Minimal Change Disease Is Associated with Mitochondrial Injury and STING Pathway Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical and Laboratory Data Collection
2.3. Urinary mtDNA Copy Number Quantification
2.4. Immunohistochemical Detection of STING
2.5. Clinical Outcome Measures
2.6. Statistical Analysis
3. Results
3.1. Study Population
3.2. Urinary mtDNA Copy Numbers Comparison in the Three Groups in the Prospective Study
3.3. STING IHC Stain Comparison in the MCD and IgAN Groups in the Retrospective Study
3.4. Treatment Response Analysis Based on the STING IHC Stain Signal Intensity in Patients with MCD
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cameron, J.S. Nephrotic syndrome in the elderly. Semin. Nephrol. 1996, 16, 319–329. [Google Scholar] [PubMed]
- Johnson, R.J.; Feehally, J.; Floege, J. Comprehensive Clinical Nephrology; Saunders: Philadelphia, PA, USA, 2014. [Google Scholar]
- Elie, V.; Fakhoury, M.; Deschênes, G.; Jacqz-Aigrain, E. Physiopathology of idiopathic nephrotic syndrome: Lessons from glucocorticoids and epigenetic perspectives. Pediatr. Nephrol. 2012, 27, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Shalhoub, R.J. Pathogenesis of lipoid nephrosis: A disorder of T-cell function. Lancet 1974, 2, 556–560. [Google Scholar] [CrossRef]
- Mathieson, P.W. Immune dysregulation in minimal change nephropathy. Nephrol. Dial. Transplant. 2003, 18 (Suppl. S6), vi26–vi29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Basu, B. Ofatumumab for rituximab-resistant nephrotic syndrome. N. Engl. J. Med. 2014, 370, 1268–1270. [Google Scholar] [CrossRef]
- Vivarelli, M.; Massella, L.; Ruggiero, B.; Emma, F. Minimal Change Disease. Clin. J. Am. Soc. Nephrol. 2017, 12, 332–345. [Google Scholar] [CrossRef] [Green Version]
- Hogan, J.; Radhakrishnan, J. The treatment of minimal change disease in adults. J. Am. Soc. Nephrol. 2013, 24, 702–711. [Google Scholar] [CrossRef] [Green Version]
- Mak, S.K.; Short, C.D.; Mallick, N.P. Long-term outcome of adult-onset minimal-change nephropathy. Nephrol. Dial. Transplant. 1996, 11, 2192–2201. [Google Scholar] [CrossRef]
- Nolasco, F.; Cameron, J.S.; Heywood, E.F.; Hicks, J.; Ogg, C.; Williams, D.G. Adult-onset minimal change nephrotic syndrome: A long-term follow-up. Kidney Int. 1986, 29, 1215–1223. [Google Scholar] [CrossRef] [Green Version]
