Incidence, Risk Factors, and Outcomes of Symptomatic Bone Cement Displacement following Percutaneous Kyphoplasty for Osteoporotic Vertebral Compression Fracture: A Single Center Study
Abstract
:1. Introduction
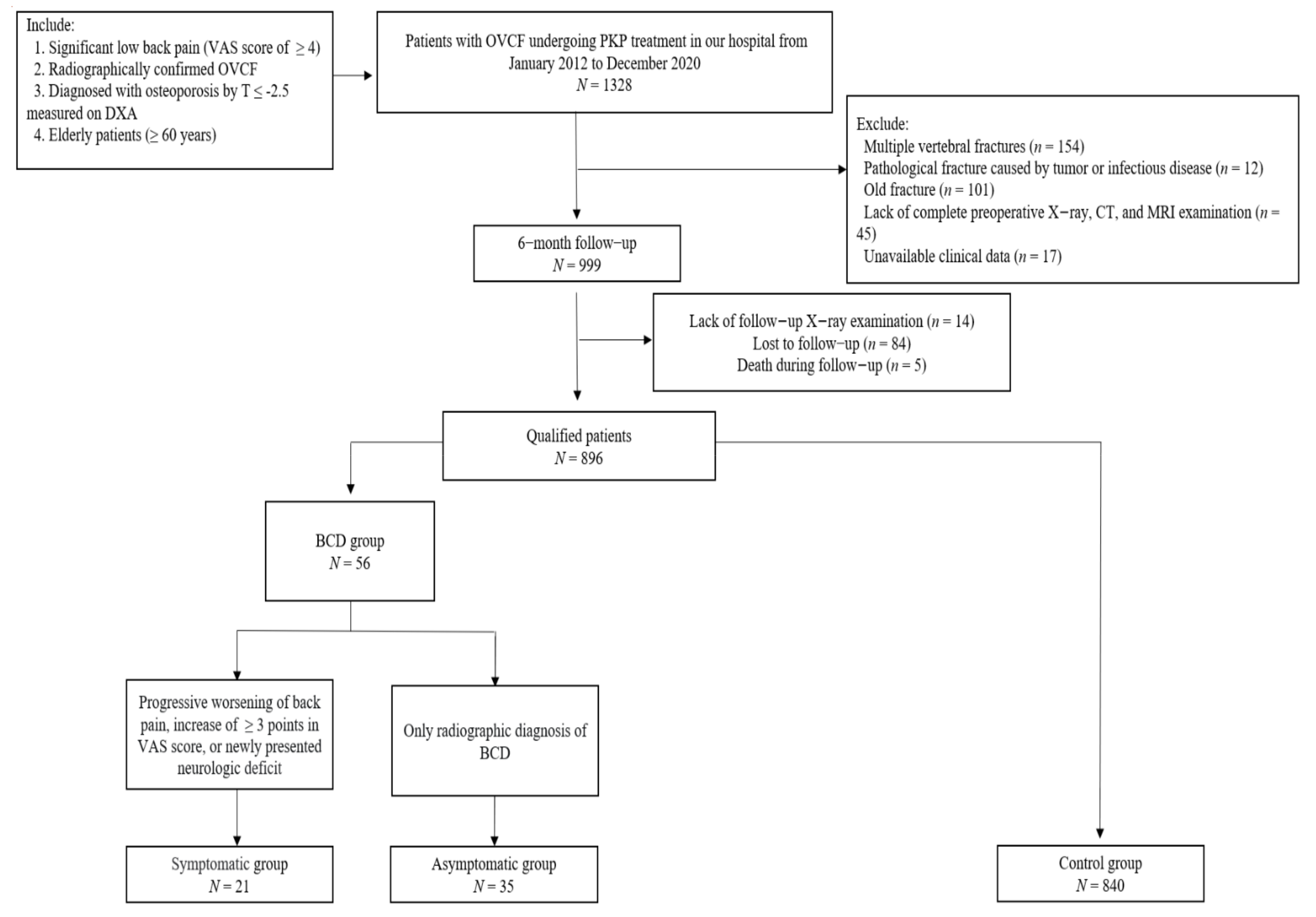
2. Materials and Methods
2.1. Subjects
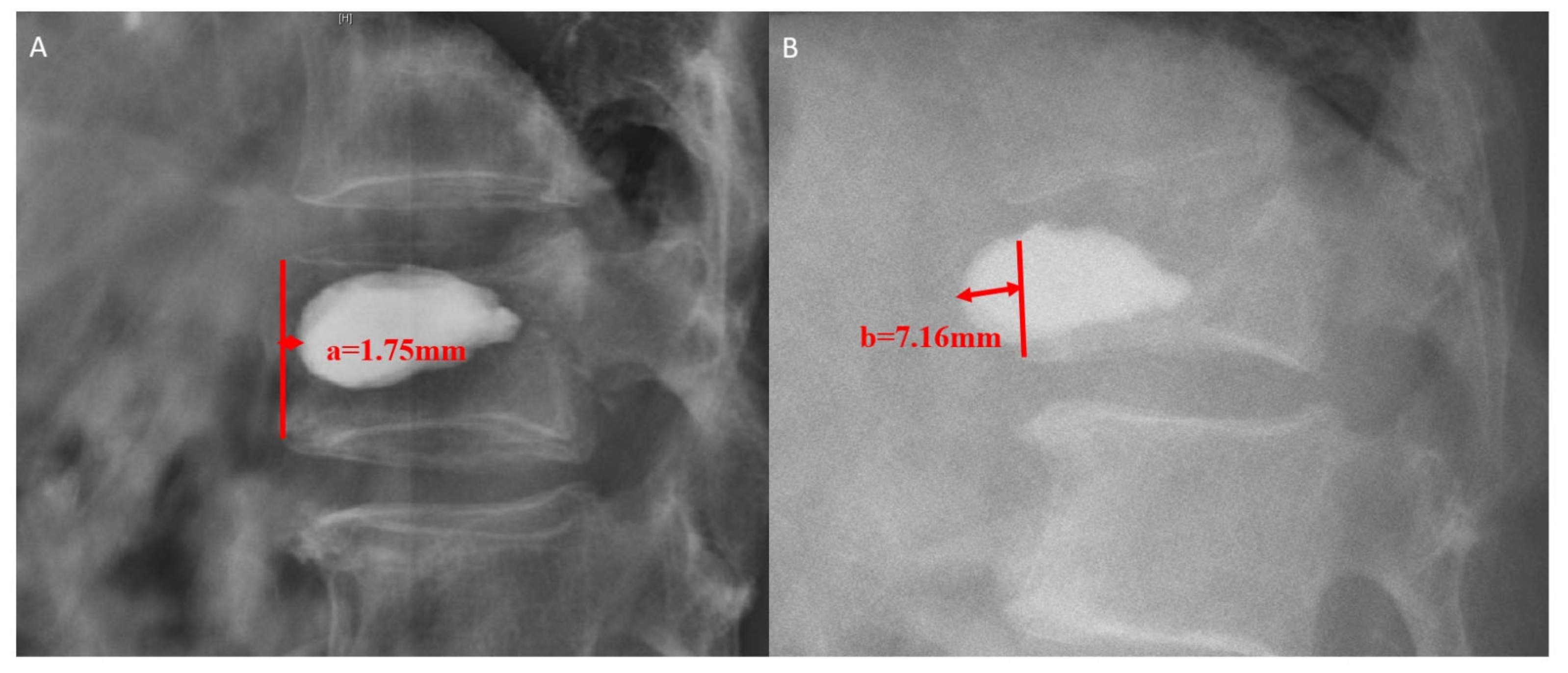
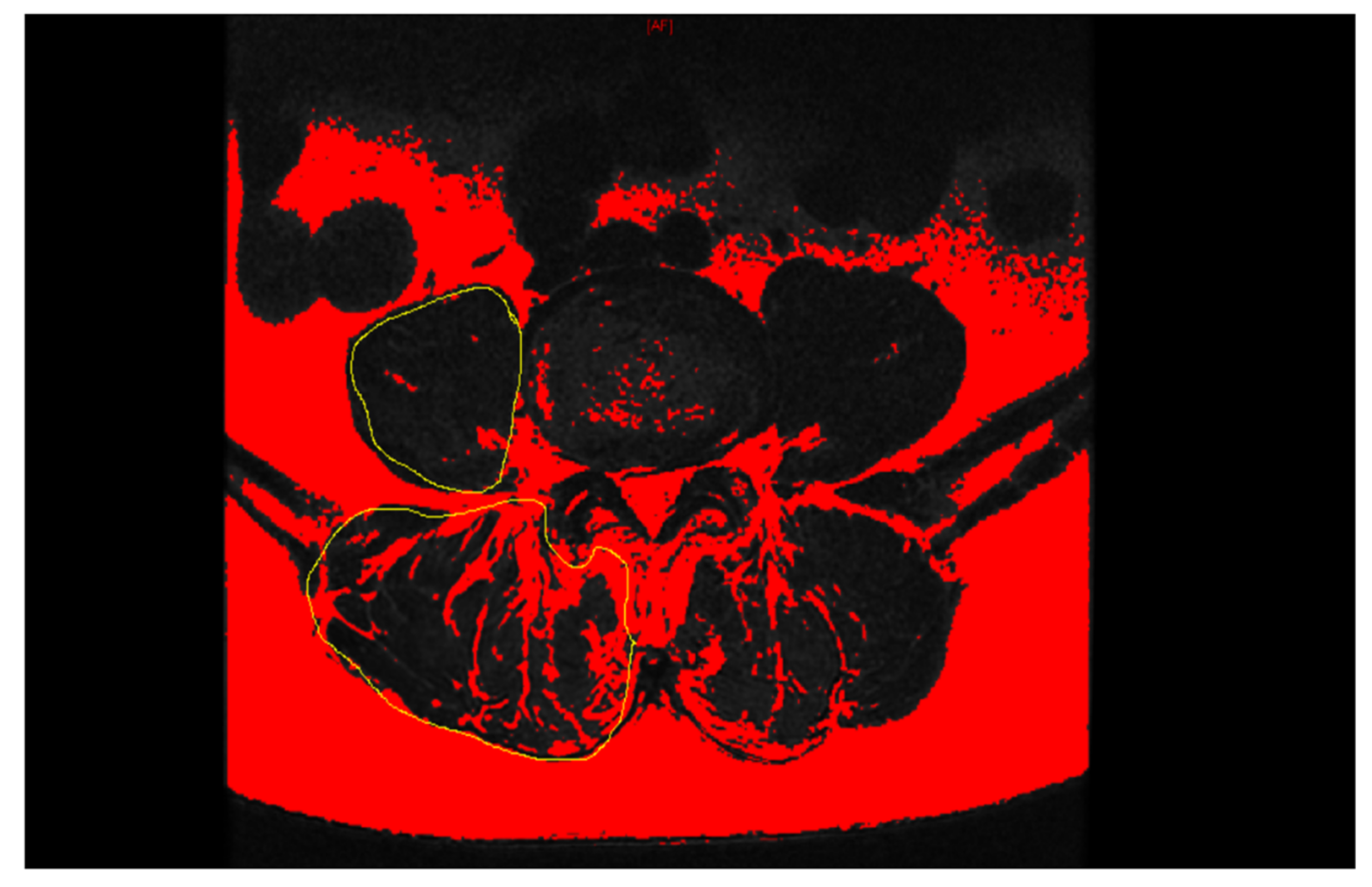
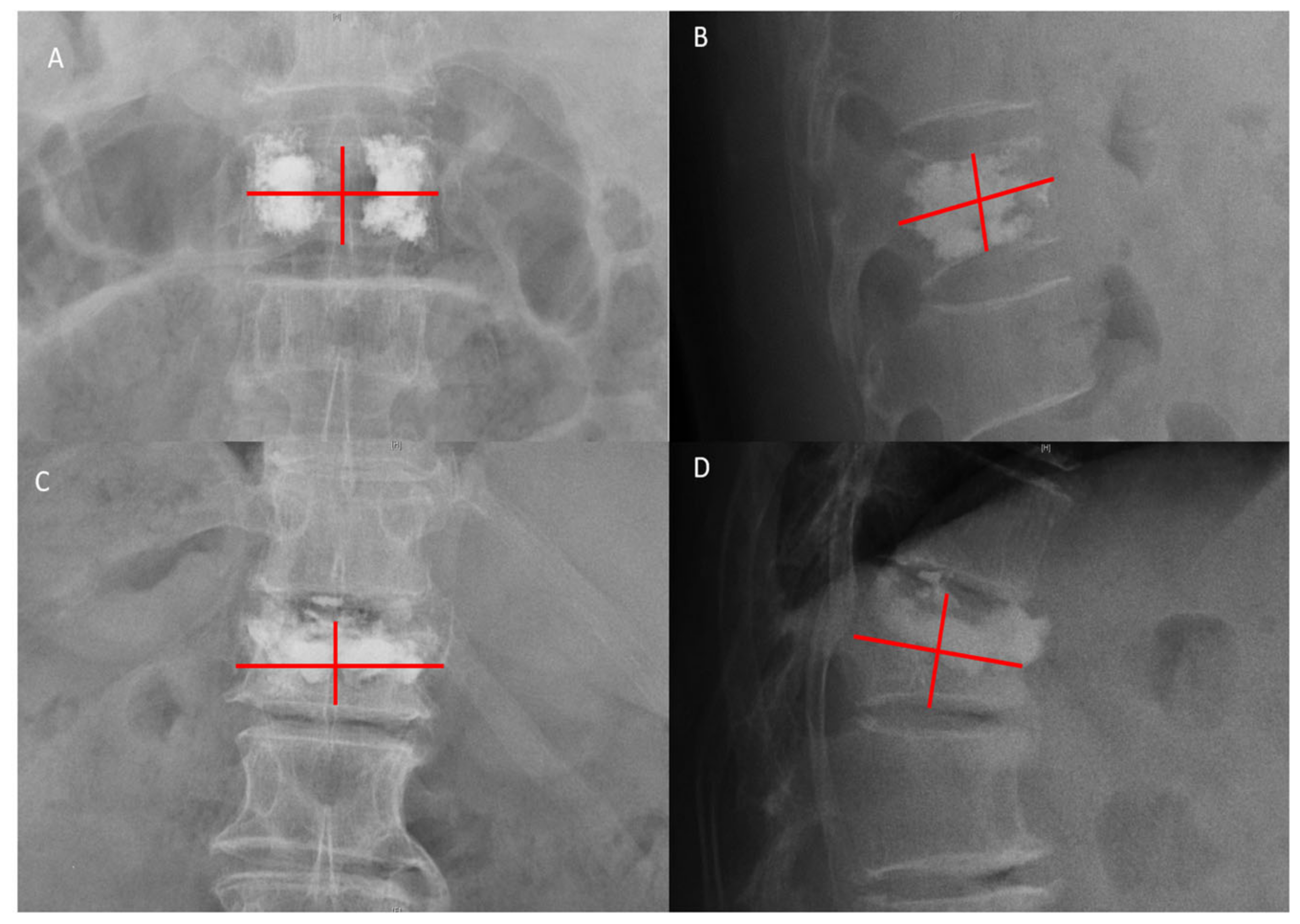
2.2. Data Collection
2.3. Review of the Literature
2.4. Statistical Analyses
3. Results
3.1. Univariate Analysis
3.2. Multiple Logistic Regression Analysis
3.3. Review of the Literature
4. Discussion
4.1. Incidence
4.2. Risk Factors
4.3. Outcomes
4.4. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kushchayev, S.V.; Wiener, P.C.; Teytelboym, O.M.; Arrington, J.A.; Khan, M.; Preul, M.C. Percutaneous vertebroplasty: A history of procedure, technology, culture, specialty, and economics. Neuroimaging Clin. N. Am. 2019, 29, 481–494. [Google Scholar] [CrossRef] [PubMed]
- Zhu, R.S.; Kan, S.L.; Ning, G.Z.; Chen, L.X.; Cao, Z.G.; Jiang, Z.H.; Zhang, X.L.; Hu, W. Which is the best treatment of osteoporotic vertebral compression fractures: Balloon kyphoplasty, percutaneous vertebroplasty, or non-surgical treatment? A Bayesian network meta-analysis. Osteoporos. Int. 2019, 30, 287–298. [Google Scholar] [CrossRef] [PubMed]
- Minamide, A.; Maeda, T.; Yamada, H.; Murakami, K.; Okada, M.; Enyo, Y.; Nakagawa, Y.; Iwasaki, H.; Tsutsui, S.; Takami, M.; et al. Early versus delayed kyphoplasty for thoracolumbar osteoporotic vertebral fractures: The effect of timing on clinical and radiographic outcomes and subsequent compression fractures. Clin. Neurol. Neurosurg. 2018, 173, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Hoyt, D.; Urits, I.; Orhurhu, V.; Orhurhu, M.S.; Callan, J.; Powell, J.; Manchikanti, L.; Kaye, A.D.; Kaye, R.J.; Viswanath, O. Current concepts in the management of vertebral compression fractures. Curr. Pain Headache Rep. 2020, 24, 16. [Google Scholar] [CrossRef]
- Zhan, Y.; Jiang, J.; Liao, H.; Tan, H.; Yang, K. Risk factors for cement leakage after vertebroplasty or kyphoplasty: A meta-analysis of published evidence. World Neurosurg. 2017, 101, 633–642. [Google Scholar] [CrossRef] [PubMed]
- Fang, M.; Zhou, J.; Yang, D.; He, Y.; Xu, Y.; Liu, X.; Zeng, Y. Management and outcomes of spinal epidural hematoma during vertebroplasty: Case series. Medicine 2018, 97, e10732. [Google Scholar] [CrossRef]
- Sidhu, G.S.; Kepler, C.K.; Savage, K.E.; Eachus, B.; Albert, T.J.; Vaccaro, A.R. Neurological deficit due to cement extravasation following a vertebral augmentation procedure. J. Neurosurg. Spine 2013, 19, 61–70. [Google Scholar] [CrossRef] [Green Version]
- Soto, E.; Galperin, M.; Portenoy, R.K. Transient thermal sympathectomy as a possible mechanism for hypotension after kyphoplasty: A case report. Clin. J. Pain 2013, 29, e49-53. [Google Scholar] [CrossRef]
