Sex Differences in Glioblastoma—Findings from the Swedish National Quality Registry for Primary Brain Tumors between 1999–2018
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bruhn, H.; Strandeus, M.; Milos, P.; Hallbeck, M.; Vrethem, M.; Lind, J. Improved survival of Swedish glioblastoma patients treated according to Stupp. Acta Neurol. Scand. 2018, 138, 332–337. [Google Scholar] [CrossRef]
- Ostrom, Q.T.; Cote, D.J.; Ascha, M.; Kruchko, C.; Barnholtz-Sloan, J.S. Adult Glioma Incidence and Survival by Race or Ethnicity in the United States From 2000 to 2014. JAMA Oncol. 2018, 4, 1254–1262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stupp, R.; Taillibert, S.; Kanner, A.; Read, W.; Steinberg, D.; Lhermitte, B.; Toms, S.; Idbaih, A.; Ahluwalia, M.S.; Fink, K.; et al. Effect of Tumor-Treating Fields Plus Maintenance Temozolomide vs Maintenance Temozolomide Alone on Survival in Patients With Glioblastoma: A Randomized Clinical Trial. JAMA 2017, 318, 2306–2316. [Google Scholar] [CrossRef] [Green Version]
- Ostrom, Q.T.; Gittleman, H.; Truitt, G.; Boscia, A.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2011–2015. Neuro Oncol. 2018, 20, iv1–iv86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Liao, J.; Zhang, X.; Zhao, E.; Liang, X.; Luo, S.; Shi, J.; Yu, F.; Xu, J.; Shen, W.; et al. Sex difference of mutation clonality in diffuse glioma evolution. Neuro Oncol. 