Patterns and Predictors of Medication Change after Discharge from Hospital: An Observational Study in Older Adults with Neurological Disorders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting and Participantss
2.2. Statistical Analysis
3. Results
3.1. Clinical and Demographical Characteristics
3.2. Patterns of Medication Changes after Discharge
3.3. Predictors of General Medication Changes after Discharge
3.4. Predictors of Patient-Initiated Medication Changes after Discharge
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Noël, P.H.; Parchman, M.L.; Williams, J.W., Jr.; Cornell, J.E.; Shuko, L.; Zeber, J.E.; Kazis, L.E.; Lee, A.F.; Pugh, J.A. The challenges of multimorbidity from the patient perspective. J. Gen. Intern. Med. 2007, 22 (Suppl. 3), 419–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, O.; Glaubitz, S.; Hager, K.; Schleef, T.; Wiese, B.; Junius-Walker, U. Post-discharge adjustment of medication in geriatric patients: A prospective cohort study. Z. Gerontol. Geriatr. 2020, 53, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Daliri, S.; Bouhnouf, M.; van de Meerendonk, H.W.P.C.; Buurman, B.M.; Scholte op Reimer, W.J.M.; Kooij, M.J.; Karapinar–Çarkit, F. Longitudinal medication reconciliation at hospital admission, discharge and post-discharge. Res. Soc. Adm. Pharm. 2021, 17, 677–684. [Google Scholar] [CrossRef] [PubMed]
- Bo, M.; Gibello, M.; Brunetti, E.; Boietti, E.; Sappa, M.; Falcone, Y.; Aurucci, M.L.; Iacovino, M.; Fonte, G.; Cappa, G. Prevalence and predictors of inappropriate prescribing according to the Screening Tool of Older People’s Prescriptions and Screening Tool to Alert to Right Treatment version 2 criteria in older patients discharged from geriatric and internal medicine wards: A prospective observational multicenter study. Geriatr. Gerontol. Int. 2019, 19, 5–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onatade, R.; Auyeung, V.; Scutt, G.; Fernando, J. Potentially inappropriate prescribing in patients on admission and discharge from an older peoples’ unit of an acute UK hospital. Drugs Aging 2013, 30, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Capiau, A.; Foubert, K.; Van der Linden, L.; Walgraeve, K.; Hias, J.; Spinewine, A.; Sennesael, A.L.; Petrovic, M.; Somers, A. Medication Counselling in Older Patients Prior to Hospital Discharge: A Systematic Review. Drugs Aging 2020, 37, 635–655. [Google Scholar] [CrossRef]
