Lab-Based Retrospective 10-Year Analysis Shows Seasonal Variation of Vaginal Candida Infection Rates in Belgium
Abstract
:1. Introduction
2. Methods
2.1. Collection of Samples
2.2. Laboratory Techniques for Vaginal Candida Cultures
2.3. Collection of Seasonal Climate Conditions
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dan, M.; Kaneti, N.; Levin, D.; Poch, F.; Samra, Z. Vaginitis in a gynecologic practice in Israel: Causes and risk factors. Isr. Med. Assoc. J. 2003, 5, 629–632. [Google Scholar] [PubMed]
- Ferrer, J. Vaginal candidosis: Epidemiological and etiological factors. Int. J. Gynaecol. Obstet. 2000, 71 (Suppl. S1), S21–S27. [Google Scholar] [CrossRef]
- Sobel, J.D.; Wiesenfeld, H.C.; Martens, M.; Danna, P.; Hooton, T.M.; Rompalo, A.; Sperling, M.; Livengood, C., 3rd; Horowitz, B.; Von Thron, J.; et al. Maintenance fluconazole therapy for recurrent vulvovaginal candidiasis. N. Engl. J. Med. 2004, 351, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.; Bellen, G.; Byttebier, G.; Verguts, L.; Hinoul, P.; Walckiers, R.; Stalpaert, M.; Vereecken, A.; Van Eldere, J. Individualized decreasing-dose maintenance fluconazole regimen for recurrent vulvovaginal candidiasis (ReCiDiF trial). Am. J. Obstet. Gynecol. 2008, 199, 613.e1–613.e9. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Liu, X.; Wu, C.; Xu, L.; Li, J. Vaginal nystatin versus oral fluconazole for the treatment for recurrent vulvovaginal candidiasis. Mycopathologia 2015, 179, 95–101. [Google Scholar] [CrossRef]
- Horowitz, B.J.; Edelstein, S.W.; Lippman, L. Sexual transmission of Candida. Obstet. Gynecol. 1987, 69, 883–886. [Google Scholar] [CrossRef]
- Grinceviciene, S.; Ruban, K.; Bellen, G.; Donders, G.G.G. Sexual behaviour and extra-genital colonisation in women treated for recurrent Candida vulvo-vaginitis. Mycoses 2018, 61, 857–860. [Google Scholar] [CrossRef]
- Mardh, P.A.; Novikova, N.; Stukalova, E. Colonisation of extragenital sites by Candida in women with recurrent vulvovaginal candidosis. BJOG 2003, 110, 934–937. [Google Scholar] [CrossRef]
- Donders, G.G.G.; Grinceviciene, S.; Bellen, G.; Ruban, K. Is multiple-site colonization with Candida spp. related to inadequate response to individualized fluconazole maintenance therapy in women with recurrent Candida vulvovaginitis? Diagn. Microbiol. Infect. Dis. 2018, 92, 226–229. [Google Scholar] [CrossRef]
- Donders, G.G.; Bellen, G.; Mendling, W. Management of recurrent vulvo-vaginal candidosis as a chronic illness. Gynecol. Obstet. Investig. 2010, 70, 306–321. [Google Scholar] [CrossRef]
- Stein, G.E.; Sheridan, V.L.; Magee, B.B.; Magee, P.T. Use of rDNA restriction fragment length polymorphisms to differentiate strains of Candida albicans in women with vulvovaginal candidiasis. Diagn. Microbiol. Infect. Dis. 1991, 14, 459–464. [Google Scholar] [CrossRef]