- Park, J.-W.; Park, S.-M.; Lee, H.J.; Lee, C.-K.; Chang, B.-S.; Kim, H. Infection following percutaneous vertebral augmentation with polymethylmethacrylate. Arch. Osteoporos. 2018, 13, 47. [Google Scholar] [CrossRef]
- Li, Y.-X.; Guo, D.-Q.; Zhang, S.-C.; Liang, D.; Yuan, K.; Mo, G.-Y.; Li, D.-X.; Guo, H.-Z.; Tang, Y.; Luo, P.-J. Risk factor analysis for re-collapse of cemented vertebrae after percutaneous vertebroplasty (PVP) or percutaneous kyphoplasty (PKP). Int. Orthop. 2018, 42, 2131–2139. [Google Scholar] [CrossRef]
- Ha, K.-Y.; Kim, Y.-H.; Yoo, S.-R.; Molon, J.N. Bone cement dislodgement: One of complications following bone cement augmentation procedures for osteoporotic spinal fracture. J. Korean Neurosurg. Soc. 2015, 57, 367–370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, G.; Liu, X.; Li, Y.; Sun, J. Failed percutaneous kyphoplasty in treatment of stage 3 Kummell disease: A case report and literature review. Medicine 2017, 96, e8895. [Google Scholar] [CrossRef] [PubMed]
- Mueller, M.; Daniels-Wredenhagen, M.; Besch, L.; Decher, C.; Seekamp, A. Postoperative aseptic osteonecrosis in a case of kyphoplasty. Eur. Spine J. 2009, 18 (Suppl. S2), 213–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, X.; Du, J.; Gao, L.; Hao, D.; Hui, H.; He, B.; Yan, L. Risk factors for bone cement displacement after percutaneous vertebral augmentation for osteoporotic vertebral compression fractures. Front. Surg. 2022, 9, 947212. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Liang, D.; Yao, Z.; Qiu, T.; Ye, L.; Jiang, X. The therapeutic effect of intravertebral vacuum cleft with osteoporotic vertebral compression fractures: A systematic review and meta-analysis. Int. J. Surg. 2017, 40, 17–23. [Google Scholar] [CrossRef]
- Jeon, I.; Kim, S.W.; Yu, D. Paraspinal muscle fatty degeneration as a predictor of progressive vertebral collapse in osteoporotic vertebral compression fractures. Spine J. 2022, 22, 313–320. [Google Scholar] [CrossRef]
- Zou, D.; Ye, K.; Tian, Y.; Li, W.; Zhou, F.; Zhang, Z.; Lu, Z.; Xu, Z. Characteristics of vertebral CT Hounsfield units in elderly patients with acute vertebral fragility fractures. Eur. Spine J. 2020, 29, 1092–1097. [Google Scholar] [CrossRef]
- Bo, J.; Zhao, X.; Hua, Z.; Li, J.; Qi, X.; Shen, Y. Impact of sarcopenia and sagittal parameters on the residual back pain after percutaneous vertebroplasty in patients with osteoporotic vertebral compression fracture. J. Orthop. Surg. Res. 2022, 17, 111. [Google Scholar] [CrossRef]
- Han, G.; Wang, W.; Zhou, S.; Li, W.; Zhang, B.; Sun, Z.; Li, W. Paraspinal muscle degeneration as an independent risk for loss of local alignment in degenerative lumbar scoliosis patients after corrective surgery. Glob. Spine J. 2021, 21925682211022284. [Google Scholar] [CrossRef]