- Waldman, M.; Crew, R.J.; Valeri, A.; Busch, J.; Stokes, B.; Markowitz, G.; D’Agati, V.; Appel, G. Adult minimal-change disease: Clinical characteristics, treatment, and outcomes. Clin. J. Am. Soc. Nephrol. 2007, 2, 445–453. [Google Scholar] [CrossRef]
- Nakayama, M.; Katafuchi, R.; Yanase, T.; Ikeda, K.; Tanaka, H.; Fujimi, S. Steroid responsiveness and frequency of relapse in adult-onset minimal change nephrotic syndrome. Am. J. Kidney Dis. 2002, 39, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Korbet, S.M.; Schwartz, M.M.; Lewis, E.J. Minimal-change glomerulopathy of adulthood. Am. J. Nephrol. 1988, 8, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Galvan, D.L.; Green, N.H.; Danesh, F.R. The hallmarks of mitochondrial dysfunction in chronic kidney disease. Kidney Int. 2017, 92, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Chung, K.P.; Nakahira, K.; Patino, E.; Rice, M.C.; Torres, L.K.; Muthukumar, T.; Choi, A.M.; Akchurin, O.M.; Choi, M.E. Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis. JCI Insight 2019, 4, e132826. [Google Scholar] [CrossRef] [Green Version]
- Wei, P.Z.; Kwan, B.C.; Chow, K.M.; Cheng, P.M.; Luk, C.C.; Li, P.K.; Szeto, C.C. Urinary mitochondrial DNA level is an indicator of intra-renal mitochondrial depletion and renal scarring in diabetic nephropathy. Nephrol. Dial. Transplant. 2018, 33, 784–788. [Google Scholar] [CrossRef] [Green Version]
- Whitaker, R.M.; Stallons, L.J.; Kneff, J.E.; Alge, J.L.; Harmon, J.L.; Rahn, J.J.; Arthur, J.M.; Beeson, C.C.; Chan, S.L.; Schnellmann, R.G. Urinary mitochondrial DNA is a biomarker of mitochondrial disruption and renal dysfunction in acute kidney injury. Kidney Int. 2015, 88, 1336–1344. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, H.; Inoue, T.; Ouchi, H.; Jao, T.M.; Inoue, R.; Nishi, H.; Fujii, R.; Ishidate, F.; Tanaka, T.; Tanaka, Y.; et al. Mitochondrial Damage Causes Inflammation via cGAS-STING Signaling in Acute Kidney Injury. Cell Rep. 2019, 29, 1261–1273.e6. [Google Scholar] [CrossRef] [Green Version]
- Che, R.; Yuan, Y.; Huang, S.; Zhang, A. Mitochondrial dysfunction in the pathophysiology of renal diseases. Am. J. Physiol. Ren. Physiol. 2014, 306, F367–F378. [Google Scholar] [CrossRef]
- Chung, K.W.; Dhillon, P.; Huang, S.; Sheng, X.; Shrestha, R.; Qiu, C.; Kaufman, B.A.; Park, J.; Pei, L.; Baur, J.; et al. Mitochondrial Damage and Activation of the STING Pathway Lead to Renal Inflammation and Fibrosis. Cell Metab. 2019, 30, 784–799.e5. [Google Scholar] [CrossRef]
