconditioning: How exercise protects your achy-breaky heart. J. Appl. Physiol. 2011, 111, 905–915. [Google Scholar] [CrossRef]
- Del Buono, M.G.; Arena, R.; Borlaug, B.A.; Carbone, S.; Canada, J.M.; Kirkman, D.L.; Garten, R.; Rodriguez-Miguelez, P.; Guazzi, M.; Lavie, C.J.; et al. Exercise Intolerance in Patients with Heart Failure: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 73, 2209–2225. [Google Scholar] [CrossRef]
- Houstis, N.E.; Eisman, A.S.; Pappagianopoulos, P.P.; Wooster, L.; Bailey, C.S.; Wagner, P.D.; Lewis, G.D. Exercise Intolerance in Heart Failure with Preserved Ejection Fraction: Diagnosing and Ranking Its Causes Using Personalized O2 Pathway Analysis. Circulation 2018, 137, 148–161. [Google Scholar] [CrossRef]
- Dhakal, B.P.; Malhotra, R.; Murphy, R.M.; Pappagianopoulos, P.P.; Baggish, A.L.; Weiner, R.B.; Houstis, N.E.; Eisman, A.S.; Hough, S.S.; Lewis, G.D. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: The role of abnormal peripheral oxygen extraction. Circ. Heart Fail. 2015, 8, 286–294. [Google Scholar] [CrossRef]
- Pelliccia, A.; Sharma, S.; Gati, S.; Back, M.; Borjesson, M.; Caselli, S.; Collet, J.P.; Corrado, D.; Drezner, J.A.; Halle, M.; et al. 2020 ESC Guidelines on sports cardiology and exercise in patients with cardiovascular disease. Eur. Heart J. 2021, 42, 17–96. [Google Scholar] [CrossRef]
- Guazzi, M.; Arena, R.; Halle, M.; Piepoli, M.F.; Myers, J.; Lavie, C.J. 2016 Focused Update: Clinical Recommendations for Cardiopulmonary Exercise Testing Data Assessment in Specific Patient Populations. Circulation 2016, 133, e694–e711. [Google Scholar] [CrossRef]
- Ismail, H.; McFarlane, J.R.; Nojoumian, A.H.; Dieberg, G.; Smart, N.A. Clinical outcomes and cardiovascular responses to different exercise training intensities in patients with heart failure: A systematic review and meta-analysis. JACC Heart Fail. 2013, 1, 514–522. [Google Scholar] [CrossRef] [PubMed]
- Gomes Neto, M.; Duraes, A.R.; Conceicao, L.S.R.; Saquetto, M.B.; Ellingsen, O.; Carvalho, V.O. High intensity interval training versus moderate intensity continuous training on exercise capacity and quality of life in patients with heart failure with reduced ejection fraction: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 261, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Piccini, J.P.; Hellkamp, A.S.; Whellan, D.J.; Ellis, S.J.; Keteyian, S.J.; Kraus, W.E.; Hernandez, A.F.; Daubert, J.P.; Pina, L.; O’Connor, C.M.; et al. Exercise training and implantable cardioverter-defibrillator shocks in patients with heart failure: Results from HF-ACTION (Heart Failure and A Controlled Trial Investigating Outcomes of Exercise TraiNing). JACC Heart Fail. 2013, 1, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Schindler, M.J.; Adams, V.; Halle, M. Exercise in Heart Failure-What Is the Optimal Dose to Improve Pathophysiology and Exercise Capacity? Curr. Heart Fail. Rep. 2019, 16, 98–107. [Google Scholar] [CrossRef]
- Harwood, A.E.; Russell, S.; Okwose, N.C.; McGuire, S.; Jakovljevic, D.G.; McGregor, G. A systematic review of rehabilitation in chronic heart failure: Evaluating the reporting of exercise interventions. ESC Heart Fail. 2021, 8, 3458–3471. [Google Scholar] [CrossRef]
- Fukuta, H.; Goto, T.; Wakami, K.; Kamiya, T.; Ohte, N. Effect of renin-angiotensin system inhibition on cardiac structure and function and exercise capacity in heart failure with preserved ejection fraction: A meta-analysis of randomized controlled trials. Heart Fail. Rev. 2021, 26, 1477–1484. [Google Scholar] [CrossRef]