- Liu, J.; Tang, J.; Liu, H.; Gu, Z.; Zhang, Y.; Yu, S. A novel and convenient method to evaluate bone cement distribution following percutaneous vertebral augmentation. Sci. Rep. 2020, 10, 16320. [Google Scholar] [CrossRef]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, T.T.; Chen, W.J.; Lai, P.L.; Chen, L.H.; Niu, C.C.; Fu, T.S.; Wong, C.B. Polymethylmethacrylate cement dislodgment following percutaneous vertebroplasty: A case report. Spine 2003, 28, E457–E460. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.; Fang, S.; Wang, L.; Xu, R.; Shen, J.; Zhu, G.; Miao, Y.; Zou, T. Vertebral collapse and polymethylmethacrylate breakage after vertebroplasty: A case report. Medicine 2019, 98, e16831. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Choi, S.S.; Lee, M.K.; Lee, D.K.; Cho, S.I. Failed percutaneous vertebroplasty due to insufficient correction of intravertebral instability in kummell’s disease: A case report. Pain Pract. 2017, 17, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.L.; Baskurt, E. Refracture with cement extrusion following percutaneous vertebroplasty of a large interbody cleft. Am. J. Neuroradiol. 2006, 27, 230–231. [Google Scholar]
- Yoshii, T.; Ueki, H.; Kato, T.; Tomizawa, S.; Okawa, A. Severe kyphotic deformity resulting from collapses of cemented and adjacent vertebrae following percutaneous vertebroplasty using calcium phosphate cement. A case report. Skelet. Radiol. 2014, 43, 1477–1480. [Google Scholar] [CrossRef]
- Shin, D.A.; Kim, K.N.; Shin, H.C.; Kim, S.H.; Yoon, D.H. Progressive collapse of PMMA-augmented vertebra: A report of three cases. Zent. Neurochir. 2008, 69, 43–46. [Google Scholar] [CrossRef]
- Nüchterlein, M.; Bail, H.J. A rare complication of kyphoplasty is PMMA-cement loosening-case report and literature review. Z. Orthop. Unf. 2013, 151, 463–467. [Google Scholar] [CrossRef]
- Jeong, Y.H.; Lee, C.J.; Yeon, J.T.; Bae, J.; Choi, E.; Lee, P.B.; Nahm, F.S. Insufficient penetration of bone cement into the trabecular bone: A potential risk for delayed bone cement displacement after kyphoplasty? Reg. Anesth. Pain Med. 2016, 41, 616–618. [Google Scholar] [CrossRef]
- Chiu, Y.C.; Yang, S.C.; Chen, H.S.; Kao, Y.H.; Tu, Y.K. Posterior transpedicular approach with circumferential debridement and anterior reconstruction as a salvage procedure for symptomatic failed vertebroplasty. J. Orthop. Surg. Res. 2015, 10, 28. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; Thng, P.L.K.; Gupta, S.; Saini, N.; Saini, Y. Cement dislodgment after percutaneous vertebroplasty—A rare complication. J. Spine Surg. 2012, 6, 2. [Google Scholar]
- Ding, J.; Zhang, Q.; Zhu, J.; Tao, W.; Wu, Q.; Chen, L.; Shi, P.; Zhang, H. Risk factors for predicting cement leakage following percutaneous vertebroplasty for osteoporotic vertebral compression fractures. Eur. Spine J. 2016, 25, 3411–3417. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, H.; Hoshino, M.; Tsujio, T.; Terai, H.; Namikawa, T.; Kato, M.; Matsumura, A.; Suzuki, A.; Takayama, K.; Takahashi, S.; et al. Difference of clinical course between cases with bone union and those with delayed union following osteoporotic vertebral fractures. Arch. Osteoporos. 