- Eirin, A.; Saad, A.; Tang, H.; Herrmann, S.M.; Woollard, J.R.; Lerman, A.; Textor, S.C.; Lerman, L.O. Urinary Mitochondrial DNA Copy Number Identifies Chronic Renal Injury in Hypertensive Patients. Hypertension 2016, 68, 401–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.C.; Cho, N.J.; Park, S.; Kim, H.; Choi, S.J.; Kim, J.K.; Hwang, S.D.; Gil, H.W.; Lee, E.Y.; Jeon, J.S.; et al. IgA nephropathy is associated with elevated urinary mitochondrial DNA copy numbers. Sci. Rep. 2019, 9, 16068. [Google Scholar] [CrossRef] [Green Version]
- Levey, A.S.; Stevens, L.A.; Schmid, C.H.; Zhang, Y.L.; Castro, A.F., 3rd; Feldman, H.I.; Kusek, J.W.; Eggers, P.; Van Lente, F.; Greene, T.; et al. A new equation to estimate glomerular filtration rate. Ann. Intern. Med. 2009, 150, 604–612. [Google Scholar] [CrossRef]
- Andrews, R.M.; Kubacka, I.; Chinnery, P.F.; Lightowlers, R.N.; Turnbull, D.M.; Howell, N. Reanalysis and revision of the Cambridge reference sequence for human mitochondrial DNA. Nat. Genet. 1999, 23, 147. [Google Scholar] [CrossRef] [PubMed]
- KDIGO. Chapter 5: Minimal-change disease in adults. Kidney Int. Suppl. 2012, 2, 177–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, B.C.; Cho, N.J.; Park, S.; Kim, H.; Gil, H.W.; Lee, E.Y.; Kwon, S.H.; Jeon, J.S.; Noh, H.; Han, D.C.; et al. Minor Glomerular Abnormalities are Associated with Deterioration of Long-Term Kidney Function and Mitochondrial Injury. J. Clin. Med. 2019, 9, 33. [Google Scholar] [CrossRef] [Green Version]
- Chadban, S.J.; Atkins, R.C. Glomerulonephritis. Lancet 2005, 365, 1797–1806. [Google Scholar] [CrossRef]
- Li, S.; Lin, Q.; Shao, X.; Zhu, X.; Wu, J.; Wu, B.; Zhang, M.; Zhou, W.; Zhou, Y.; Jin, H.; et al. Drp1-regulated PARK2-dependent mitophagy protects against renal fibrosis in unilateral ureteral obstruction. Free Radic. Biol. Med. 2020, 152, 632–649. [Google Scholar] [CrossRef]
- Patil, N.K.; Parajuli, N.; MacMillan-Crow, L.A.; Mayeux, P.R. Inactivation of renal mitochondrial respiratory complexes and manganese superoxide dismutase during sepsis: Mitochondria-targeted antioxidant mitigates injury. Am. J. Physiol. Ren. Physiol. 2014, 306, F734–F743. [Google Scholar] [CrossRef] [Green Version]