2019, 21, 201–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansen, M.L.; Stetson, L.C.; Vadmal, V.; Waite, K.; Berens, M.E.; Connor, J.R.; Lathia, J.; Rubin, J.B.; Barnholtz-Sloan, J.S. Gliomas display distinct sex-based differential methylation patterns based on molecular subtype. Neurooncol. Adv. 2020, 2, vdaa002. [Google Scholar] [CrossRef]
- Yang, W.; Warrington, N.M.; Taylor, S.J.; Whitmire, P.; Carrasco, E.; Singleton, K.W.; Wu, N.; Lathia, J.D.; Berens, M.E.; Kim, A.H.; et al. Sex differences in GBM revealed by analysis of patient imaging, transcriptome, and survival data. Sci. Transl. Med. 2019, 11, eaao5253. [Google Scholar] [CrossRef] [Green Version]
- Ippolito, J.E.; Yim, A.K.; Luo, J.; Chinnaiyan, P.; Rubin, J.B. Sexual dimorphism in glioma glycolysis underlies sex differences in survival. JCI Insight 2017, 2, e92142. [Google Scholar] [CrossRef] [Green Version]
- Smits, A.; Lysiak, M.; Magnusson, A.; Rosell, J.; Soderkvist, P.; Malmstrom, A. Sex Disparities in MGMT Promoter Methylation and Survival in Glioblastoma: Further Evidence from Clinical Cohorts. J. Clin. Med. 2021, 10, 556. [Google Scholar] [CrossRef]
- Franceschi, E.; Tosoni, A.; Minichillo, S.; Depenni, R.; Paccapelo, A.; Bartolini, S.; Michiara, M.; Pavesi, G.; Urbini, B.; Crisi, G.; et al. The Prognostic Roles of Gender and O6-Methylguanine-DNA Methyltransferase Methylation Status in Glioblastoma Patients: The Female Power. World Neurosurg. 2018, 112, e342–e347. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Rubin, J.B.; Lathia, J.D.; Berens, M.E.; Barnholtz-Sloan, J.S. Females have the survival advantage in glioblastoma. Neuro Oncol. 2018, 20, 576–577. [Google Scholar] [CrossRef] [PubMed]