- Haynes, R.B.; Yao, X.; Degani, A.; Kripalani, S.; Garg, A.; McDonald, H.P. Interventions to enhance medication adherence. Cochrane Database Syst. Rev. 2005, Cd000011. [Google Scholar] [CrossRef]
- Sabaté, E. Adherence to Long-Term Therapies: Evidence for Action; World Health Organization: Geneva, Switzerland, 2003. [Google Scholar]
- Yap, A.F.; Thirumoorthy, T.; Kwan, Y.H. Systematic review of the barriers affecting medication adherence in older adults. Geriatr. Gerontol. Int. 2016, 16, 1093–1101. [Google Scholar] [CrossRef]
- Budnitz, D.S.; Lovegrove, M.C.; Shehab, N.; Richards, C.L. Emergency hospitalizations for adverse drug events in older Americans. N. Engl. J. Med. 2011, 365, 2002–2012. [Google Scholar] [CrossRef]
- DiMatteo, M.R.; Giordani, P.J.; Lepper, H.S.; Croghan, T.W. Patient adherence and medical treatment outcomes: A meta-analysis. Med. Care 2002, 40, 794–811. [Google Scholar] [CrossRef]
- Brown, M.T.; Bussell, J.; Dutta, S.; Davis, K.; Strong, S.; Mathew, S. Medication Adherence: Truth and Consequences. Am. J. Med. Sci. 2016, 351, 387–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhtar, O.; Weinman, J.; Jackson, S.H. Intentional non-adherence to medications by older adults. Drugs Aging 2014, 31, 149–157. [Google Scholar] [CrossRef] [PubMed]
- McLeod, L.A. Patient transitions from inpatient to outpatient: Where are the risks? Can we address them? J. Healthc. Risk Manag. 2013, 32, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.M.; Sridharan, A.; Landis, R.; Howell, E.; Wright, S. What happens to the medication regimens of older adults during and after an acute hospitalization? J. Patient Saf. 2013, 9, 150–153. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.T.; Steer, R.A.; Brown, G.K. Manual for the Beck Depression Inventory-II; Psychological Corporation: San Antonio, TX, USA, 1996. [Google Scholar]
- Kühner, C.; Bürger, C.; Keller, F.; Hautzinger, M. Reliabilität und Validität des revidierten Beck-Depressionsinventars (BDI-II). Der Nervenarzt 2007, 78, 651–656. [Google Scholar] [CrossRef] [PubMed]
- Prell, T. Data on medication adherence in adults with neurological disorders: The NeuroGerAd study. Dryad 2022. [Google Scholar] [CrossRef]
- John, O.P.; Donahue, E.M.; Kentle, R.L. The Big Five Inventory—Versions 4a and 54; University of California, Institute of Personality and Social Research: Berkeley, CA, USA, 1991. [Google Scholar]