2017, 13, 3. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, Q.; Liu, W.J.; Li, Q.B.; Cai, L.; Wang, Z.K. Different Performance of intravertebral vacuum clefts in Kümmell’s disease and relevant treatment strategies. Orthop. Surg. 2020, 12, 199–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.H.; Kim, E.S.; Eoh, W. Cement augmented anterior reconstruction with short posterior instrumentation: A less invasive surgical option for Kummell’s disease with cord compression. J. Clin. Neurosci. 2011, 18, 509–514. [Google Scholar] [CrossRef]
- Lin, J.; Qian, L.; Jiang, C.; Chen, X.; Feng, F.; Lao, L. Bone cement distribution is a potential predictor to the reconstructive effects of unilateral percutaneous kyphoplasty in OVCFs: A retrospective study. J. Orthop. Surg. Res. 2018, 13, 140. [Google Scholar] [CrossRef] [Green Version]
- Tang, S.; Fu, W.; Zhang, H.; Zhang, H.; Liang, B. Efficacy and safety of high-viscosity bone cement vertebroplasty in treatment of osteoporotic vertebral compression fractures with intravertebral cleft. World Neurosurg. 2019, 132, e739–e745. [Google Scholar] [CrossRef]
- Lv, B.; Ji, P.; Fan, X.; Yuan, J.; Xu, T.; Yao, X.; Huang, A.; Zou, T. Clinical efficacy of different bone cement distribution patterns in percutaneous kyphoplasty: A retrospective study. Pain Physician 2020, 23, e409–e416. [Google Scholar]
- Lin, D.; Hao, J.; Li, L.; Wang, L.; Zhang, H.; Zou, W.; Lian, K. Effect of bone cement volume fraction on adjacent vertebral fractures after unilateral percutaneous kyphoplasty. Clin. Spine Surg. 2017, 30, E270–E275. [Google Scholar] [CrossRef]
- Sun, H.B.; Jing, X.S.; Liu, Y.Z.; Qi, M.; Wang, X.K.; Hai, Y. The optimal volume fraction in percutaneous vertebroplasty evaluated by pain relief, cement dispersion, and cement leakage: A prospective cohort study of 130 patients with painful osteoporotic vertebral compression fracture in the thoracolumbar vertebra. World Neurosurg. 2018, 114, e677–e688. [Google Scholar] [CrossRef]
- Bleiler, C.; Wagner, A.; Stadelmann, V.A.; Windolf, M.; Köstler, H.; Boger, A.; Gueorguiev-Rüegg, B.; Ehlers, W.; Röhrle, O. Multiphasic modelling of bone-cement injection into vertebral cancellous bone. Int. J. Numer. Method Biomed. Eng. 2015, 31, e02696. [Google Scholar] [CrossRef] [PubMed]
- Widmer, R.P.; Ferguson, S.J. A mixed boundary representation to simulate the displacement of a biofluid by a biomaterial in porous media. J. Biomech. Eng. 2011, 133, 051007. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, I.H. Sarcopenia: Origins and clinical relevance. Clin. Geriatr. Med. 2011, 27, 337–339. [Google Scholar] [CrossRef]
