Functional Network Connectivity Reveals the Brain Functional Alterations in Breast Cancer Survivors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Exclusion Criteria
2.3. Rs-fMRI
2.4. Data Processing and Statistical Analyses
3. Results
- (1)
- Comparison of the connectivity differences between all patients after breast cancer treatment who participated in the study and the control group;
- (2)
- Comparison between patients after breast cancer treatment with and without lymphedema;
- (3)
- Comparison between patients after breast cancer treatment with the presence of pain in the upper limb and without;
- (4)
- Comparison between patients after breast cancer treatment with vestibulocerebellar ataxia and without;
- (5)
- Comparison between patients after breast cancer treatment with depression and without depression.
3.1. Resting State Functional MRI Results
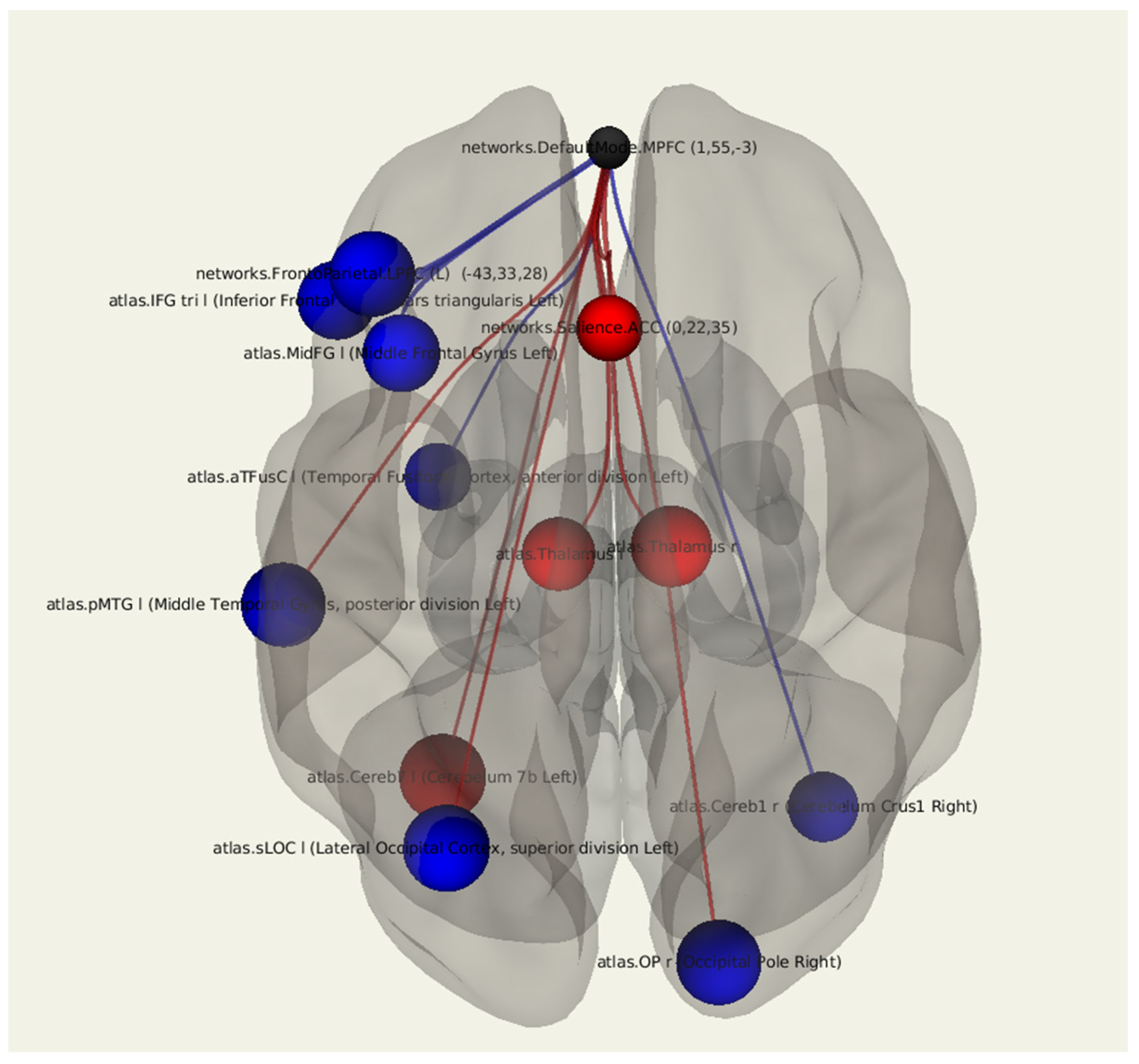
3.1.1. All Patients after Breast Cancer Treatment in Comparison with Control Group
3.1.2. Lymphedema
3.1.3. Postmastectomy Pain Syndrome
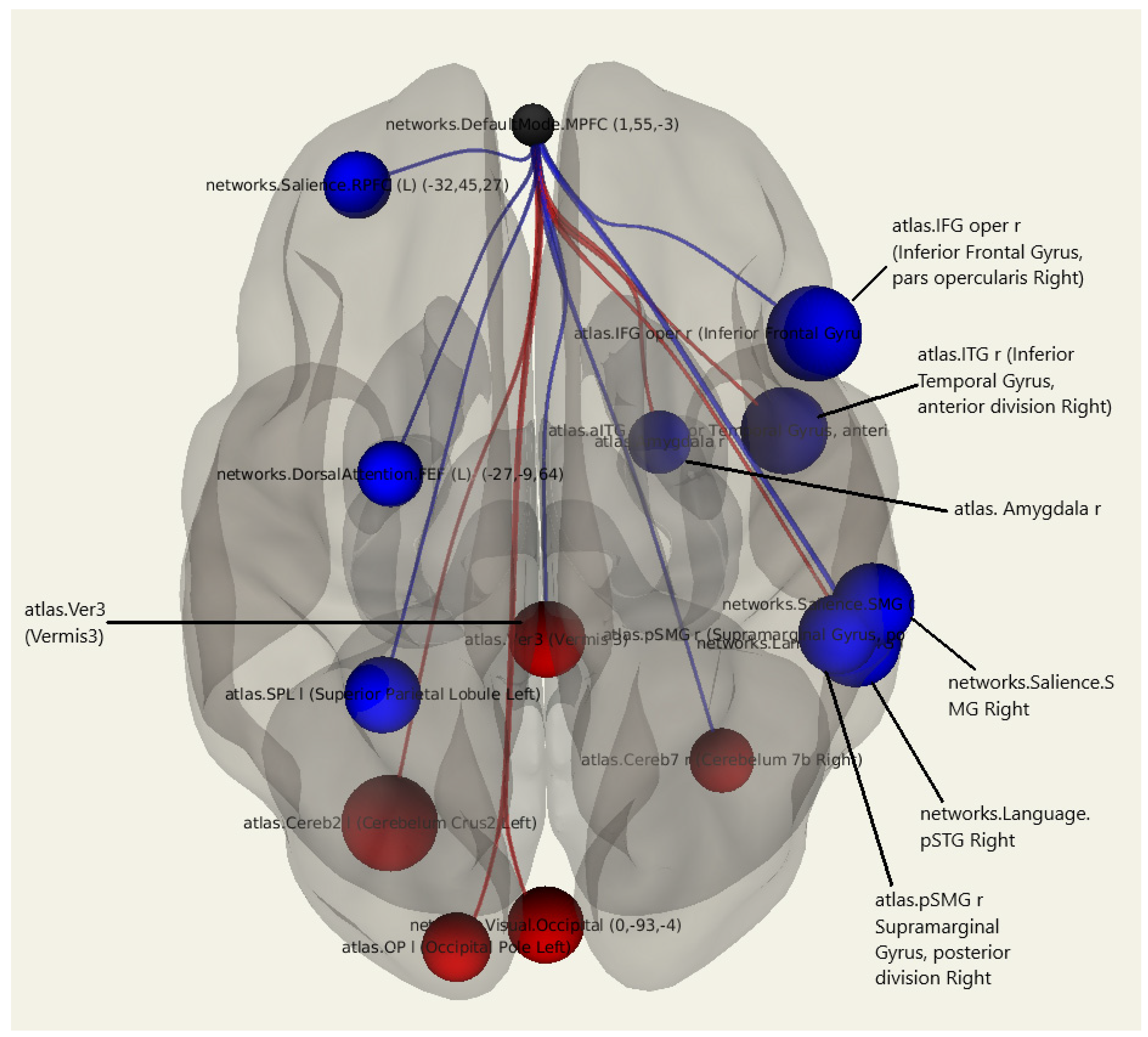
3.1.4. Vestibulocerebellar Ataxia
3.1.5. Depression
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Shirokova, I.; Yu, E. Breast cancer: The view of the Remedium experts. J. Russ. Market Med. Med. Equipment 2016, 10, 53–58. [Google Scholar]
- Fakhari, S.; Atashkhoei, S.; Pourfathi, H.; Farzin, H.; Bilehjani, E. Postmastectomy pain syndrome. Int. J. Women’s Health Reprod. Sci. 2017, 5, 18–23. [Google Scholar] [CrossRef] [Green Version]
- Tishakova, V.E.; Filonenko, E.V.; Chissov, V.I.; Efimenko, N.A.; Urlova, A.N. Physical methods of rehabilitation in cancer patients after combined modality treatment for breast cancer. Biomed. Photon. 2017, 6, 28–37. [Google Scholar] [CrossRef] [Green Version]
- Obmanov, I.V.; Yarygin, M.L.; Shmyrev, V.I.; Yarygin, L.M. Neurological disorders in patients with breast cancer after surgical treatment. J. Neurol. Psychiatry 2015, 115, 42–44. [Google Scholar]
- Stubblefield, M.D.; Keole, N. Upper body pain and functional disorders in patients with breast cancer. PM&R 2014, 6, 170–183. [Google Scholar] [CrossRef]