- Dare, A.J.; Bolton, E.A.; Pettigrew, G.J.; Bradley, J.A.; Saeb-Parsy, K.; Murphy, M.P. Protection against renal ischemia-reperfusion injury in vivo by the mitochondria targeted antioxidant MitoQ. Redox Biol. 2015, 5, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Soong, Y.; Seshan, S.V.; Szeto, H.H. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am. J. Physiol. Ren. Physiol. 2014, 306, F970–F980. [Google Scholar] [CrossRef] [Green Version]
- Szeto, H.H.; Liu, S.; Soong, Y.; Alam, N.; Prusky, G.T.; Seshan, S.V. Protection of mitochondria prevents high-fat diet-induced glomerulopathy and proximal tubular injury. Kidney Int. 2016, 90, 997–1011. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Liu, Y.J.; Liu, Z.R.; Tang, D.D.; Chen, X.W.; Chen, Y.H.; Zhou, R.N.; Chen, S.Q.; Niu, H.X. Role of mitochondrial dysfunction in renal fibrosis promoted by hypochlorite-modified albumin in a remnant kidney model and protective effects of antioxidant peptide SS-31. Eur. J. Pharmacol. 2017, 804, 57–67. [Google Scholar] [CrossRef]
- Caliò, A.; Brunelli, M.; Gobbo, S.; Pedron, S.; Segala, D.; Argani, P.; Martignoni, G. Stimulator of interferon genes (STING) immunohistochemical expression in the spectrum of perivascular epithelioid cell (PEC) lesions of the kidney. Pathology 2021, 53, 579–585. [Google Scholar] [CrossRef]

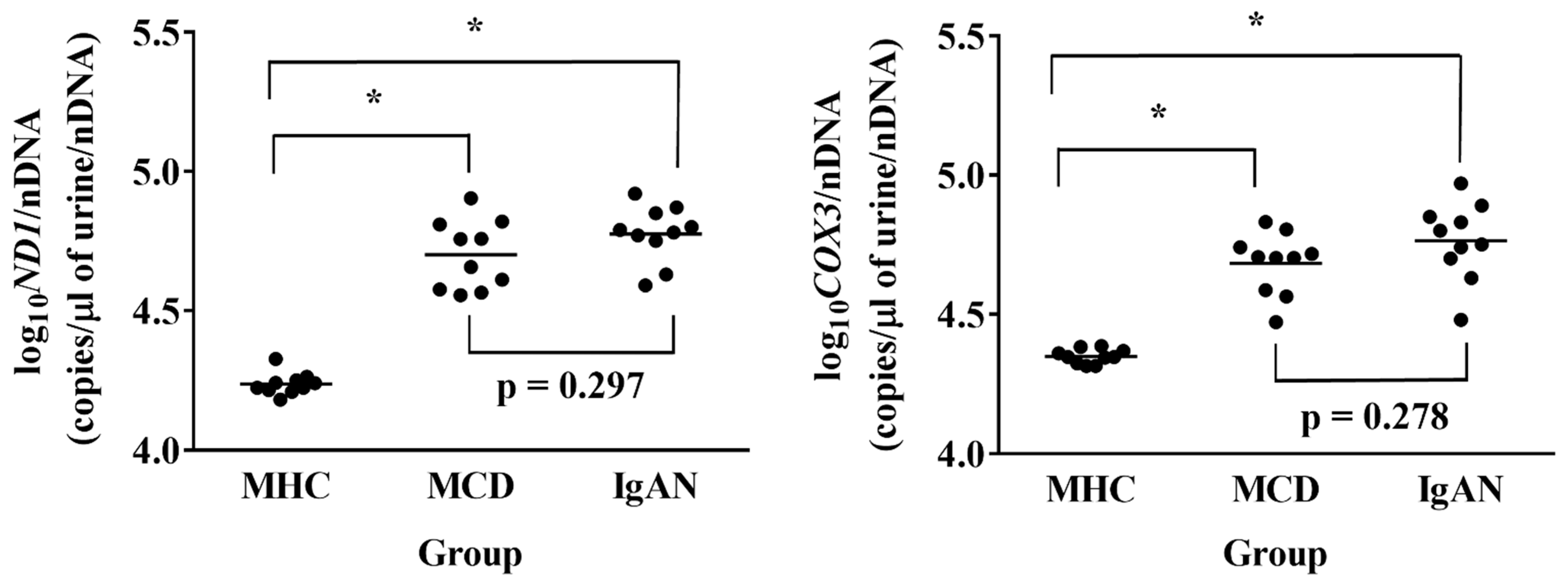
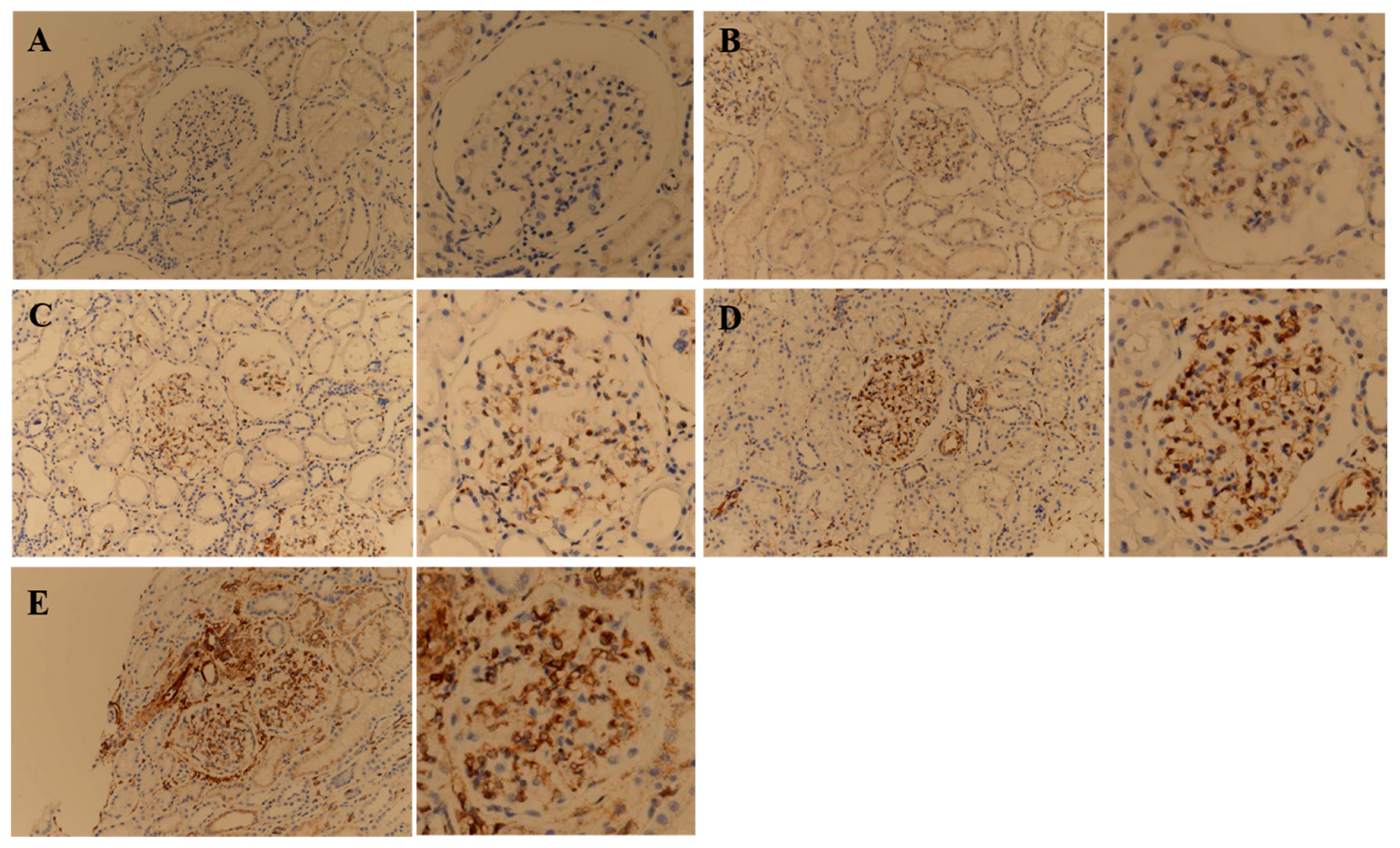
| Variable | MHC Group (n = 10) | MCD Group (n = 10) | IgAN Group (n = 10) | p-Value * |
|---|---|---|---|---|
| Age (years) | 45.8 ± 15.2 | 45.8 ± 22.7 | 45.8 ± 16.3 | >0.999 |
| Sex (male) | 4 (40.0) | 4 (40.0) | 4 (40.0) | >0.999 |
| Body mass index (kg/m2) | 22.7 ± 3.1 | 26.0 ± 5.4 | 24.5 ± 4.2 | 0.236 |
| Hypertension | 0 (0.0) | 4 (40.0) | 5 (50.0) | 0.038 |
| Systolic blood pressure (mmHg) | 119.1 ± 7.9 | 126.9 ± 21.5 | 131.4 ± 19.4 | 0.291 |
| Diastolic blood pressure (mmHg) | 77.2 ± 7.1 | 76.0 ± 10.0 | 81.5 ± 10.6 | 0.396 |
| Mean arterial pressure (mmHg) | 91.2 ± 6.2 | 93.0 ± 12.3 | 98.1 ± 11.8 | 0.320 |
| Baseline eGFR (mL/min/1.73 m2) | 88.7 ± 17.6 | 73.0 ± 34.1 | 71.96 ± 18.1 | 0.250 |
| Baseline proteinuria (mg/day) | 80.8 ± 30.7 | 8659.4 ± 5559.8 | 902.3 ± 629.0 | <0.001 |
| Use of ARBs or ACE inhibitors | 0 (0.0) | 4 (40.0) | 5 (50.0) | 0.038 |
| Stain Site | MCD Group (n = 20) | IgAN Group (n = 20) | p-Value |
|---|---|---|---|
| Glomerulus | 13 (65.0) | 13 (65.0) | >0.999 |
| Tubule | 0 (0.0) | 18 (90.0) | <0.001 |
| Interstitium | 0 (0.0) | 17 (85.0) | <0.001 |
| Variable | High-Intensity Group (n = 6) | Low-Intensity Group (n = 14) | p-Value |