- Schmidt, K.; Gensichen, J.; Petersen, J.J.; Szecsenyi, J.; Walther, M.; Williams, G.; Freund, T. Autonomy support in primary care—Validation of the German version of the Health Care Climate Questionnaire. J. Clin. Epidemiol. 2012, 65, 206–211. [Google Scholar] [CrossRef]
- Ware, J.E., Jr.; Sherbourne, C.D. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med. Care 1992, 30, 473–483. [Google Scholar] [CrossRef]
- Franke, G.H.; Nentzl, J.; Jagla-Franke, M. SAMS. Stendal Adherence to Medication Score. Testmanual 2020. [Google Scholar] [CrossRef]
- Podsiadlo, D.; Richardson, S. The timed “Up & Go”: A test of basic functional mobility for frail elderly persons. J. Am. Geriatr. Soc. 1991, 39, 142–148. [Google Scholar] [CrossRef]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef] [PubMed]
- Prell, T.; Grosskreutz, J.; Mendorf, S.; Franke, G.H.; Witte, O.W.; Kunze, A. Clusters of non-adherence to medication in neurological patients. Res. Soc. Adm. Pharm. 2019, 15, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Box, G.E.P.; Tidwell, P.W. Transformation of the Independent Variables. Technometrics 1962, 4, 531–550. [Google Scholar] [CrossRef]
- Tabachnick, B.G.; Fidell, L.S. Using Multivariate Statistics, 7th ed.; Pearson Education: New York, NY, USA, 2018. [Google Scholar]
- Mitchell, B.; Chong, C.; Lim, W.K. Medication adherence 1 month after hospital discharge in medical inpatients. Intern. Med. J. 2016, 46, 185–192. [Google Scholar] [CrossRef]
- Mansur, N.; Weiss, A.; Hoffman, A.; Gruenewald, T.; Beloosesky, Y. Continuity and adherence to long-term drug treatment by geriatric patients after hospital discharge: A prospective cohort study. Drugs Aging 2008, 25, 861–870. [Google Scholar] [CrossRef]
- Feldmann, F.; Zipprich, H.M.; Witte, O.W.; Prell, T. Self-Reported Nonadherence Predicts Changes of Medication after Discharge from Hospital in People with Parkinson’s Disease. Parkinsons Dis. 2020, 2020, 4315489. [Google Scholar] [CrossRef] [PubMed]
- Groves, J.E. Taking Care of the Hateful Patient. N. Engl. J. Med. 1978, 298, 883–887. [Google Scholar] [CrossRef]
- Pentzek, M.; Santos, S.; Wollny, A.; Gummersbach, E.; Herber, O.R.; In der Schmitten, J.; Icks, A.; Abholz, H.H.; Wilm, S. Which patients with type 2 diabetes mellitus are perceived as ‘difficult’ by general practitioners? Prim. Care Diabetes 2019, 13, 353–359. [Google Scholar] [CrossRef]
