Paclitaxel-Loaded PLGA Coating Stents in the Treatment of Benign Cicatrical Airway Stenosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Equipment and Consumables
2.3. Study Design
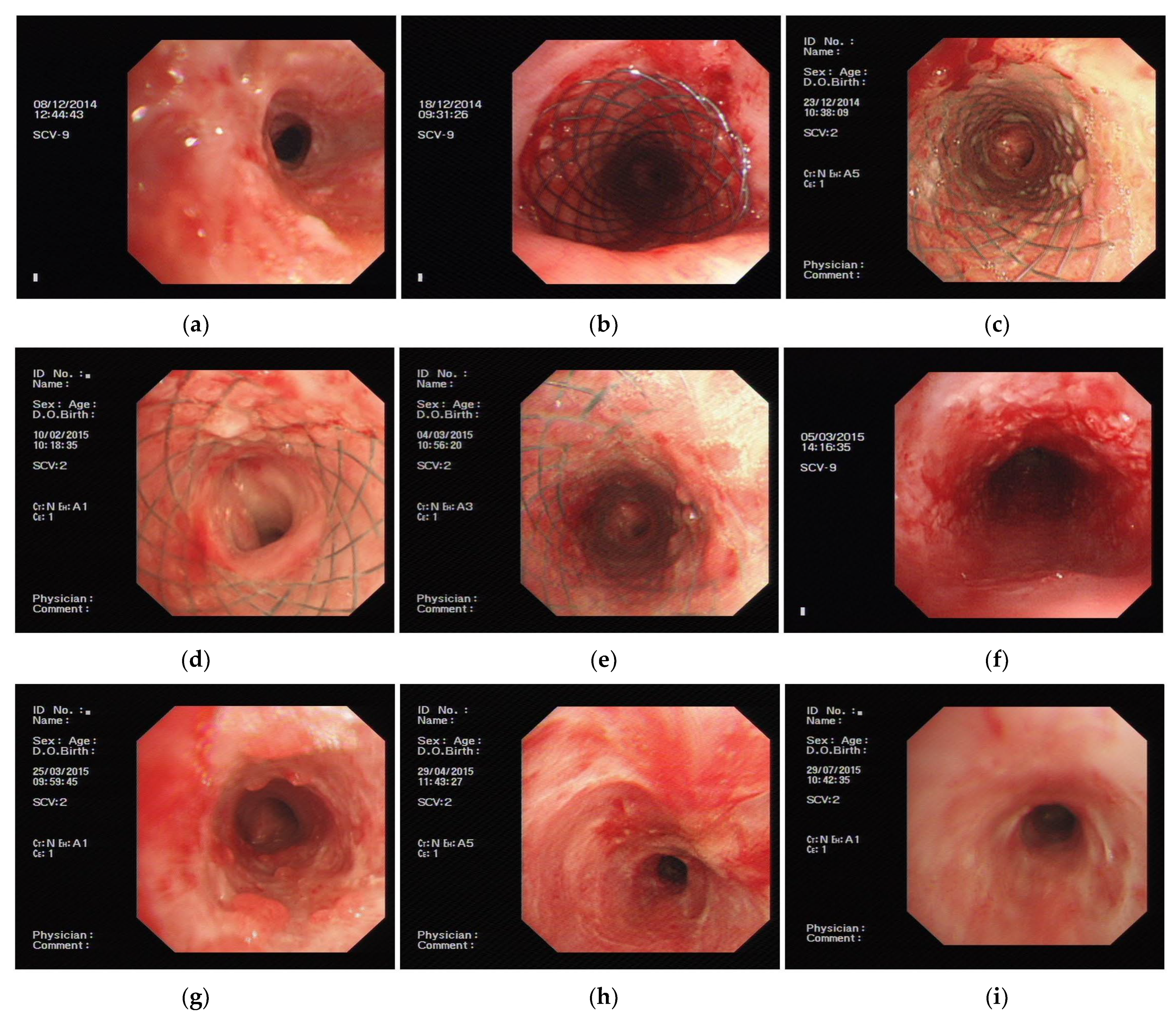
2.4. Treatment Methods
2.5. Observation Method
2.6. Patient Evaluation
2.7. Statistical Analysis
3. Results
3.1. Demographic and Clinical Features of the Patients
3.2. Evaluation of Therapeutic Effect
3.2.1. Immediate Therapeutic Effect
3.2.2. Continuous Therapeutic Effects of PLPCS
First Observation after Stent Implant for One Week
Six Months Post-Implantation Follow-Up
3.3. Evaluation of Treatment Safety
3.3.1. Local Complications
3.3.2. Systemic Complications
3.3.3. Effect of Paclitaxel on Blood Routine, Liver and Kidney Functions
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tsakiridis, K.; Darwiche, K.; Visouli, A.N.; Zarogoulidis, P.; Machairiotis, N.; Christofis, C.; Stylianaki, A.; Katsikogiannis, N.; Mpakas, A.; Courcoutsakis, N.; et al. Management of complex benign post-tracheostomy tracheal stenosis with bronchoscopic insertion of silicon tracheal stents, in patients with failed or contraindicated surgical reconstruction of trachea. J. Thorac. Dis. 2012, 4 (Suppl. S1), 32–40. [Google Scholar] [CrossRef] [PubMed]
- Singh, T.; Sandulache, V.C.; Otteson, T.D.; Barsic, M.; Klein, E.C.; Dohar, J.E.; Hebda, P.A. Subglottic stenosis examined as a fibrotic response to airway injury characterized by altered mucosal fibroblast activity. Arch. Otolaryngol. Head Neck Surg. 2010, 136, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, Y.J.; Kim, H.; Yu, C.M.; Choi, J.C.; Kwon, Y.S.; Kwon, O.J. Use of silicone stents for the management of post-tuberculosis tracheobronchial stenosis. Eur. Respir. J. 2006, 28, 1029–1035. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Um, S.W.; Koh, W.J.; Suh, G.Y.; Chung, M.P.; Kwon, O.J.; Kim, H. Long-term tolerance of airway silicone stent in patients with post-tuberculosis tracheobronchial stenosis. ASAIO J. 2012, 58, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Fortin, M.; Lacasse, Y.; Elharrar, X.; Tazi-Mezalek, R.; Laroumagne, S.; Guinde, J.; Astoul, P.; Dutau, H. Safety and Efficacy of a Fully Covered Self-Expandable Metallic Stent in Benign Airway Stenosis. Respiration 2017, 93, 430–435. [Google Scholar] [CrossRef] [PubMed]
- Makkar, P.; Revelo, A.; Lee, R.; Chawla, M. A Single Center Experience of Feasibility of a Novel Self-Expanding Metallic Airway Stent (Bonastent): A Case Series. J. Bronchol. Interv. Pulmonol. 2019, 26, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Lemaire, A.; Burfeind, W.R.; Toloza, E.; Balderson, S.; Petersen, R.P.; Harpole, D.H., Jr.; D’Amico, T.A. Outcomes of tracheobronchial stents in patients with malignant airway disease. Ann. Thorac. Surg. 2005, 80, 434–437; discussion 437–438. [Google Scholar] [CrossRef] [PubMed]