|---|---|---|---|
| Age (years) | 35.3 ± 8.8 | 47.5 ± 19.3 | 0.179 |
| Sex (male) | 3 (50.0) | 9 (64.3) | 0.642 |
| Body mass index (kg/m2) | 24.9 ± 3.8 | 26.4 ± 3.5 | 0.368 |
| Hypertension | 1 (16.7) | 3 (21.4) | >0.999 |
| Systolic blood pressure (mmHg) | 126.7 ± 5.2 | 127.7 ± 13.0 | 0.898 |
| Diastolic blood pressure (mmHg) | 80.0 ± 11.0 | 83.1 ± 7.5 | 0.368 |
| Mean arterial pressure (mmHg) | 95.6 ± 8.1 | 98.0 ± 8.6 | 0.639 |
| Baseline eGFR (mL/min/1.73 m2) | 95.2 ± 21.4 | 82.9 ± 19.9 | 0.368 |
| Baseline proteinuria (mg/day) | 6463.8 ± 5672.4 | 5279.3 ± 2562.9 | 0.966 |
| Use of ARBs or ACE inhibitors | 3 (50.0) | 11 (78.6) | 0.303 |
| Variable | High-Intensity Group (n = 6) | Low-Intensity Group (n = 14) | p-Value |
|---|---|---|---|
| Mean follow-up duration (years) | 10.8 ± 5.4 | 8.7 ± 5.2 | 0.179 |
| Mean annual rate of eGFR decline (mL/min/1.73 m2/year) | −1.2 ± 0.8 | −1.4 ± 2.3 | 0.701 |
| Time-averaged proteinuria (g/day) | 1.18 ± 0.54 | 0.57 ± 0.45 | 0.022 |
| Time to complete or partial remission after treatment (month) | 4.4 ± 7.1 | 6.0 ± 7.4 | 0.579 |
| Time to first relapse after complete or partial remission (month) | 15.1 ± 18.8 | 22.1 ± 31.0 | 0.905 |
| Frequency of relapses during follow-up duration (episodes/year) | 0.72 ± 0.60 | 0.09 ± 0.22 | 0.022 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, B.C.; Moon, A.; Lee, K.H.; Oh, Y.S.; Park, M.Y.; Choi, S.J.; Kim, J.K. Minimal Change Disease Is Associated with Mitochondrial Injury and STING Pathway Activation. J. Clin. Med. 2022, 11, 577. https://doi.org/10.3390/jcm11030577
Yu BC, Moon A, Lee KH, Oh YS, Park MY, Choi SJ, Kim JK. Minimal Change Disease Is Associated with Mitochondrial Injury and STING Pathway Activation. Journal of Clinical Medicine. 2022; 11(3):577. https://doi.org/10.3390/jcm11030577
Chicago/Turabian StyleYu, Byung Chul, Ahrim Moon, Kyung Ho Lee, Young Seung Oh, Moo Yong Park, Soo Jeong Choi, and Jin Kuk Kim. 2022. "Minimal Change Disease Is Associated with Mitochondrial Injury and STING Pathway Activation" Journal of Clinical Medicine 11, no. 3: 577. https://doi.org/10.3390/jcm11030577
APA StyleYu, B. C., Moon, A., Lee, K. H., Oh, Y. S., Park, M. Y., Choi, S. J., & Kim, J. K. (2022). Minimal Change Disease Is Associated with Mitochondrial Injury and STING Pathway Activation. Journal of Clinical Medicine, 11(3), 577. https://doi.org/10.3390/jcm11030577