- Thakkar, J.P.; Dolecek, T.A.; Horbinski, C.; Ostrom, Q.T.; Lightner, D.D.; Barnholtz-Sloan, J.S.; Villano, J.L. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol. Biomarkers. Prev. 2014, 23, 1985–1996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Ohgaki, H.; Wiestler, O.D.; Cavenee, W.K.; Burger, P.C.; Jouvet, A.; Scheithauer, B.W.; Kleihues, P. The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007, 114, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Wick, W.; Roth, P.; Hartmann, C.; Hau, P.; Nakamura, M.; Stockhammer, F.; Sabel, M.C.; Wick, A.; Koeppen, S.; Ketter, R.; et al. Long-term analysis of the NOA-04 randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with PCV or temozolomide. Neuro Oncol. 2016, 18, 1529–1537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tesileanu, C.M.S.; van den Bent, M.J.; Sanson, M.; Wick, W.; Brandes, A.A.; Clement, P.M.; Erridge, S.C.; Vogelbaum, M.A.; Nowak, A.K.; Baurain, J.F.; et al. Prognostic significance of genome-wide DNA methylation profiles within the randomized, phase 3, EORTC CATNON trial on non-1p/19q deleted anaplastic glioma. Neuro Oncol. 2021, 23, 1547–1559. [Google Scholar] [CrossRef]
- Weller, M.; van den Bent, M.; Preusser, M.; Le Rhun, E.; Tonn, J.C.; Minniti, G.; Bendszus, M.; Balana, C.; Chinot, O.; Dirven, L.; et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat. Rev. Clin. Oncol. 2021, 18, 170–186. [Google Scholar] [CrossRef]