- Lin, E.H.; Katon, W.; Von Korff, M.; Bush, T.; Lipscomb, P.; Russo, J.; Wagner, E. Frustrating patients: Physician and patient perspectives among distressed high users of medical services. J. Gen. Intern. Med. 1991, 6, 241–246. [Google Scholar] [CrossRef]
- Dingwall, R.; Murray, T. Categorization in accident departments: ‘Good’ patients, ‘bad’ patients and ‘children’. Sociol. Health Illn. 1983, 5, 127–148. [Google Scholar] [CrossRef]
- Heimrich, K.G.; Prell, T. Short- and Long-Term Effect of Parkinson’s Disease Multimodal Complex Treatment. Brain Sci. 2021, 11, 1460. [Google Scholar] [CrossRef] [PubMed]
- Briesacher, B.A.; Andrade, S.E.; Fouayzi, H.; Chan, K.A. Comparison of drug adherence rates among patients with seven different medical conditions. Pharmacotherapy 2008, 28, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Yeaw, J.; Benner, J.S.; Walt, J.G.; Sian, S.; Smith, D.B. Comparing adherence and persistence across 6 chronic medication classes. J. Manag. Care Pharm. 2009, 15, 728–740. [Google Scholar] [CrossRef] [PubMed]
- DiMatteo, M.R. Variations in patients’ adherence to medical recommendations: A quantitative review of 50 years of research. Med. Care 2004, 42, 200–209. [Google Scholar] [CrossRef] [PubMed]
- Schönenberg, A.; Prell, T. Factors Influencing Self-Reported Medication Use in the Survey of Health Aging and Retirement in Europe (SHARE) Dataset. Healthcare 2021, 9, 1752. [Google Scholar] [CrossRef] [PubMed]
- Bortz, J.; Döring, N. Forschungsmethoden und Evaluation für Human- und Sozialwissenschaftler; Springer: Berlin, Germany, 2007. [Google Scholar]
- Stirratt, M.J.; Dunbar-Jacob, J.; Crane, H.M.; Simoni, J.M.; Czajkowski, S.; Hilliard, M.E.; Aikens, J.E.; Hunter, C.M.; Velligan, D.I.; Huntley, K.; et al. Self-report measures of medication adherence behavior: Recommendations on optimal use. Transl. Behav. Med. 2015, 5, 470–482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forster, A.J.; Murff, H.J.; Peterson, J.F.; Gandhi, T.K.; Bates, D.W. Adverse drug events occurring following hospital discharge. J. Gen. Intern. Med. 2005, 20, 317–323. [Google Scholar] [CrossRef] [Green Version]
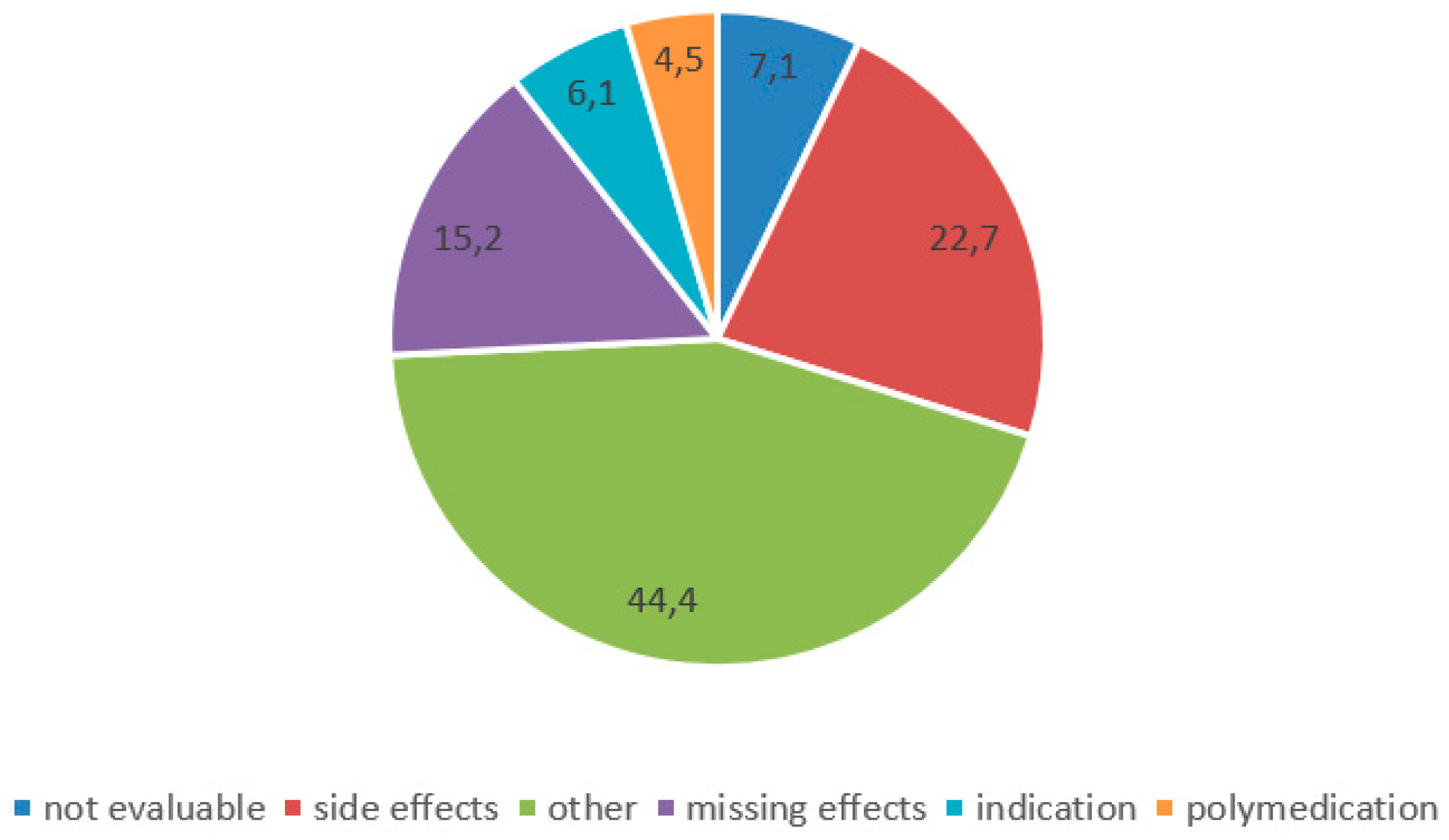
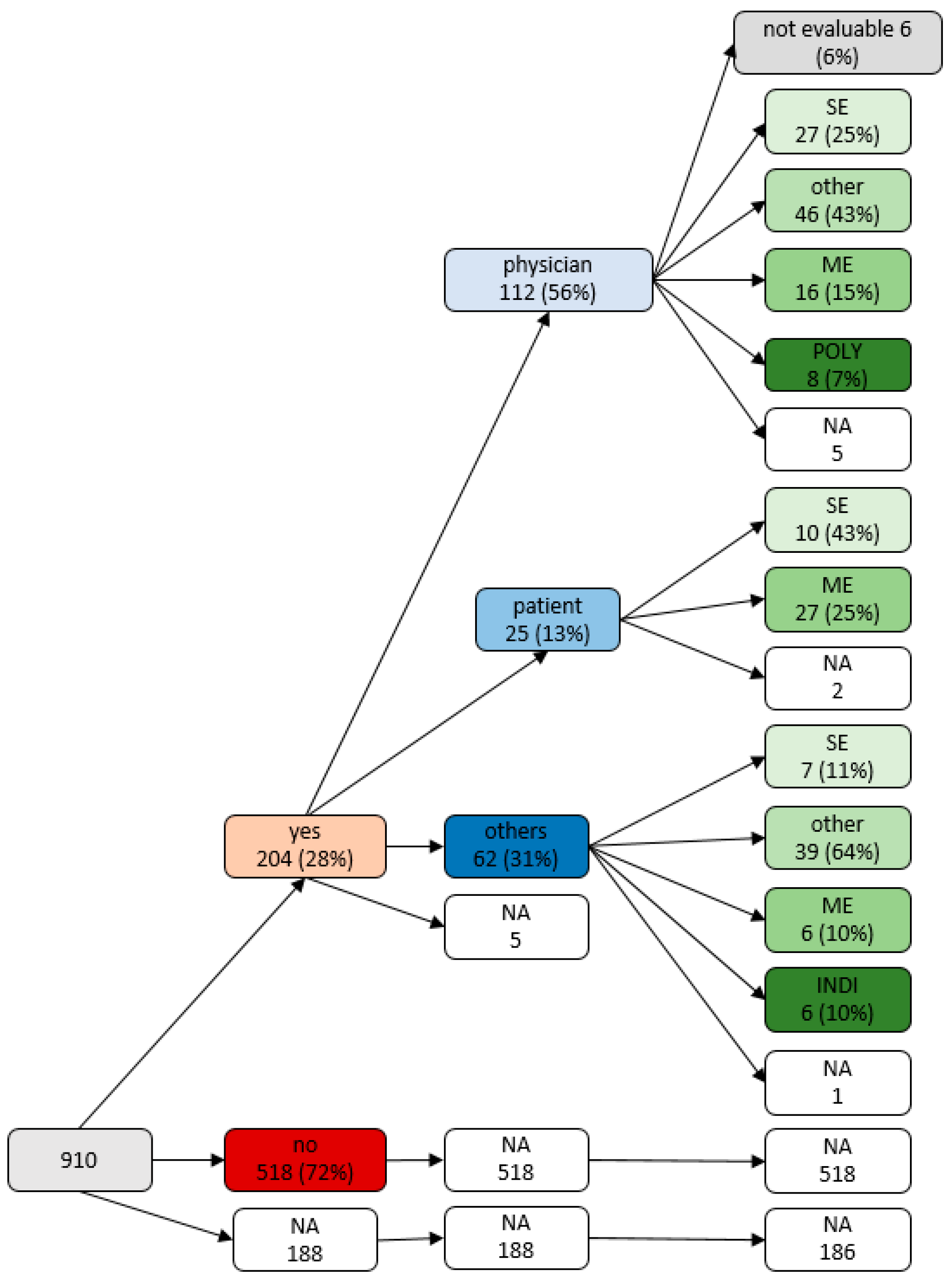
| n | Medication Change: Yes (n = 204) | Medication Change: No (n = 518) | p | |
|---|---|---|---|---|
| % (Frequency) | % (Frequency) | |||
| sex | 910 | 0.99 1 | ||