- Shikhkerimov, R.K.; Savin, A.A.; Welsher, L.Z.; Stakhanov, M.L.; Stulin, I.D.; Savin, L.A.; Strazhev, S.V. Pathology of the brachial neurovascular bundle in the clinical manifestations of post-mastectomy syndrome. Bull. Natl. Med. Surg. Center 2011, 6, 86–90. [Google Scholar]
- Beyaz, S.G.; Ergönenç, J.Ş.; Ergönenç, T.; Sönmez, Ö.U.; Erkorkmaz, Ü. Postmastectomy pain: A cross-sectional study of prevalence, pain characteristics, and effects on quality of life. Chin. Med. J. 2016, 129, 66–71. [Google Scholar] [CrossRef]
- Fomberstein, K.; Qadri, S.; Ramani, R. Functional MRI and pain. Curr. Opin. Anaesthesiol. 2013, 26, 588–593. [Google Scholar] [CrossRef]
- Castillo, E.M.; Suarez, A.B.; Vazquez, A.S.; Ramírez, M.G. C0041—Can lymphedema cause a thoracic outlet syndrome? Case Ser. Br. J. Sports Med. 2018, 52, A8–A9. [Google Scholar]
- Maksimova, M.Y.; Skrylev, S.I.; Kosheev, A.Y.; Shchipakin, V.L.; Sinitsyn, I.A.; Chechetkin, A.O. Insufficiency of blood flow in the arteries of the vertebral-basilar system in anterior stair muscle syndrome. Ann. Clin. Exp. Neurol. 2018, 12, 5–11. [Google Scholar]
- Jones, M.R.; Prabhakar, A.; Viswanath, O.; Urits, I.; Green, J.B.; Kendrick, J.B.; Brunk, A.J.; Eng, M.R.; Orhurhu, V.; Cornett, E.M.; et al. Thoracic outlet syndrome: A comprehensive review of pathophysiology, diagnosis, and treatment. Pain Ther. 2019, 8, 5–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wisotzky, E.; Hanrahan, N.; Lione, T.P.; Maltser, S. Deconstructing postmastectomy syndrome: Implications for physiatric management. Phys. Med. Rehab. Clin. N. Am. 2017, 28, 153–169. [Google Scholar] [CrossRef] [PubMed]
- Masljakov, V.V.; Ljovina, V.A.; Nakaeva, E.J. Quality of life and postoperative rehabilitation of patients with cancer of the mammary gland. Med. News N. Caucasus 2014, 9, 26–29. (In Russian) [Google Scholar] [CrossRef]
- Ahmed, R.L.; Prizment, A.; Lazovich, D.; Schmitz, K.H.; Folsom, A.R. Lymphedema and quality of life in breast cancer survivors: The Iowa Women’s Health Study. J. Clin. Oncol. 2008, 26, 5689–5696. [Google Scholar] [CrossRef]
- Glover, G.H. Overview of functional magnetic resonance imaging. Neurosurg. Clin. N. Am. 2011, 22, 133. [Google Scholar] [CrossRef] [Green Version]