- Wen, P.Y.; Packer, R.J. The 2021 WHO Classification of Tumors of the Central Nervous System: Clinical implications. Neuro Oncol. 2021, 23, 1215–1217. [Google Scholar] [CrossRef]
- Gittleman, H.; Ostrom, Q.T.; Stetson, L.C.; Waite, K.; Hodges, T.R.; Wright, C.H.; Wright, J.; Rubin, J.B.; Berens, M.E.; Lathia, J.; et al. Sex is an important prognostic factor for glioblastoma but not for nonglioblastoma. Neurooncol. Pract. 2019, 6, 451–462. [Google Scholar] [CrossRef] [PubMed]
- Matteoni, S.; Abbruzzese, C.; Villani, V.; Malorni, W.; Pace, A.; Matarrese, P.; Paggi, M.G. The influence of patient sex on clinical approaches to malignant glioma. Cancer Lett. 2020, 468, 41–47. [Google Scholar] [CrossRef]
- Sun, T.; Plutynski, A.; Ward, S.; Rubin, J.B. An integrative view on sex differences in brain tumors. Cell Mol. Life Sci. 2015, 72, 3323–3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fyllingen, E.H.; Hansen, T.I.; Jakola, A.S.; Haberg, A.K.; Salvesen, O.; Solheim, O. Does risk of brain cancer increase with intracranial volume? A population-based case control study. Neuro Oncol. 2018, 20, 1225–1230. [Google Scholar] [CrossRef]
- Sun, T.; Warrington, N.M.; Luo, J.; Brooks, M.D.; Dahiya, S.; Snyder, S.C.; Sengupta, R.; Rubin, J.B. Sexually dimorphic RB inactivation underlies mesenchymal glioblastoma prevalence in males. J. Clin. Investig. 2014, 124, 4123–4133. [Google Scholar] [CrossRef] [Green Version]