| male | 58.3% (119/204) | 58.3% (302/518) | ||
| female | 41.7% (85/204) | 41.7% (216/518) | ||
| Education level | 896 | 0.42 1 | ||
| High | 36.2% (72/199) | 34.8% (179/513) | ||
| Middle | 31.6% (63/199) | 36.6% (188/513) | ||
| Low | 32.2% (64/199) | 28.6% (146/513) | ||
| Diagnosis group | 910 | 0.71 1 | ||
| movement disorder | 34.3% (70/204) | 35.5% (184/518) | ||
| cerebrovascular disorder | 27.9% (57/204) | 23.1% (120/518) | ||
| Epilepsy | 4.4% (9/204) | 4.4% (23/518) | ||
| neuromuscular | 18.1% (37/204) | 18.9% (98/518) | ||
| Others | 15.2 (31/204) | 17.9 (93/518) | ||
| BFI | 843 | 0.55 1 | ||
| Neuroticism | 15.3% (28/182) | 10.7% (51/476) | ||
| Openness | 13.7% (25/182) | 16.1% (77/476) | ||
| Conscientiousness | 42.3% (77/182) | 44.3% (211/476) | ||
| Extraversion | 20.8% (38/182) | 21.0% (100/476) | ||
| Agreeableness | 7.6% (14/182) | 7.7% (37/476) | ||
| Medication change in the last 6 months: yes | 844 | 52.7% (95/180) | 44.1% (212/480) | 0.05 1 |
| Q1 Mdn Q3 | Q1 Mdn Q3 | p | ||
| Age | 910 | 84.0 71.0 78.0 | 63.0 70.0 77.0 | 0.41 2 |
| TuG | 585 | 8.0 9.0 11.0 | 8.0 9.0 11.0 | 0.67 2 |
| Frequency of doctor appointments (quarterly) | 838 | 1.0 1.0 3.0 | 1.0 1.0 3.0 | 0.78 2 |
| BDI | 909 | 6.0 9.2 15.0 | 4.0 8.0 14.0 | <0.01 2 |
| MoCA | 910 | 22.0 24.0 25.0 | 22.0 23.0 26.0 | 0.84 2 |
| SAMS | 755 | 1.0 5.0 10.0 | 1.0 4.0 8.0 | 0.01 2 |
| Number of Medications | 910 | 1.0 5.0 9.6 | 1.0 4.0 8.0 | 0.01 2 |
| HCCQ | 831 | 5.0 5.7 6.4 | 5.1 5.9 6.4 | 0.24 2 |
| SF36 | ||||
| Physical functioning | 903 | 18.3 45.0 70.0 | 25.0 50.0 75.0 | 0.02 2 |
| Social functioning | 907 | 50.0 75.0 100.0 | 50.0 75.0 100.0 | 0.75 2 |
| Role limitations due to physical health | 874 | 0.0 0.0 75.0 | 0.0 0.0 75.0 | 0.91 2 |
| Role limitations due to emotional problem | 875 | 0.0 100.0 100.0 | 0.0 100.0 100.0 | 0.28 2 |
| Emotional well-being | 899 | 52.0 68.0 80.0 | 52.0 68.0 80.0 | 0.68 2 |
| Energy/fatigue | 899 | 30.0 45.0 60.0 | 35.0 50.0 65.0 | 0.12 2 |
| Pain | 907 | 22.4 44.9 77.6 | 32.7 55.1 77.7 | 0.24 2 |
| General health | 896 | 35.0 45.0 55.0 | 35.0 45.0 55.0 | 0.35 2 |
| Health change | 899 | 0.0 25.0 50.0 | 25.0 25.0 50.0 | 0.01 2 |
| Physical Health component score | 849 | 24.2 33.1 41.0 | 26.6 33.7 43.0 | 0.20 2 |
| Mental Health component score | 849 | 38.7 50.4 57.1 | 39.5 51.0 57.4 | 0.81 2 |
| Step | 95% Confidence Interval | Nagelkerkes R2 | ||||||