- Lv, H.; Wang, Z.; Tong, E.; Williams, L.M.; Zaharchuk, G.; Zeineh, M.; Goldstein-Piekarski, A.N.; Ball, T.M.; Liao, C.; Wintermark, M. Resting-state functional MRI: Everything that nonexperts have always wanted to know. ANJR Am. J. Neuroradiol. 2018, 39, 390–1399. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Smyser, C.D.; Shimony, J.S. Resting-state fMRI: A review of methods and clinical applications. AJNR Am. J. Neuroradiol. 2013, 34, 1866–1872. [Google Scholar] [CrossRef] [Green Version]
- Buckner, R.L.; DiNicola, L.M. The brain’s default network: Updated anatomy, physiology and evolving insights. Nat. Rev. Neurosci. 2019, 20, 593–608. [Google Scholar] [CrossRef]
- Mak, L.E.; Minuzzi, L.; MacQueen, G.; Hall, G.; Kennedy, S.H.; Milev, R. The default mode network in healthy individuals: A systematic review and meta-analysis. Brain Connect. 2017, 7, 25–33. [Google Scholar] [CrossRef]
- Whitfield-Gabrieli, S.; Ford, J.M. Default mode network activity and connectivity in psychopathology. Annu. Rev. Clin. Psychol. 2012, 8, 49–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Q.; Su, Y.; Meng, J.; Qiu, J.; Zheng, W. Pain in the default mode network: A voxel-based morphometry study on thermal pain sensitivity. NeuroReport 2020, 31, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Jones, S.A.; Morales, A.M.; Holley, A.L.; Wilson, A.C.; Nagel, B.J. Default mode network connectivity is related to pain frequency and intensity in adolescents. Neuroimage Clin. 2020, 27, 102326. [Google Scholar] [CrossRef]
- Javaheripour, N.; Li, M.; Chand, T.; Krug, A.; Kircher, T.; Dannlowski, U.; Nenadić, I.; Hamilton, J.P.; Sacchet, M.D.; Gotlib, I.H.; et al. Altered resting-state functional connectome in major depressive disorder: A mega-analysis from the PsyMRI consortium. Transl. Psychiatry 2021, 11, 511. [Google Scholar] [CrossRef] [PubMed]
- Klingner, C.M.; Volk, G.F.; Brodoehl, S.; Witte, O.W.; Guntinas-Lichius, O. Disrupted functional connectivity of the default mode network due to acute vestibular deficit. Neuroimage Clin. 2014, 6, 109–114. [Google Scholar] [CrossRef] [Green Version]