- Haupt, S.; Caramia, F.; Herschtal, A.; Soussi, T.; Lozano, G.; Chen, H.; Liang, H.; Speed, T.P.; Haupt, Y. Identification of cancer sex-disparity in the functional integrity of p53 and its X chromosome network. Nat. Commun. 2019, 10, 5385. [Google Scholar] [CrossRef] [PubMed]
- Ostrom, Q.T.; Coleman, W.; Huang, W.; Rubin, J.B.; Lathia, J.D.; Berens, M.E.; Speyer, G.; Liao, P.; Wrensch, M.R.; Eckel-Passow, J.E.; et al. Sex-specific gene and pathway modeling of inherited glioma risk. Neuro Oncol. 2019, 21, 71–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hirtz, A.; Rech, F.; Dubois-Pot-Schneider, H.; Dumond, H. Astrocytoma: A Hormone-Sensitive Tumor? Int. J. Mol. Sci. 2020, 21, 9114. [Google Scholar] [CrossRef] [PubMed]
- Massey, S.C.; Whitmire, P.; Doyle, T.E.; Ippolito, J.E.; Mrugala, M.M.; Hu, L.S.; Canoll, P.; Anderson, A.R.A.; Wilson, M.A.; Fitzpatrick, S.M.; et al. Sex differences in health and disease: A review of biological sex differences relevant to cancer with a spotlight on glioma. Cancer Lett. 2021, 498, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Qi, Z.Y.; Shao, C.; Zhang, X.; Hui, G.Z.; Wang, Z. Exogenous and endogenous hormones in relation to glioma in women: A meta-analysis of 11 case-control studies. PLoS ONE 2013, 8, e68695. [Google Scholar] [CrossRef] [Green Version]
- Asad, A.S.; Nicola Candia, A.J.; Gonzalez, N.; Zuccato, C.F.; Abt, A.; Orrillo, S.J.; Lastra, Y.; De Simone, E.; Boutillon, F.; Goffin, V.; et al. Prolactin and its receptor as therapeutic targets in glioblastoma multiforme. Sci. Rep. 2019, 9, 19578. [Google Scholar] [CrossRef]