- Han, A.; Bokshan, S.L.; Marcaccio, S.E.; DePasse, J.M.; Daniels, A.H. Diagnostic criteria and clinical outcomes in sarcopenia research: A literature review. J. Clin. Med. 2018, 7, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talar, K.; Hernández-Belmonte, A.; Vetrovsky, T.; Steffl, M.; Kałamacka, E.; Courel-Ibáñez, J. Benefits of resistance training in early and late stages of frailty and sarcopenia: A systematic review and meta-analysis of randomized controlled studies. J. Clin. Med. 2021, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Lidar, S.; Salame, K.; Chua, M.; Khashan, M.; Ofir, D.; Grundstein, A.; Hochberg, U.; Lidar, Z.; Regev, G.J. Sarcopenia Is an independent risk factor for subsequent osteoporotic vertebral fractures following percutaneous cement augmentation in elderly patients. J. Clin. Med. 2022, 11, 5778. [Google Scholar] [CrossRef]
- Wang, W.-F.; Lin, C.-W.; Xie, C.-N.; Liu, H.-T.; Zhu, M.-Y.; Huang, K.-L.; Teng, H.-L. The association between sarcopenia and osteoporotic vertebral compression refractures. Osteoporos. Int. 2019, 30, 2459–2467. [Google Scholar] [CrossRef]
- Yokoyama, K.; Kawanishi, M.; Yamada, M.; Tanaka, H.; Ito, Y.; Hirano, M.; Kuroiwa, T. Safety and therapeutic efficacy of the second treatment for new fractures developed after initial vertebroplasty performed for painful vertebral compression fractures. Neurol. Res. 2013, 35, 608–613. [Google Scholar] [CrossRef]
- Cheng, Y.; Yang, H.; Hai, Y.; Liu, Y.; Guan, L.; Pan, A.; Zhang, Y. Low paraspinal lean muscle mass is an independent predictor of adjacent vertebral compression fractures after percutaneous kyphoplasty: A propensity score–matched case-control study. Front. Surg. 2022, 9, 965332. [Google Scholar] [CrossRef]
- Perna, A.; Santagada, D.A.; Bocchi, M.B.; Zirio, G.; Proietti, L.; Tamburrelli, F.C.; Genitiempo, M. Early loss of angular kyphosis correction in patients with thoracolumbar vertebral burst (A3–A4) fractures who underwent percutaneous pedicle screws fixation. J. Orthop. 2021, 24, 77–81. [Google Scholar] [CrossRef]
- Reginster, J.-Y.; Beaudart, C.; Buckinx, F.; Bruyère, O. Osteoporosis and sarcopenia: Two diseases or one? Curr. Opin. Clin. Nutr. Metab. Care 2016, 19, 31–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sollmann, N.; Franz, D.; Burian, E.; Löffler, M.T.; Probst, M.; Gersing, A.; Schwaiger, B.; Pfeiffer, D.; Kirschke, J.S.; Baum, T.; et al. Assessment of paraspinal muscle characteristics, lumbar BMD, and their associations in routine multi-detector CT of patients with and without osteoporotic vertebral fractures. Eur. J. Radiol. 2020, 125, 108867. [Google Scholar] [CrossRef]
- Yang, S.-C.; Chen, W.-J.; Yu, S.-W.; Tu, Y.-K.; Kao, Y.-H.; Chung, K.-C. Revision strategies for complications and failure of vertebroplasties. Eur. Spine J. 2008, 17, 982–988. [Google Scholar] [CrossRef] [PubMed]
- Ha, K.-Y.; Kim, Y.-H.; Chang, D.-G.; Son, I.-N.; Kim, K.-W.; Kim, S.-E. Causes of late revision surgery after bone cement augmentation in osteoporotic vertebral compression fractures. Asian Spine J. 2013, 7, 294–300. [Google Scholar] [CrossRef] [PubMed]

| ICC | 95% CI | p | |
|---|---|---|---|
| Radiographic diagnosis of BCD | 0.944 | 0.910–0.957 | <0.001 |
| IVC sign | 0.951 | 0.939–0.982 | <0.001 |