- Lunn, W.; Feller-Kopman, D.; Wahidi, M.; Ashiku, S.; Thurer, R.; Ernst, A. Endoscopic removal of metallic airway stents. Chest 2005, 127, 2106–2112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casal, R.F. Update in airway stents. Curr. Opin. Pulm. Med. 2010, 16, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Ernst, A.; Feller-Kopman, D.; Becker, H.D.; Mehta, A.C. Central airway obstruction. Am. J. Respir. Crit. Care Med. 2004, 169, 1278–1297. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.J.; Zhang, J.; Wang, J.; Wang, Y.L.; Xu, M. Application of paclitaxel as adjuvant treatment for benign cicatricial airway stenosis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2016, 36, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.; Zhang, J.; Wang, T.; Qiu, X.; Wang, Y. Preparation and characterization of paclitaxel-loaded poly lactic acid-co-glycolic acid coating tracheal stent. Chin. Med. J. 2014, 127, 2236–2240. [Google Scholar] [PubMed]
- Zhang, J. Expert Consensus of Interventional Treatment Through Bronchoscopy of Benign Central Airway Stenosis. Chin. J. Tuberc. Respir. Dis. 2017, 40, 408–418. [Google Scholar]
- Wang, T.; Zhang, J.; Wang, J.; Pei, Y.H.; Qiu, X.J.; Wang, Y.L. Paclitaxel Drug-eluting Tracheal Stent Could Reduce Granulation Tissue Formation in a Canine Model. Chin. Med. J. 2016, 129, 2708–2713. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.J.; Zhang, J.; Wang, T.; Pei, Y.H.; Xu, M. Nonstent Combination Interventional Therapy for Treatment of Benign Cicatricial Airway Stenosis. Chin. Med. J. 2015, 128, 2154–2161. [Google Scholar] [CrossRef] [PubMed]
- Matsui, H.; Hiroma, T.; Hasegawa, H.; Ogiso, Y. Decreased granulomatous reaction by polyurethane-coated stent in the trachea. Pediatr. Int. 2014, 56, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.T.; Lin, S.M.; Chou, C.L.; Chen, H.C.; Liu, C.Y.; Yu, C.T.; Kuo, H.P. Factors leading to obstructive granulation tissue formation after ultraflex stenting in benign tracheal narrowing. Thorac. Cardiovasc. Surg. 2010, 58, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Perl, S.; Perlman, J.; Weitzel, R.P.; Phang, O.; Hsieh, M.M.; Tisdale, J. Addition of Rapamycin to Anti-CD3 Antibody Improves Long-Term Glycaemia Control in Diabetic NOD Mice. PLoS ONE 2013, 8, e67189. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Characteristic | Markers | |
|---|---|---|
| Gender (case) | male female | 5 (50.0%) 5 (50.0%) |
| Age (years) | 41.9 ± 14.43 | |
| Causes | after tuberculosis after tracheal intubation | 6 (60.0%) 4 (40.0%) |
| Dyspnea index | 2.70 ± 2.06 | |
| Stenosis degree(case) | 1 2 3 4 | 0 1 9 0 |
| Stenosis length (cm) | 2.30 ± 0.79 | |
| Stenosis position (case) | 1 2 3 | 7 2 1 |
| Test Group | ||||
|---|---|---|---|---|
| Before | After | f | p | |
| Dyspnea index | 2.70 ± 2.06 | 0.50 ± 0.71 | 3.97 | 0.003 |
| Airway stenosis classification | 2.90 ± 0.32 | 1.10 ± 0.32 | 13.50 | 0.000 |