- Michaud, D.S.; Gallo, V.; Schlehofer, B.; Tjønneland, A.; Olsen, A.; Overvad, K.; Dahm, C.C.; Kaaks, R.; Lukanova, A.; Boeing, H.; et al. Reproductive Factors and Exogenous Hormone Use in Relation to Risk of Glioma and Meningioma in a Large European Cohort Study. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2562–2569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalcman, N.; Canello, T.; Ovadia, H.; Charbit, H.; Zelikovitch, B.; Mordechai, A.; Fellig, Y.; Rabani, S.; Shahar, T.; Lossos, A.; et al. Androgen receptor: A potential therapeutic target for glioblastoma. Oncotarget 2018, 9, 19980–19993. [Google Scholar] [CrossRef] [Green Version]
- Schiffgens, S.; Wilkens, L.; Brandes, A.A.; Meier, T.; Franceschi, E.; Ermani, M.; Hartmann, C.; Sandalcioglu, I.E.; Dumitru, C.A. Sex-specific clinicopathological significance of novel (Frizzled-7) and established (MGMT, IDH1) biomarkers in glioblastoma. Oncotarget 2016, 7, 55169–55180. [Google Scholar] [CrossRef] [Green Version]
- Gupta, T.; Mohanty, S.; Moiyadi, A.; Jalali, R. Factors predicting temozolomide induced clinically significant acute hematologic toxicity in patients with high-grade gliomas: A clinical audit. Clin. Neurol. Neurosurg. 2013, 115, 1814–1819. [Google Scholar] [CrossRef]
- Asklund, T.; Malmstrom, A.; Bergqvist, M.; Bjor, O.; Henriksson, R. Brain tumors in Sweden: Data from a population-based registry 1999–2012. Acta Oncol. 