|---|---|---|---|---|---|---|---|---|
| Exp(B) | Lower CI | Upper CI | χ2 | df | Sig. | |||
| 1 | BDI | 1.011 | 0.987 | 1.035 | 0.020 | 9.568 | 4 | 0.048 |
| Number of pills/day | 0.997 | 0.949 | 1.047 | |||||
| Physical functioning | 0.995 | 0.989 | 1.002 | |||||
| Health change | 0.994 | 0.987 | 1.001 | |||||
| Constant | 0.549 | |||||||
| 2 | BDI | 1.011 | 0.987 | 1.035 | 0.020 | 9.553 | 3 | 0.023 |
| Physical functioning | 0.995 | 0.989 | 1.002 | |||||
| Health change | 0.994 | 0.987 | 1.001 | |||||
| Constant | 0.539 | |||||||
| 3 | Physical functioning | 0.995 | 0.989 | 1.000 | 0.019 | 8.784 | 2 | 0.012 |
| Health change | 0.993 | 0.986 | 1.001 | |||||
| Constant | 0.634 | |||||||
| Physician-Initiated | Patient-Initiated | p | ||||||||
| n | % | n | % | |||||||
| Sex | female | 41 | 29.9% | 16 | 11.7% | 0.012 | ||||
| male | 71 | 51.8% | 9 | 6.6% | ||||||
| Medication change | no | 39 | 34.2% | 8 | 7.0% | 0.196 | ||||
| yes | 61 | 53.5% | 6 | 5.3% | ||||||
| Diagnosis | Movement disorder | 184 | 25.5% | 70 | 9.7% | 0.714 | ||||
| cerebrovascular disorder | 120 | 16.6% | 57 | 7.9% | ||||||
| epilepsy | 23 | 3.2% | 9 | 1.2% | ||||||
| neuromuscular | 98 | 13.6% | 37 | 5.1% | ||||||
| others | 93 | 12.9% | 31 | 4.3% | ||||||
| Education level | high | 45 | 33.6% | 7 | 5.2% | 0.469 | ||||
| middle | 36 | 26.9% | 10 | 7.5% | ||||||
| low | 28 | 20.9% | 8 | 6.0% | ||||||
| M | SD | Lower 95% CI (M) | Upper 95% CI (M) | M | SD | Lower 95% CI (M) | Upper 95% CI (M) | p | ||
| Age | 70.5 | 9.4 | 68.7 | 72.3 | 72.8 | 6.7 | 70.1 | 75.6 | 0.263 | |
| Number of pills/day | 5.8 | 3.7 | 5.1 | 6.5 | 6.0 | 4.1 | 4.3 | 7.7 | 0.840 | |
| BDI | 11.3 | 7.3 | 9.9 | 12.6 | 12.9 | 7.0 | 10.0 | 15.9 | 0.206 | |
| HCCQ-D | 5.5 | 1.0 | 5.3 | 5.7 | 5.2 | 1.6 | 4.3 | 6.2 | 0.829 | |
| MoCA | 23.7 | 2.4 | 23.2 | 24.1 | 24.1 | 2.2 | 23.2 | 25.0 | 0.313 | |
| Frequency of doctor appointments (quarterly) | 2.1 | 3.0 | 1.5 | 2.7 | 1.4 | 0.9 | 0.9 | 1.9 | 0.760 | |
| SAMS total | 7.0 | 7.2 | 5.7 | 8.4 | 6.4 | 6.2 | 3.8 | 8.9 | 0.621 | |
| Others | Side Effects | Missing Effects | Sign. | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | χ2 | ||||||||
| Sex | female | 48 | 24.2% | 21 | 10.6% | 13 | 6.6% | 0.655 | ||||||
| male | 75 | 37.9% | 24 | 12.1% | 17 | 8.6% | ||||||||
| Diagnosis group | movement disorder | 32 | 16.2% | 19 | 9.6% | 17 | 8.6% | 0.082 | ||||||