- Talaei, Z.; Sheikhbahaei, S.; Ostadi, V.; Ganjalikhani Hakemi, M.; Meidani, M.; Naghshineh, E.; Yaran, M.; Emami Naeini, A.; Sherkat, R. Recurrent vulvovaginal candidiasis: Could it be related to cell-mediated immunity defect in response to candida antigen? Int. J. Fertil. Steril. 2017, 11, 134–141. [Google Scholar] [PubMed]
- Neves, N.A.; Carvalho, L.P.; De Oliveira, M.A.; Giraldo, P.C.; Bacellar, O.; Cruz, A.A.; Carvalho, E.M. Association between atopy and recurrent vaginal candidiasis. Clin. Exp. Immunol. 2005, 142, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Babula, O.; Lazdane, G.; Kroica, J.; Ledger, W.J.; Witkin, S.S. Relation between recurrent vulvovaginal candidiasis, vaginal concentrations of mannose-binding lectin, and a mannose-binding lectin gene polymorphism in Latvian women. Clin. Infect. Dis. 2003, 37, 733–737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraldo, P.C.; Babula, O.; Goncalves, A.K.; Linhares, I.M.; Amaral, R.L.; Ledger, W.J.; Witkin, S.S. Mannose-binding lectin gene polymorphism, vulvovaginal candidiasis, and bacterial vaginosis. Obstet. Gynecol. 2007, 109, 1123–1128. [Google Scholar] [CrossRef]
- Donders, G.G.; Babula, O.; Bellen, G.; Linhares, I.M.; Witkin, S.S. Mannose-binding lectin gene polymorphism and resistance to therapy in women with recurrent vulvovaginal candidiasis. BJOG 2008, 115, 1225–1231. [Google Scholar] [CrossRef]
- Chen, S.; Li, S.; Wu, Y.; Liu, Z.; Li, J. Local expression of vaginal Th1 and Th2 cytokines in murine vaginal candidiasis under different immunity conditions. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 476–479. [Google Scholar] [CrossRef]
- De Bernardis, F.; Santoni, G.; Boccanera, M.; Lucciarini, R.; Arancia, S.; Sandini, S.; Amantini, C.; Cassone, A. Protection against rat vaginal candidiasis by adoptive transfer of vaginal B lymphocytes. FEMS Yeast Res. 2010, 10, 432–440. [Google Scholar] [CrossRef] [PubMed]
- De Bernardis, F.; Santoni, G.; Boccanera, M.; Spreghini, E.; Adriani, D.; Morelli, L.; Cassone, A. Local anticandidal immune responses in a rat model of vaginal infection by and protection against Candida albicans. Infect. Immun. 2000, 68, 3297–3304. [Google Scholar] [CrossRef] [Green Version]
- Fidel, P.L., Jr.; Lynch, M.E.; Sobel, J.D. Candida-specific Th1-type responsiveness in mice with experimental vaginal candidiasis. Infect. Immun. 1993, 61, 4202–4207. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, W.; Chen, S.; Liu, Z.; Wu, Y.; Li, J. Local Th1/Th2 cytokine expression in experimental murine vaginal candidiasis. J. Huazhong Univ. Sci. Technol. Med. Sci. 2008, 28, 352–355. [Google Scholar] [CrossRef] [PubMed]