| Vertebral collapse | 0.776 | 0.704–0.841 | 0.002 |
| L1-HU | 0.889 | 0.874–0.902 | <0.001 |
| PSM rCSA | 0.818 | 0.715–0.934 | <0.001 |
| PSM fatty degeneration | 0.783 | 0.756–0.807 | <0.001 |
| Kyphotic angle | |||
| Preoperative | 0.907 | 0.854–0.966 | <0.001 |
| Postoperative | 0.861 | 0.836–0.904 | <0.001 |
| At the last follow-up | 0.881 | 0.865–0.894 | <0.001 |
| Bone cement distribution score | 0.814 | 0.790–0.835 | <0.001 |
| N (%) | Symptomatic Group (a) n = 21 (2.3%) | Asymptomatic Group (b) n = 35 (3.9%) | Control Group (c) n = 840 (93.8%) | p * | pa−c | pb−c | pa−b |
|---|---|---|---|---|---|---|---|
| Demographic | |||||||
| Age (years) | 71.48 ± 9.63 | 73.85 ± 10.06 | 72.39 ± 9.14 | 0.755 | 0.763 | 0.496 | 0.467 |
| Female (n, %) | 16 (76.1) | 27 (77.1) | 643 (76.5) | 0.989 | 0.974 | 0.895 | 0.935 |
| BMI (Kg/m2) | 24.25 ± 3.10 | 25.00 ± 3.86 | 24.23 ± 3.64 | 0.612 | 0.978 | 0.466 | 0.555 |
| Hypertension (n, %) | 7 (33.3) | 15 (46.9) | 367 (43.7) | 0.613 | 0.344 | 0.922 | 0.480 |
| Diabetes (n, %) | 2 (9.5) | 9 (25.7) | 157 (18.7) | 0.285 | 0.285 | 0.299 | 0.140 |
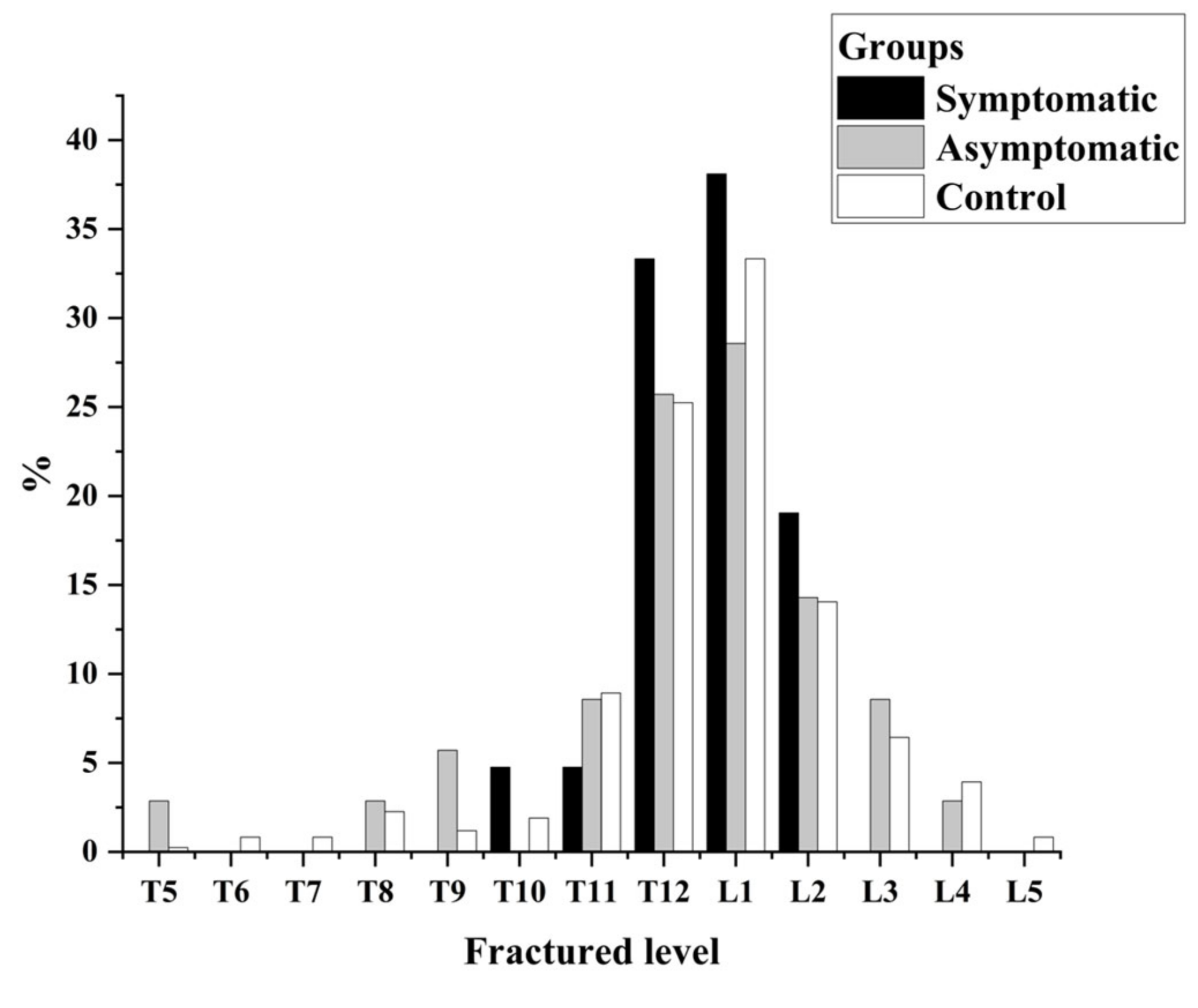
| Vertebral distribution | 0.304 | 0.328 | 0.305 | 0.168 | |||
| Thoracic region (n, %) | 0 (0) | 4 (11.4) | 45 (5.9) | ||||
| Thoracolumbar region (n, %) | 20 (95.2) | 27 (77.1) | 701 (82.6) | ||||
| Lumbar region (n, %) | 1 (4.8) | 4 (11.4) | 94 (11.4) | ||||
| IVC sign (n, %) | 3 (14.3) | 4 (11.4) | 32 (3.8) | 0.008 | 0.016 | 0.026 | 0.754 |
| Vertebral collapse (%) | 32.02 ± 11.15 | 32.41 ± 12.94 | 29.61 ± 8.66 | 0.171 | 0.212 | 0.178 | 0.841 |
| L1-HU (Hu) | 78.89 ± 14.71 | 77.84 ± 12.16 | 79.82 ± 11.63 | 0.525 | 0.717 | 0.328 | 0.740 |
| Paraspinal muscle (L4-5) | |||||||
| PSM rCSA (%) | 150.05 ± 14.41 | 159.88 ± 23.41 | 159.20 ± 17.42 | 0.043 | 0.023 | 0.825 | 0.046 |
| PSM fatty degeneration (%) | 38.52 ± 12.41 | 32.33 ± 8.27 | 34.13 ± 8.23 | 0.028 | 0.020 | 0.213 | 0.008 |
| Kyphotic angle | |||||||
| Preoperative (°) | 22.06 ± 5.87 | 21.71 ± 6.20 | 21.10 ± 3.34 | 0.313 | 0.203 | 0.519 | 0.869 |
| Postoperative (°) | 20.75 ± 7.31 | 18.39 ± 5.19 | 18.31 ± 5.83 | 0.157 | 0.076 | 0.953 | 0.054 |
| Restoration of kyphotic angle (%) | 17.21 ± 11.63 | 20.79 ± 14.34 | 23.38 ± 17.71 | 0.207 | 0.113 | 0.393 | 0.339 |
| At the last follow-up (°) | 21.51 ± 6.19 | 18.72 ± 5.38 | 18.87 ± 5.75 | 0.045 | 0.037 | 0.879 | 0.046 |
| Volume of bone cement injected (mL) | 5.33 ± 0.73 | 5.08 ± 0.76 | 5.12 ± 1.02 | 0.607 | 0.323 | 0.904 | 0.475 |
| Cement leakage | <0.001 | 0.002 | <0.001 | 0.588 | |||
| No leakage (n, %) | 8 (38.1) | 9 (25.7) | 571 (68.0) | ||||
| Anterior leakage (n, %) | 11 (52.4) | 23 (65.7) | 157 (18.7) | ||||
| Others (n, %) | 2 (9.5) | 3 (8.6) | 112 (13.3) | ||||
| Bone cement distribution score | 7.73 ± 1.88 | 7.85 ± 1.76 | 8.08 ± 1.43 | <0.001 | 0.007 | 0.042 | 0.824 |
| VAS | |||||||
| At admission | 6.06 ± 1.66 | 6.11 ± 1.72 | 6.12 ± 0.98 | 0.612 | 0.335 | 0.932 | 0.411 |