2015, 54, 377–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asklund, T.; Malmstrom, A.; Bjor, O.; Blomquist, E.; Henriksson, R. Considerable improvement in survival for patients aged 60-84 years with high grade malignant gliomas—Data from the Swedish Brain Tumour Population-based Registry. Acta Oncol. 2013, 52, 1041–1043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stepp, H.; Stummer, W. 5-ALA in the management of malignant glioma. Lasers Surg. Med. 2018, 50, 399–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ostrom, Q.T.; Cioffi, G.; Waite, K.; Kruchko, C.; Barnholtz-Sloan, J.S. CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2014–2018. Neuro Oncol. 2021, 23, iii1–iii105. [Google Scholar] [CrossRef]
- Bjorland, L.S.; Fluge, O.; Gilje, B.; Mahesparan, R.; Farbu, E. Treatment approach and survival from glioblastoma: Results from a population-based retrospective cohort study from Western Norway. BMJ Open 2021, 11, e043208. [Google Scholar] [CrossRef]
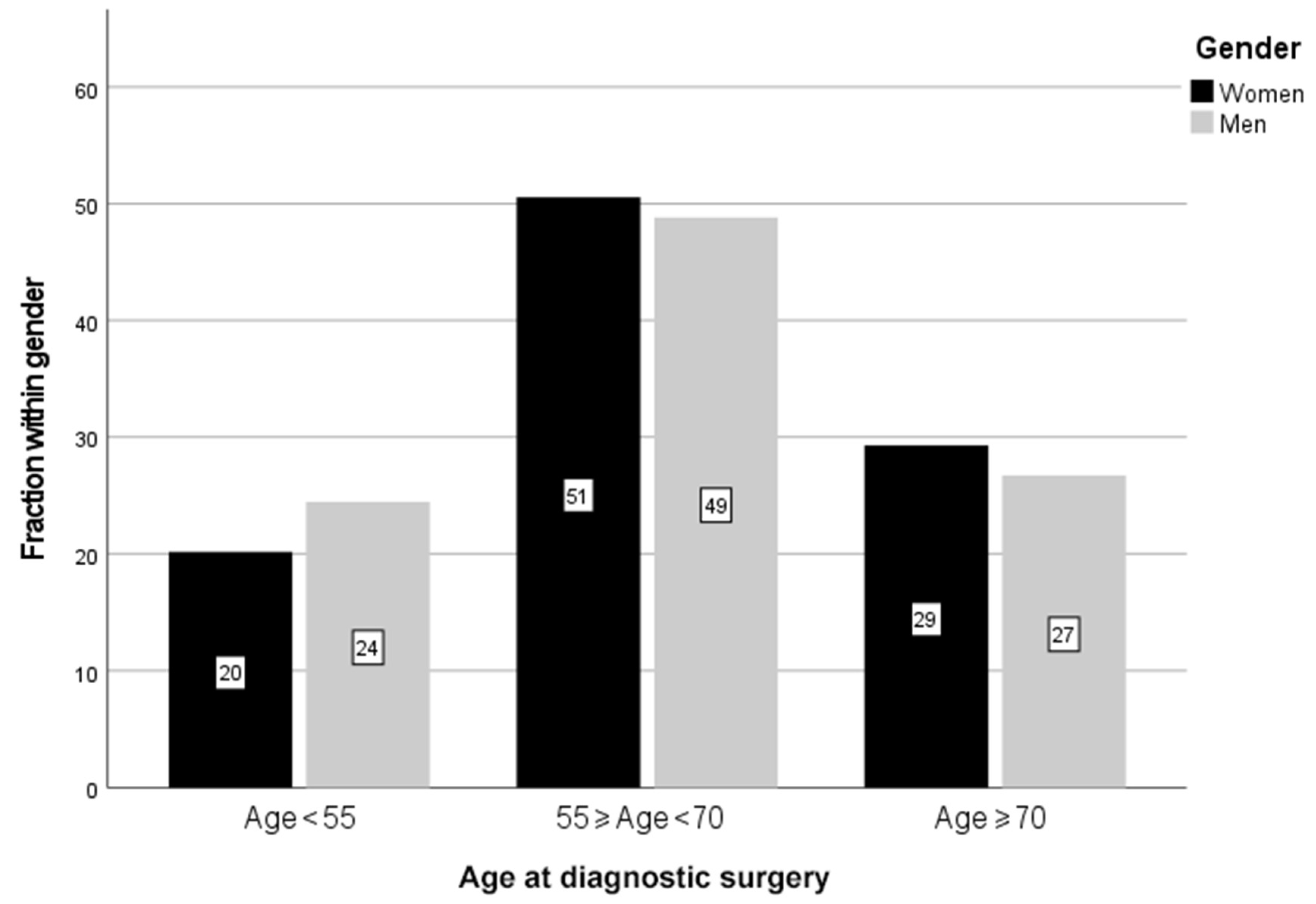
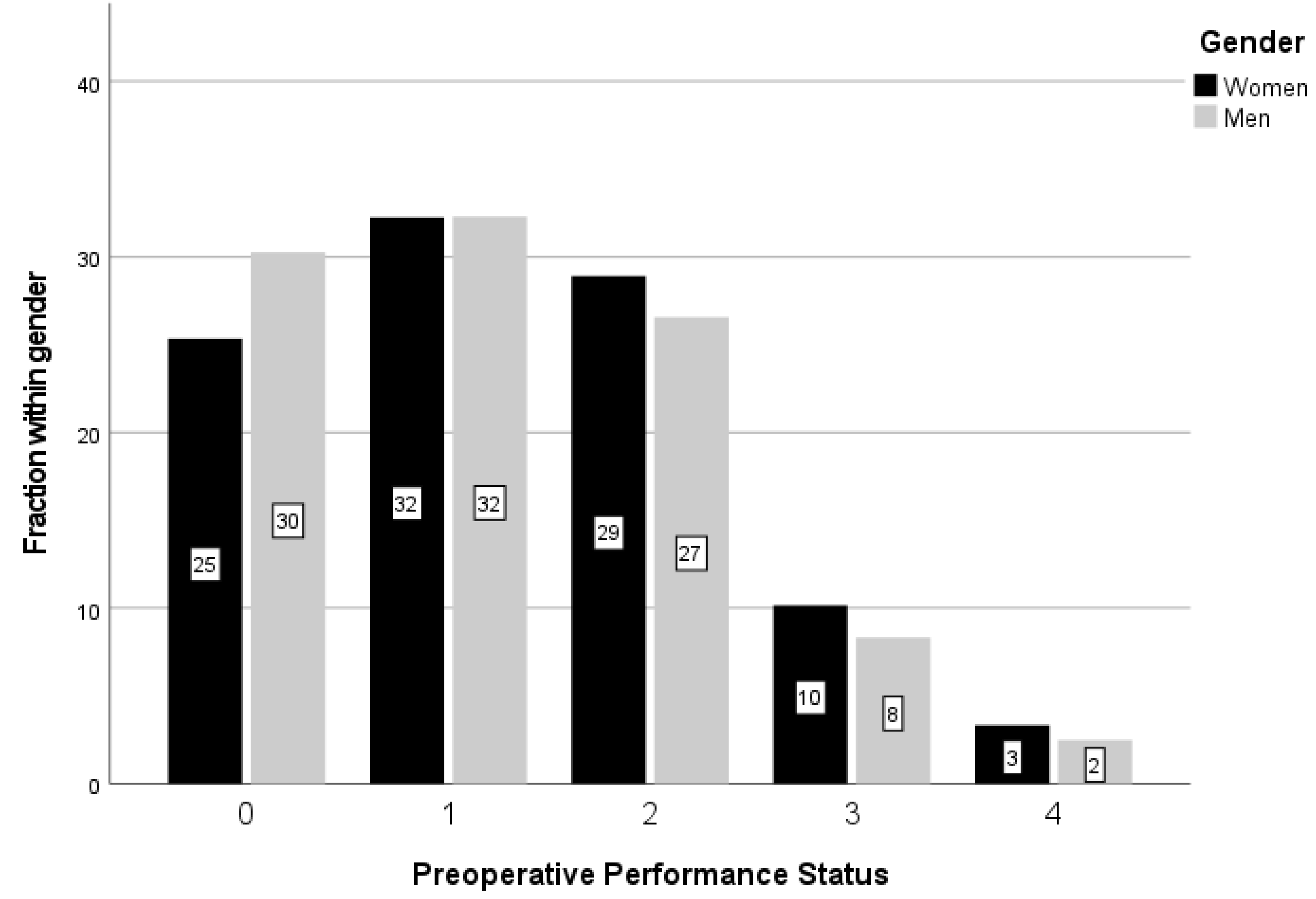
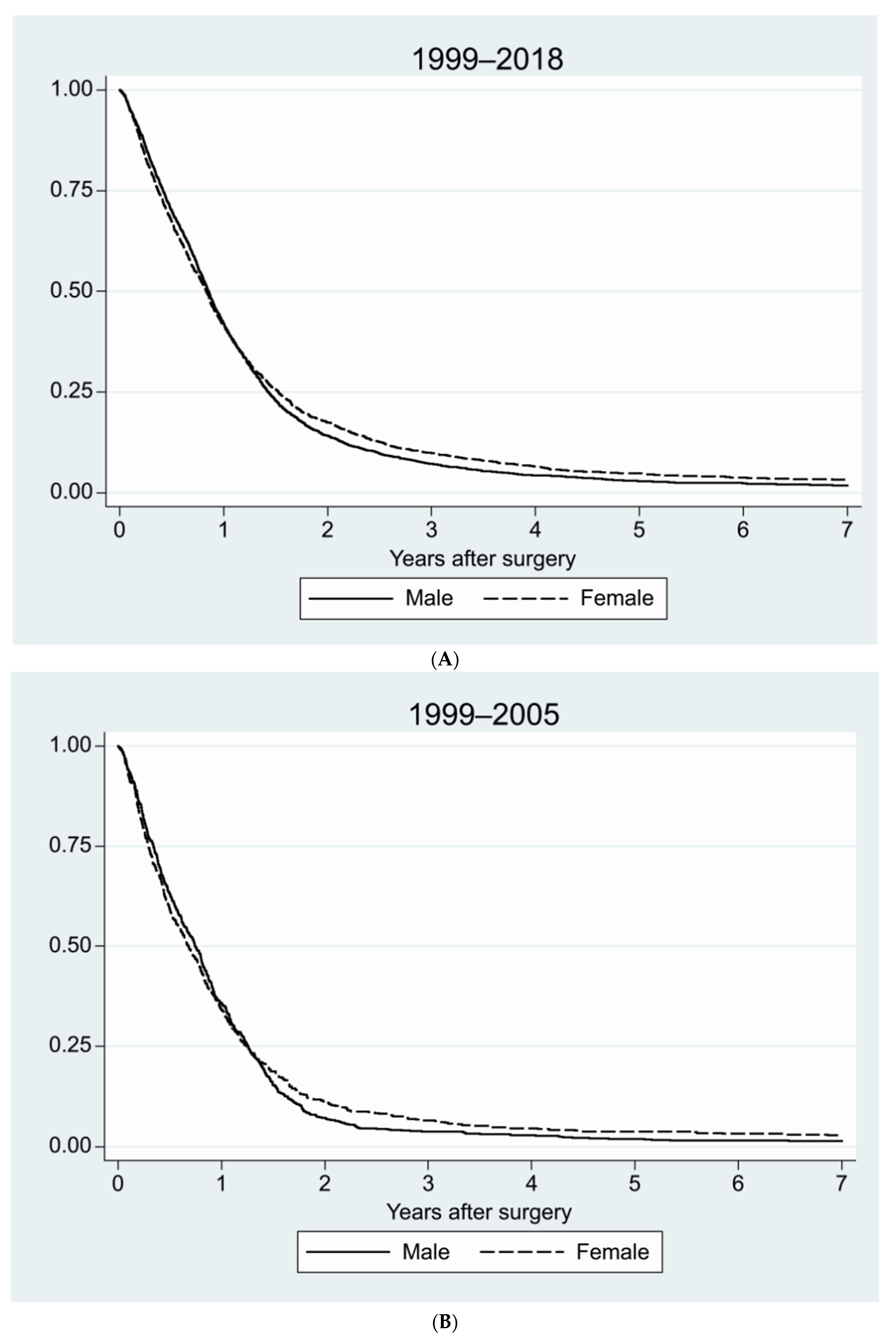
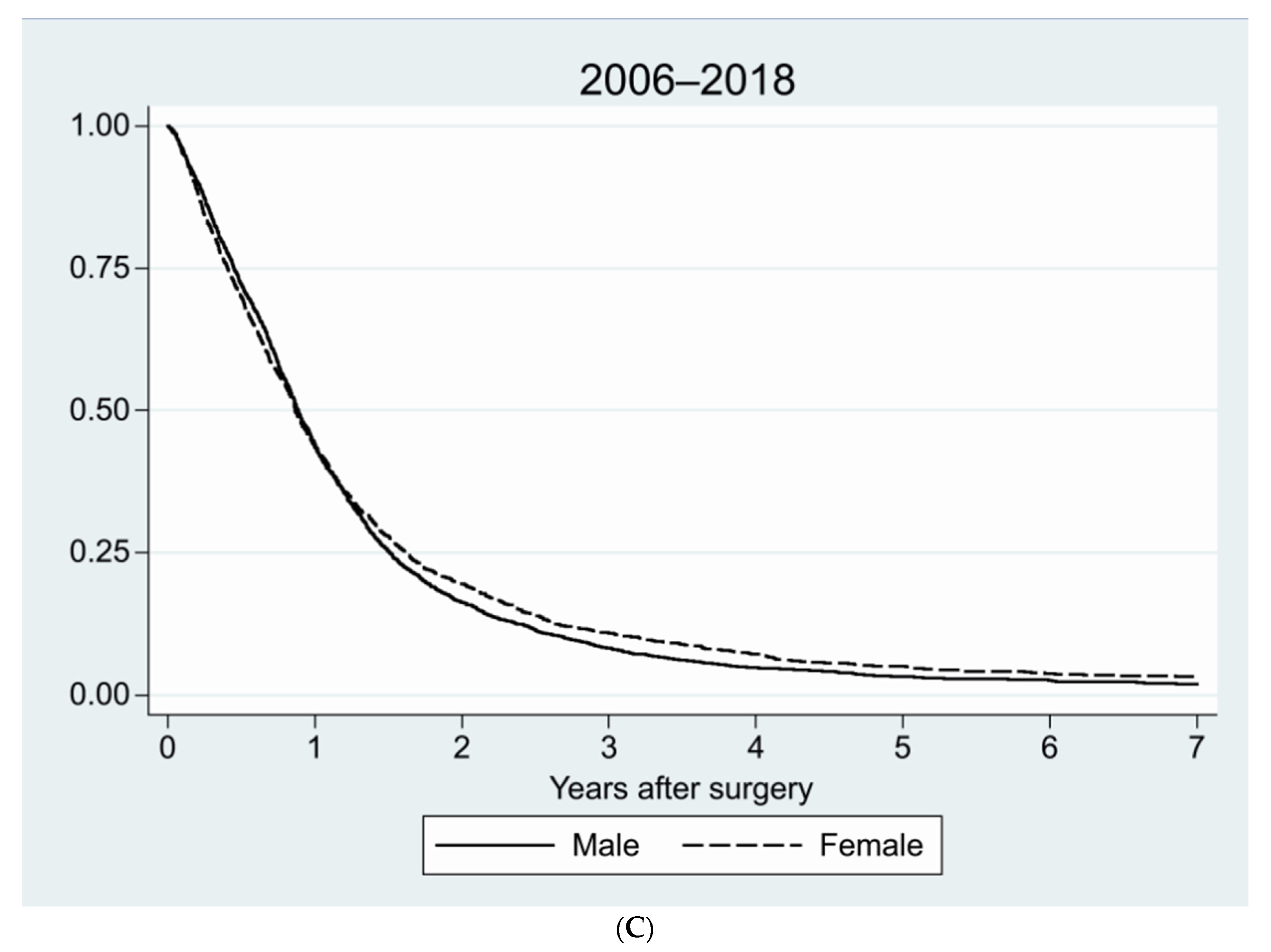
| Age, Years | Sex | Number | Percent, % |
|---|---|---|---|
| 18–39 | Men | 131 | 4.1 |
| Women | 70 | 3.4 | |
| 40–54 | Men | 642 | 20.3 |
| Women | 350 | 16.8 | |
| 55–69 | Men | 1543 | 48.8 |
| Women | 1053 | 50.6 | |
| ≥70 | Men | 844 | 26.7 |
| Women | 610 | 29.3 |
| Sex | Median Survival, Days | 95% Confidence Interval, Days | p-Value | |
|---|---|---|---|---|
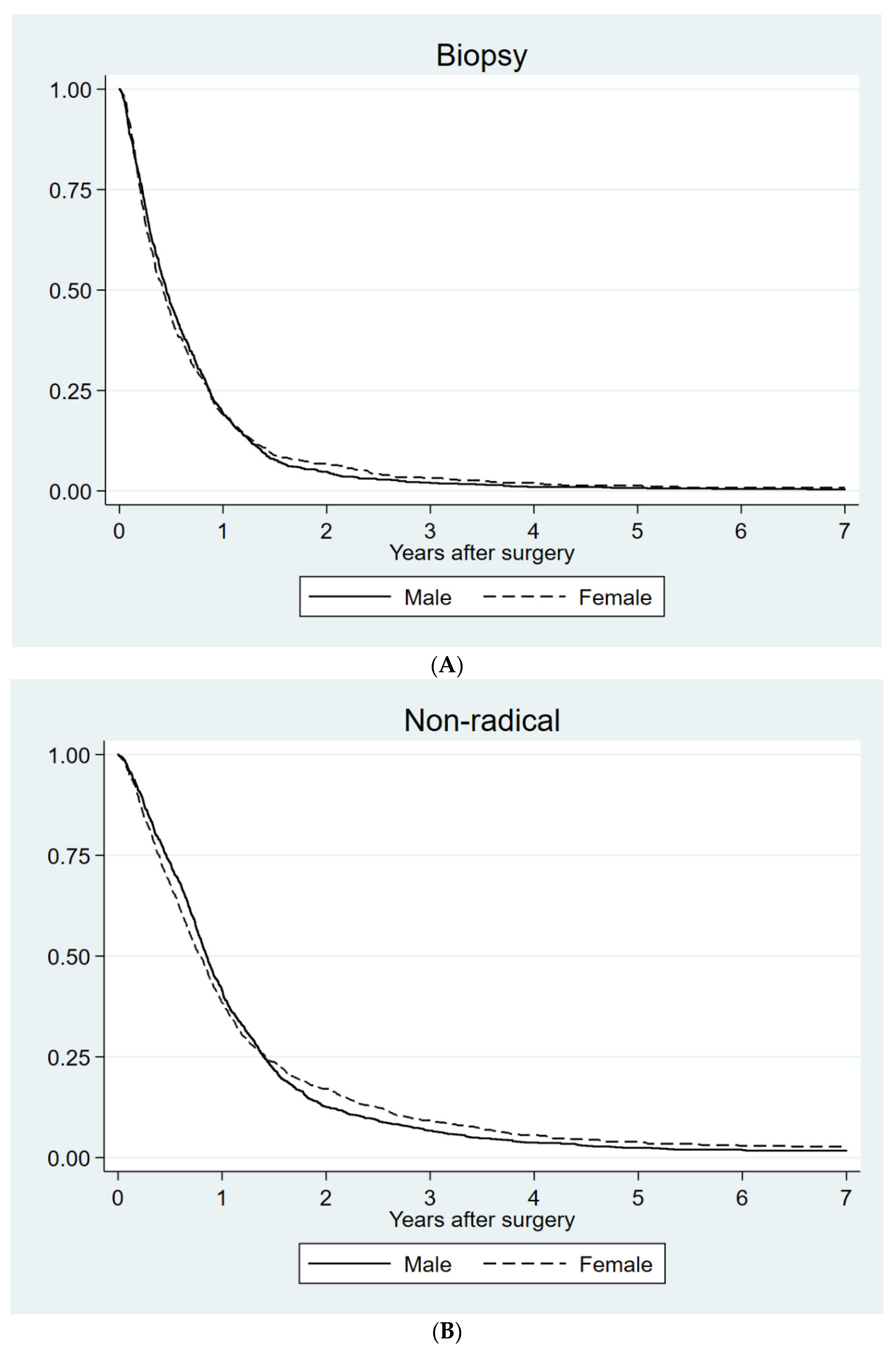
| Biopsy | Men | 165 | 149–181 | 0.86 |
| Women | 153 | 132–174 | ||