| cerebrovascular disorder | 38 | 19.2% | 11 | 5.6% | 7 | 3.5% | ||||||||
| epilepsy | 7 | 3.5% | 1 | 0.5% | 0 | 0.0% | ||||||||
| neuromuscular | 25 | 12.6% | 6 | 3.0% | 4 | 2.0% | ||||||||
| others | 21 | 10.6% | 8 | 4.0% | 2 | 1.0% | ||||||||
| Education Level | high | 47 | 24.4% | 12 | 6.2% | 13 | 6.7% | 0.145 | ||||||
| middle | 39 | 20.2% | 14 | 7.3% | 12 | 6.2% | ||||||||
| low | 34 | 17.6% | 18 | 9.3% | 4 | 2.1% | ||||||||
| M | SD | 95%CI | 95%CI | M | SD | 95%CI | 95%CI | M | SD | 95%CI | 95%CI | p | ||
| Age | 69.7 | 8.7 | 68.1 | 71.2 | 71.8 | 8.1 | 69.4 | 74.2 | 71.0 | 9.3 | 67.5 | 74.5 | <0.05 | |
| Number of pills/day | 5.8 | 3.7 | 5.1 | 6.5 | 5.6 | 3.9 | 4.5 | 6.8 | 5.6 | 3.8 | 4.2 | 7.1 | <0.05 | |
| BDI | 10.5 | 6.7 | 9.3 | 11.7 | 10.8 | 7.4 | 8.6 | 13.0 | 15.2 | 9.3 | 11.6 | 18.7 | <0.05 | |
| HCCQ-D | 5.6 | 1.0 | 5.4 | 5.7 | 5.5 | 1.2 | 5.2 | 5.9 | 5.0 | 1.6 | 4.3 | 5.8 | <0.05 | |
| MoCA | 23.6 | 2.6 | 23.1 | 24.1 | 23.8 | 2.7 | 23.0 | 24.6 | 23.8 | 2.2 | 23.0 | 24.7 | <0.05 | |
| Frequency of doctor appointments (quarterly) | 2.0 | 2.8 | 1.5 | 2.5 | 2.7 | 2.9 | 1.8 | 3.6 | 1.7 | 1.5 | 1.1 | 2.4 | <0.05 | |
| SAMS total | 7.5 | 7.7 | 6.1 | 8.8 | 5.4 | 5.6 | 3.7 | 7.1 | 6.4 | 6.5 | 3.9 | 8.8 | <0.05 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schwarzkopf, A.; Schönenberg, A.; Prell, T. Patterns and Predictors of Medication Change after Discharge from Hospital: An Observational Study in Older Adults with Neurological Disorders. J. Clin. Med. 2022, 11, 563. https://doi.org/10.3390/jcm11030563
Schwarzkopf A, Schönenberg A, Prell T. Patterns and Predictors of Medication Change after Discharge from Hospital: An Observational Study in Older Adults with Neurological Disorders. Journal of Clinical Medicine. 2022; 11(3):563. https://doi.org/10.3390/jcm11030563
Chicago/Turabian StyleSchwarzkopf, Anna, Aline Schönenberg, and Tino Prell. 2022. "Patterns and Predictors of Medication Change after Discharge from Hospital: An Observational Study in Older Adults with Neurological Disorders" Journal of Clinical Medicine 11, no. 3: 563. https://doi.org/10.3390/jcm11030563
APA StyleSchwarzkopf, A., Schönenberg, A., & Prell, T. (2022). Patterns and Predictors of Medication Change after Discharge from Hospital: An Observational Study in Older Adults with Neurological Disorders. Journal of Clinical Medicine, 11(3), 563. https://doi.org/10.3390/jcm11030563