| At discharge | 3.68 ± 1.12 | 3.50 ± 0.59 | 3.57 ± 0.57 | 0.571 | 0.217 | 0.960 | 0.397 |
| ODI | |||||||
| At admission | 47.98 ± 9.31 | 50.50 ± 5.22 | 49.52 ± 5.65 | 0.284 | 0.216 | 0.573 | 0.433 |
| At discharge | 21.15 ± 7.46 | 21.66 ± 5.68 | 20.96 ± 3.99 | 0.812 | 0.835 | 0.534 | 0.751 |
| Preoperative drug consumption | 0.147 | 0.096 | 0.359 | 0.115 | |||
| None (n, %) | 6 (28.6) | 3 (8.6) | 126 (15.0) | ||||
| NSAIDs (n, %) | 10 (47.6) | 18 (51.4) | 341 (40.6) | ||||
| Opioids (n, %) | 5 (23.8) | 14 (40.0) | 373 (44.4) | ||||
| Postoperative drug consumption | 0.418 | 0.491 | 0.304 | 0.187 | |||
| None (n, %) | 9 (42.9) | 21 (60.0) | 452 (53.8) | ||||
| NSAIDs (n, %) | 11 (52.4) | 12 (28.6) | 332 (39.5) | ||||
| Opioids (n, %) | 1 (4.8) | 4 (11.4) | 56 (6.7) | ||||
| Brace wearing time | 0.582 | 0.444 | 0.602 | 0.362 | |||
| <1 month (n,%) | 5 (23.8) | 13 (37.1) | 266 (31.7) | ||||
| ≥1 month (n,%) | 16 (76.2) | 22 (62.9) | 574 (68.3) | ||||
| Osteoporosis medication (n, %) | 16 (76.2) | 26 (74.3) | 609 (72.5) | 0.775 | 0.708 | 0.817 | 0.873 |
| N (%) | Symptomatic Group (a) n = 21 (2.3%) | Asymptomatic Group (b) n = 35 (3.9%) | Control Group (c) n = 840 (93.8%) | p * | pa−c | pb−c | pa−b |
|---|---|---|---|---|---|---|---|
| VAS | 5.47 ± 1.53 | 3.38 ± 1.23 | 3.33 ± 0.96 | <0.001 | <0.001 | 0.577 | <0.001 |
| ODI | 34.89 ± 8.42 | 21.98 ± 7.10 | 20.66 ± 4.12 | <0.001 | <0.001 | 0.148 | <0.001 |
| New vertebral fractures (n, %) | 9 (42.9) | 8 (22.9) | 178 (21.2) | 0.048 | 0.017 | 0.813 | 0.115 |
| Treatment | - | - | - | ||||
| Reoperation (n, %) | 16 (76.1) | - | - | - | - | - | |
| Conservative treatment (n, %) | 5 (23.8) | - | - | - | - | - |
| Adjusted OR | 95% CI | p | |
|---|---|---|---|
| Symptomatic BCD | |||
| Anterior leakage | 1.737 | 1.215–3.300 | 0.022 |
| IVC | 3.361 | 1.605–13.036 | 0.008 |
| Bone cement distribution score | 0.476 | 0.225–0.904 | 0.025 |
| PSM rCSA | 0.953 | 0.917–0.992 | 0.017 |
| PSM fatty degeneration | 1.061 | 1.005–1.119 | 0.009 |
| Asymptomatic BCD | |||
| Anterior leakage | 1.839 | 1.206–2.803 | 0.013 |
| IVC | 2.936 | 1.174–9.018 | 0.032 |
| Bone cement distribution score | 0.632 | 0.295–0.858 | 0.006 |
| Reference | Age (yr)/Sex | Affected Level/ Intervention | Time to BCD | Symptom | Cause | Treatment | FU Time/Outcome |
|---|---|---|---|---|---|---|---|
| Ha et al. [11] | 73/F | T11/PKP | 6 weeks | WBP | No trauma | Surgical treatment, fixation T8–L3 | 2 years/Cured |
| Tsai et al. [22] | 69/M | T12/PVP | 1 month | WBP and ND | No trauma | Surgical treatment, fixation T11–L1 | NA |
| Zhang C [12] | 73/F | T12/PVP | 1 month | WBP and ND | No trauma | Surgical treatment, fixation T10–L2 | 1 year/BBP but similar muscle strength |
| Mueller et al. [13] | 73/F | L1/PKP | 3 weeks | WBP | No trauma | Surgical treatment, fixation T12–L2 | NA |
| Huang et al. [23] | 72/M | L1/PVP | 30 months | WBP | No trauma | Surgical treatment, fixation T10–L4 | >2 years/Cured |