| Positions | Cases |
|---|---|
| In the upper edge of stent | 2 |
| In the middle of stent | 2 |
| All | 6 |
| Case | Gender | Age | Stenosis Conditions | Stent Size | Granulation Hyperplasia Positions | Degree of Granulation Hyperplasia | Epithelialization (Week) | Time of Stent Cannot Move (Week) | Implant Time (Week) | Outcome | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Causes | Positions (Under Glottis) (cm) | Degree | Length (cm) | ||||||||||
| 1 | F | 26 | 1 | 4.5 | 3 | 3.5 | 14 × 40 | 2 | 1 | no | / | 12 | cure |
| 2 | M | 54 | 2 | 1.5 | 3 | 3.0 | 16 × 50 | 1 | 1 | 3 | 8 | 8 | improvement |
| 3 | M | 62 | 2 | 1.5 | 2 | 2.5 | 18 × 30 | 1 + 2 | 1 | no | 1 | 2 | ineffectiveness |
| 4 | M | 46 | 2 | 1.5 | 3 | 2.5 | 20 × 30 | 1 + 2 | 1 | no | 2 | 6 | improvement |
| 5 | F | 24 | 1 | 0.5 | 3 | 1.0 | 16 × 30 | 1 + 2 | 1 | no | 1 | 10 | ineffectiveness |
| 6 | M | 62 | 2 | 5.5 | 3 | 1.5 | 18 × 40 | 1 | 1 | no | 3 | 3 | ineffectiveness |
| 7 | M | 41 | 1 | 0.5 | 3 | 1.5 | 16 × 40 | 1 + 2 | 1 | no | 4 | 4 | ineffectiveness |
| 8 | F | 24 | 1 | 2.5 | 3 | 3.0 | 16 × 60 | 1 + 2 | 1 | no | 5 | 5 | improvement |
| 9 | F | 41 | 1 | 1.5 | 3 | 2.0 | 16 × 40 | 2 | 1 | no | 12 | 12 | ineffectiveness |
| 10 | F | 39 | 1 | 0.5 | 3 | 2.5 | 16 × 60 | 1 + 2 | 1 | no | 12 | 12 | ineffectiveness |
| Case | |
|---|---|
| Granulation hyperplasia | 10 |
| Mucosal hyperemia | 10 |
| Sputum retention | 10 |
| Mucosal edema | 7 |
| Mucosal necrosis or ulceration | 6 |
| Before Treatment | One Week after Treatment | One Month after Treatment | f | p | |
|---|---|---|---|---|---|
| WBC (109/L) | 5.15 ± 1.59 | 5.25 ± 1.46 | 4.80 ± 0.65 | 0.201 | 0.819 |
| ALT (U/L) | 15.68 ± 6.82 | 15.17 ± 5.18 | 19.83 ± 5.42 | 1.261 | 0.303 |
| AST (U/L) | 19.27 ± 7.42 | 17.74 ± 6.61 | 22.50 ± 3.89 | 0.982 | 0.390 |
| Cr (umol/L) | 46.48 ± 16.97 | 50.46 ± 10.12 | 47.5 ± 6.95 | 0.236 | 0.792 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, X.; Liu, Y.; Zhang, J.; Wang, T.; Wang, J. Paclitaxel-Loaded PLGA Coating Stents in the Treatment of Benign Cicatrical Airway Stenosis. J. Clin. Med. 2022, 11, 517. https://doi.org/10.3390/jcm11030517
Qiu X, Liu Y, Zhang J, Wang T, Wang J. Paclitaxel-Loaded PLGA Coating Stents in the Treatment of Benign Cicatrical Airway Stenosis. Journal of Clinical Medicine. 2022; 11(3):517. https://doi.org/10.3390/jcm11030517
Chicago/Turabian StyleQiu, Xiaojian, Yan Liu, Jie Zhang, Ting Wang, and Juan Wang. 2022. "Paclitaxel-Loaded PLGA Coating Stents in the Treatment of Benign Cicatrical Airway Stenosis" Journal of Clinical Medicine 11, no. 3: 517. https://doi.org/10.3390/jcm11030517
APA StyleQiu, X., Liu, Y., Zhang, J., Wang, T., & Wang, J. (2022). Paclitaxel-Loaded PLGA Coating Stents in the Treatment of Benign Cicatrical Airway Stenosis. Journal of Clinical Medicine, 11(3), 517. https://doi.org/10.3390/jcm11030517