- Gunther, L.S.; Martins, H.P.; Gimenes, F.; Abreu, A.L.; Consolaro, M.E.; Svidzinski, T.I. Prevalence of Candida albicans and non-albicans isolates from vaginal secretions: Comparative evaluation of colonization, vaginal candidiasis and recurrent vaginal candidiasis in diabetic and non-diabetic women. Sao Paulo Med. J. 2014, 132, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Akimoto-Gunther, L.; de Souza Bonfim-Mendonca, P.; Takahachi, G.; Irie, M.M.; Miyamoto, S.; Consolaro, M.E.; Svidzinsk, T.I. Highlights regarding host predisposing factors to recurrent vulvovaginal candidiasis: Chronic stress and reduced antioxidant capacity. PLoS ONE 2016, 11, e0158870. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G. Lower genital tract infections in diabetic women. Curr. Infect. Dis. Rep. 2002, 4, 536–539. [Google Scholar] [CrossRef] [PubMed]
- Donders, G.G.; Prenen, H.; Verbeke, G.; Reybrouck, R. Impaired tolerance for glucose in women with recurrent vaginal candidiasis. Am. J. Obstet. Gynecol. 2002, 187, 989–993. [Google Scholar] [CrossRef]
- Grinceviciene, S.; Bellen, G.; Ruban, K.; Donders, G. Non-response to fluconazole maintenance treatment (ReCiDiF regimen) for recurrent vulvovaginal candidosis is not related to impaired glucose metabolism. Mycoses 2017, 60, 546–551. [Google Scholar] [CrossRef]
- Donders, G.G.; Mertens, I.; Bellen, G.; Pelckmans, S. Self-elimination of risk factors for recurrent vaginal candidosis. Mycoses 2011, 54, 39–45. [Google Scholar] [CrossRef]
- Corsello, S.; Spinillo, A.; Osnengo, G.; Penna, C.; Guaschino, S.; Beltrame, A.; Blasi, N.; Festa, A. An epidemiological survey of vulvovaginal candidiasis in Italy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2003, 110, 66–72. [Google Scholar] [CrossRef]
- Rhodus, N.L.; Bloomquist, C.; Liljemark, W.; Bereuter, J. A comparison of three methods for detecting Candida albicans in patients with Sjogren’s syndrome. Quintessence Int. 1998, 29, 107–113. [Google Scholar]
- Kim, T.H.; Park, B.R.; Kim, H.R.; Lee, M.K. Candida dubliniensis screening using the germ tube test in clinical yeast isolates and prevalence of C. dubliniensis in Korea. J. Clin. Lab. Anal. 2010, 24, 145–148. [Google Scholar] [CrossRef]
- Wright, R.A.; Judson, F.N. Relative and seasonal incidences of the sexually transmitted diseases. A two-year statistical review. Br. J. Vener. Dis. 1978, 54, 433–440. [Google Scholar] [CrossRef] [Green Version]