- Marinus, N.; Hansen, D.; Feys, P.; Meesen, R.; Timmermans, A.; Spildooren, J. The Impact of Different Types of Exercise Training on Peripheral Blood Brain-Derived Neurotrophic Factor Concentrations in Older Adults: A Meta-Analysis. Sports Med. 2019, 49, 1529–1546. [Google Scholar] [CrossRef]
- Vona, M.; Codeluppi, G.M.; Iannino, T.; Ferrari, E.; Bogousslavsky, J.; von Segesser, L.K. Effects of different types of exercise training followed by detraining on endothelium-dependent dilation in patients with recent myocardial infarction. Circulation 2009, 119, 1601–1608. [Google Scholar] [CrossRef]
- Oja, P.; Kelly, P.; Pedisic, Z.; Titze, S.; Bauman, A.; Foster, C.; Hamer, M.; Hillsdon, M.; Stamatakis, E. Associations of specific types of sports and exercise with all-cause and cardiovascular-disease mortality: A cohort study of 80 306 British adults. Br. J. Sports Med. 2017, 51, 812–817. [Google Scholar] [CrossRef]
- Strain, T.; Wijndaele, K.; Dempsey, P.C.; Sharp, S.J.; Pearce, M.; Jeon, J.; Lindsay, T.; Wareham, N.; Brage, S. Wearable-device-measured physical activity and future health risk. Nat. Med. 2020, 26, 1385–1391. [Google Scholar] [CrossRef]
- Savikj, M.; Gabriel, B.M.; Alm, P.S.; Smith, J.; Caidahl, K.; Bjornholm, M.; Fritz, T.; Krook, A.; Zierath, J.R.; Wallberg-Henriksson, H. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: A randomised crossover trial. Diabetologia 2019, 62, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.A.; Zanesco, A. Early physical activity promotes lower prevalence of chronic diseases in adulthood. Hypertens. Res. 2010, 33, 926–931. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.E.; Baer, L.A.; Stanford, K.I. Maternal Exercise Improves the Metabolic Health of Adult Offspring. Trends Endocrinol. Metab. 2018, 29, 164–177. [Google Scholar] [CrossRef] [PubMed]
- Stanford, K.I.; Takahashi, H.; So, K.; Alves-Wagner, A.B.; Prince, N.B.; Lehnig, A.C.; Getchell, K.M.; Lee, M.Y.; Hirshman, M.F.; Goodyear, L.J. Maternal Exercise Improves Glucose Tolerance in Female Offspring. Diabetes 2017, 66, 2124–2136. [Google Scholar] [CrossRef]
- Stanford, K.I.; Rasmussen, M.; Baer, L.A.; Lehnig, A.C.; Rowland, L.A.; White, J.D.; So, K.; De Sousa-Coelho, A.L.; Hirshman, M.F.; Patti, M.E.; et al. Paternal Exercise Improves Glucose Metabolism in Adult Offspring. Diabetes 2018, 67, 2530–2540. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Gan, L.; Deng, Y.; Zhu, S.; Li, G.; Nasser, M.I.; Liu, N.; Zhu, P. RETRACTED: Cardiovascular Disease and Exercise: From Molecular Mechanisms to Clinical Applications. J. Clin. Med. 2022, 11, 7511. https://doi.org/10.3390/jcm11247511
Wang B, Gan L, Deng Y, Zhu S, Li G, Nasser MI, Liu N, Zhu P. RETRACTED: Cardiovascular Disease and Exercise: From Molecular Mechanisms to Clinical Applications. Journal of Clinical Medicine. 2022; 11(24):7511. https://doi.org/10.3390/jcm11247511
Chicago/Turabian StyleWang, Bo, Lin Gan, Yuzhi Deng, Shuoji Zhu, Ge Li, Moussa Ide Nasser, Nanbo Liu, and Ping Zhu. 2022. "RETRACTED: Cardiovascular Disease and Exercise: From Molecular Mechanisms to Clinical Applications" Journal of Clinical Medicine 11, no. 24: 7511. https://doi.org/10.3390/jcm11247511
APA StyleWang, B., Gan, L., Deng, Y., Zhu, S., Li, G., Nasser, M. I., Liu, N., & Zhu, P. (2022). RETRACTED: Cardiovascular Disease and Exercise: From Molecular Mechanisms to Clinical Applications. Journal of Clinical Medicine, 11(24), 7511. https://doi.org/10.3390/jcm11247511