| Partial resection | Men | 311 | 296–326 | 0.66 |
| Women | 291 | 269–313 | ||
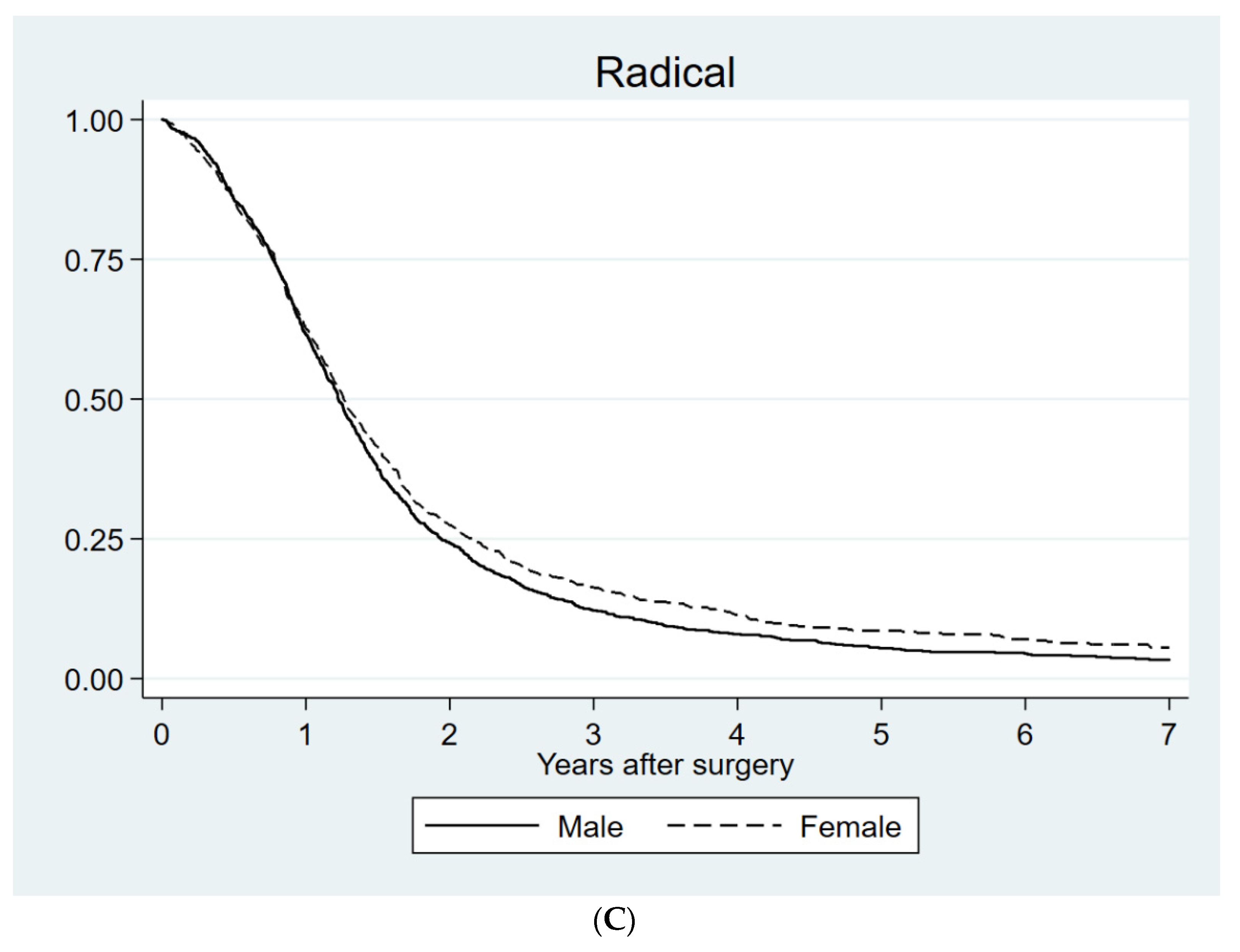
| Radical resection | Men | 447 | 424–470 | 0.045 |
| Women | 461 | 431–491 |
| Prognostic Factor | Hazard Ratio | 95% Confidence Interval | Significance, p-Value | |
|---|---|---|---|---|
| Preoperative Performance status (WHO) | 0–1 | 1.00 | ||
| 2 | 1.27 | 1.19–1.36 | <0.001 | |
| 3–4 | 1.89 | 1.72–2.07 | <0.001 | |
| Age | ≤54 | 1.00 | ||
| 55–69 | 1.59 | 1.47–1.71 | <0.001 | |
| ≥70 | 2.46 | 2.26–2.68 | <0.001 | |
| Surgery | Radical | 1.00 | ||
| Partial | 1.47 | 1.37–1.57 | <0.001 | |
| Biopsy | 2.43 | 2.25–2.62 | <0.001 | |
| Sex | Male | 1.00 | ||
| Female | 0.92 | 0.87–0.98 | 0.006 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tavelin, B.; Malmström, A. Sex Differences in Glioblastoma—Findings from the Swedish National Quality Registry for Primary Brain Tumors between 1999–2018. J. Clin. Med. 2022, 11, 486. https://doi.org/10.3390/jcm11030486
Tavelin B, Malmström A. Sex Differences in Glioblastoma—Findings from the Swedish National Quality Registry for Primary Brain Tumors between 1999–2018. Journal of Clinical Medicine. 2022; 11(3):486. https://doi.org/10.3390/jcm11030486
Chicago/Turabian StyleTavelin, Björn, and Annika Malmström. 2022. "Sex Differences in Glioblastoma—Findings from the Swedish National Quality Registry for Primary Brain Tumors between 1999–2018" Journal of Clinical Medicine 11, no. 3: 486. https://doi.org/10.3390/jcm11030486