| Kim JE et al. [24] | 75/F | L1/PVP | 10 weeks | WBP | No trauma | Surgical treatment, repeat PVP | 1 month/BBP |
| Wagner et al. [25] | 75/F | L3/PVP | 1 month | WBP | No trauma | Non-surgical treatment | Died soon |
| Yoshii T et al. [26] | 74/F | L3/PVP | 1 month | WBP | No trauma | Surgical treatment, fixation T12–S1 | 1 year/Cured |
| Shin DA et al. [27] | 78/M | L4/PVP | 1 month | WBP | No trauma | Non-surgical treatment | NA |
| Nüchterlein et al. [28] | 72/M | L4/PKP | 2 months | WBP | Trauma | Surgical treatment, fixation L2–S1 | 1 year/Cured |
| Sharma et al. [31] | 70/F | L4/PVP | 6 months | WBP | No trauma | Non-surgical treatment | NA |
| Jeong YH et al. [29] | 74/F | L4/PKP | 1 month | WBP | No trauma | Non-surgical treatment | 3 months/BBP but worse kyphosis |
| Chiu YC et al. [30] | 78/M | T11 and T12/PVP | 18 months | WBP | No trauma | Surgical treatment, fixation T9–L2 | Mean of 1 year/Symptomatic relief |
| 76/F | T12/PVP | 12 months | WBP | No trauma | Surgical treatment, fixation T10–L2 | ||
| 69/F | T12/PVP | 25 months | WBP | No trauma | Surgical treatment, fixation T9–L4 | ||
| 89/M | T12 and L1/PVP | 22 months | WBP | No trauma | Surgical treatment, fixation T10–L4 | ||
| 71/F | L2 and L3/PVP | 9 months | WBP | No trauma | Surgical treatment, fixation T12–L5 | ||
| 90/M | L3/PVP | 20 months | WBP | No trauma | Surgical treatment, fixation L1–L5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qi, J.; Hu, Y.; Yang, Z.; Dong, Y.; Zhang, X.; Hou, G.; Lv, Y.; Guo, Y.; Zhou, F.; Liu, B.; et al. Incidence, Risk Factors, and Outcomes of Symptomatic Bone Cement Displacement following Percutaneous Kyphoplasty for Osteoporotic Vertebral Compression Fracture: A Single Center Study. J. Clin. Med. 2022, 11, 7530. https://doi.org/10.3390/jcm11247530
Qi J, Hu Y, Yang Z, Dong Y, Zhang X, Hou G, Lv Y, Guo Y, Zhou F, Liu B, et al. Incidence, Risk Factors, and Outcomes of Symptomatic Bone Cement Displacement following Percutaneous Kyphoplasty for Osteoporotic Vertebral Compression Fracture: A Single Center Study. Journal of Clinical Medicine. 2022; 11(24):7530. https://doi.org/10.3390/jcm11247530
Chicago/Turabian StyleQi, Junbo, Yuanyu Hu, Zhongwei Yang, Yanlei Dong, Xin Zhang, Guojin Hou, Yang Lv, Yan Guo, Fang Zhou, Bingchuan Liu, and et al. 2022. "Incidence, Risk Factors, and Outcomes of Symptomatic Bone Cement Displacement following Percutaneous Kyphoplasty for Osteoporotic Vertebral Compression Fracture: A Single Center Study" Journal of Clinical Medicine 11, no. 24: 7530. https://doi.org/10.3390/jcm11247530
APA StyleQi, J., Hu, Y., Yang, Z., Dong, Y., Zhang, X., Hou, G., Lv, Y., Guo, Y., Zhou, F., Liu, B., & Tian, Y. (2022). Incidence, Risk Factors, and Outcomes of Symptomatic Bone Cement Displacement following Percutaneous Kyphoplasty for Osteoporotic Vertebral Compression Fracture: A Single Center Study. Journal of Clinical Medicine, 11(24), 7530. https://doi.org/10.3390/jcm11247530