- Schmid, J.; Rotman, M.; Reed, B.; Pierson, C.L.; Soll, D.R. Genetic similarity of Candida albicans strains from vaginitis patients and their partners. J. Clin. Microbiol. 1993, 31, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Goncalves, B.; Ferreira, C.; Alves, C.T.; Henriques, M.; Azeredo, J.; Silva, S. Vulvovaginal candidiasis: Epidemiology, microbiology and risk factors. Crit. Rev. Microbiol. 2016, 42, 905–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behall, K.M.; Scholfield, D.J.; Hallfrisch, J.G.; Kelsay, J.L.; Reiser, S. Seasonal variation in plasma glucose and hormone levels in adult men and women. Am. J. Clin. Nutr. 1984, 40, 1352–1356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donahoo, W.T.; Jensen, D.R.; Shepard, T.Y.; Eckel, R.H. Seasonal variation in lipoprotein lipase and plasma lipids in physically active, normal weight humans. J. Clin. Endocrinol. Metab. 2000, 85, 3065–3068. [Google Scholar]
- Suarez, L.; Barrett-Connor, E. Seasonal variation in fasting plasma glucose levels in man. Diabetologia 1982, 22, 250–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, R.; Brot, C.; Jakobsen, J.; Mejborn, H.; Molgaard, C.; Skovgaard, L.T.; Trolle, E.; Tetens, I.; Ovesen, L. Seasonal changes in vitamin D status among Danish adolescent girls and elderly women: The influence of sun exposure and vitamin D intake. Eur. J. Clin. Nutr. 2013, 67, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Yavuz, T.; Kaya, D.; Behcet, M.; Ozturk, E.; Yavuz, O. Effects of melatonin on Candida sepsis in an experimental rat model. Adv. Ther. 2007, 24, 91–100. [Google Scholar] [CrossRef]
- Terron, M.P.; Paredes, S.D.; Barriga, C.; Ortega, E.; Rodriguez, A.B. Comparative study of the heterophil phagocytic function in young and old ring doves (Streptopelia risoria) and its relationship with melatonin levels. J. Comp. Physiol. B 2004, 174, 421–427. [Google Scholar] [CrossRef]
- Yang, H.P.; Tsang, P.C.; Tsang, P.W. Melatonin inhibits biofilm formation in Candida parapsilosis. J. Mycol. Med. 2014, 24, 360–361. [Google Scholar] [CrossRef]
- Khoo, A.L.; Chai, L.Y.; Koenen, H.J.; Kullberg, B.J.; Joosten, I.; van der Ven, A.J.; Netea, M.G. 1,25-dihydroxyvitamin D3 modulates cytokine production induced by Candida albicans: Impact of seasonal variation of immune responses. J. Infect. Dis. 2011, 203, 122–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khoo, A.L.; Chai, L.Y.; Koenen, H.J.; Sweep, F.C.; Joosten, I.; Netea, M.G.; van der Ven, A.J. Regulation of cytokine responses by seasonality of vitamin D status in healthy individuals. Clin. Exp. Immunol. 2011, 164, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Berman, B.A. Seasonal allergic vulvovaginitis caused by pollen. Ann. Allergy 1964, 22, 594–597. [Google Scholar] [PubMed]
- Donders, G.G.; Grinceviciene, S.; Bellen, G.; Jaeger, M.; ten Oever, J.; Netea, M. Is non-response to fluconazole maintenance therapy for recurrent Candida vaginitis related to sensitization to atopic reactions? Am. J. Reprod. Immmunol. 2018; in press. [Google Scholar]
- Carvalho, L.P.; Bacellar, O.; Neves, N.; de Jesus, A.R.; Carvalho, E.M. Downregulation of IFN-gamma production in patients with recurrent vaginal candidiasis. J. Allergy Clin. Immunol. 2002, 109, 102–105. [Google Scholar] [CrossRef]
- Neves, N.A.; Carvalho, L.P.; Lopes, A.C.; Cruz, A.; Carvalho, E.M. Successful treatment of refractory recurrent vaginal candidiasis with cetirizine plus fluconazole. J. Low Genit. Tract. Dis. 2005, 9, 167–170. [Google Scholar] [CrossRef]
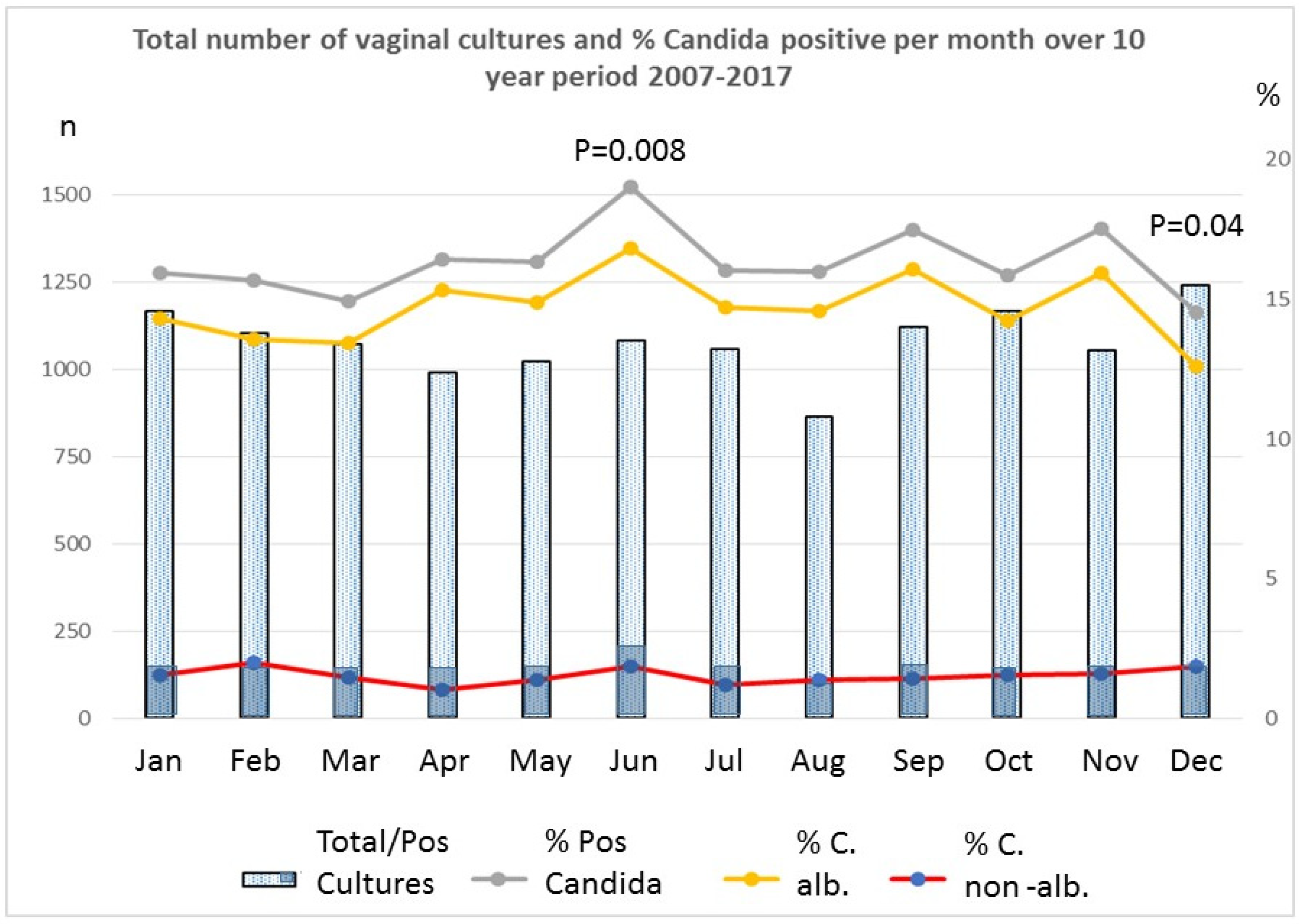
| MONTH | % Tot Candida | % C. albicans | % C. Non-albicans | Insolation, kWh/m²/Day | Clearness (0–1) | Wet Days | Temp °C |
| January | 15.94 | 14.31 | 1.54 | 0.74 | 0.32 | 20 | 3.77 |
| February | 15.67 | 13.59 | 1.99 | 1.37 | 0.36 | 15.2 | 3.84 |
| March | 14.91 | 13.42 | 1.49 | 2.46 | 0.4 | 18.4 | 6.11 |
| April | 16.45 | 15.34 | 1.01 | 3.72 | 0.43 | 16.7 | 8.72 |
| May | 16.36 | 14.89 | 1.37 | 4.77 | 0.45 | 16.7 | 13.04 |
| June | 19.04 | 16.82 | 1.85 | 4.89 | 0.43 | 15.2 | 15.87 |
| July | 16.05 | 14.73 | 1.23 | 4.85 | 0.44 | 15 | 18.44 |
| August | 15.99 | 14.60 | 1.39 | 4.24 | 0.45 | 15.4 | 18.76 |
| September | 17.50 | 16.07 | 1.43 | 2.84 | 0.4 | 15.3 | 15.56 |
| October | 15.87 | 14.24 | 1.54 | 1.67 | 0.36 | 17 | 12.05 |
| November | 17.54 | 15.92 | 1.61 | 0.86 | 0.31 | 19.3 | 7.38 |
| December | 14.52 | 12.58 | 1.85 | 0.55 | 0.29 | 19.2 | 4.78 |
| Regression % Total Candida infection (r², p-value) | R² 0.17, p 0.2 | R² 0.08, p 0.4 | R ²0.13, p 0.2 | R² 0.21, p 0.13 | |||
| Regression % C. albicans infection (r², p-value) | R² 0.24 p 0.1 | R² 0.16, p 0.2 | R² 0.14, p 0.2 | R² 0.29, p 0.07 | |||
| Regression % TC. non-albicans infection (r², p-value) | R² 0.23 p 0.1 | R² 0.30, p 0.06 | R² 0.01, p 0.7 | R² 0.16, p 0.2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donders, G.G.G.; Ruban, K.; Donders, F.; Reybrouck, R. Lab-Based Retrospective 10-Year Analysis Shows Seasonal Variation of Vaginal Candida Infection Rates in Belgium. J. Clin. Med. 2022, 11, 574. https://doi.org/10.3390/jcm11030574
Donders GGG, Ruban K, Donders F, Reybrouck R. Lab-Based Retrospective 10-Year Analysis Shows Seasonal Variation of Vaginal Candida Infection Rates in Belgium. Journal of Clinical Medicine. 2022; 11(3):574. https://doi.org/10.3390/jcm11030574
Chicago/Turabian StyleDonders, Gilbert G. G., Kateryna Ruban, Francesca Donders, and Reinhilde Reybrouck. 2022. "Lab-Based Retrospective 10-Year Analysis Shows Seasonal Variation of Vaginal Candida Infection Rates in Belgium" Journal of Clinical Medicine 11, no. 3: 574. https://doi.org/10.3390/jcm11030574