- Xu, P.; Chen, A.; Li, Y.; Xing, X.; Lu, H. Medial prefrontal cortex in neurological diseases. Physiol. Genom. 2019, 51, 432–442. [Google Scholar] [CrossRef]
- Cai, S.; Chong, T.; Zhang, Y.; Li, J.; von Deneen, K.M.; Ren, J.; Dong, M.; Huang, L. Alzheimer’s disease neuroimaging initiative. altered functional connectivity of fusiform gyrus in subjects with amnestic mild cognitive impairment: A resting-state fMRI study. Front. Hum. Neurosci. 2015, 9, 471. [Google Scholar] [CrossRef] [Green Version]
- Kühn, S.; Gallinat, J. Resting-state brain activity in schizophrenia and major depression: A quantitative meta-analysis. Schizophr. Bull. 2013, 39, 358–365. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Cheng, C.; Cao, X.; Xiang, J.; Chen, J.; Zhang, K. Resting-state functional connectivity abnormalities in first-onset unmedicated depression. Neural Regen. Res. 2014, 9, 153–163. [Google Scholar] [CrossRef]
- Greicius, M.D.; Krasnow, B.; Reiss, A.L.; Menon, V. Functional connectivity in the resting brain: A network analysis of the default mode hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 253–258. [Google Scholar] [CrossRef] [Green Version]
- Fransson, P. Spontaneous low-frequency BOLD signal fluctuations: An fMRI investigation of the resting-state default mode of brain function hypothesis. Hum. Brain Mapp. 2005, 26, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Keller, J.B.; Hedden, T.; Thompson, T.W.; Anteraper, S.A.; Gabrieli, J.D.; Whitfield-Gabrieli, S. Resting-state anticorrelations between medial and lateral prefrontal cortex: Association with working memory, aging, and individual differences. Cortex 2015, 64, 271–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marek, S.; Hwang, K.; Foran, W.; Hallquist, M.N.; Luna, B. The contribution of network organization and integration to the development of cognitive control. PLoS Biol. 2015, 13, e1002328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantini, D.; Caulo, M.; Ferretti, A.; Romani, G.L.; Tartaro, A. Noxious somatosensory stimulation affects the default mode of brain function: Evidence from functional MR imaging. Radiology 2009, 253, 797–804. [Google Scholar] [CrossRef]
- Ward, A.M.; Schultz, A.P.; Huijbers, W.; Van Dijk, K.R.; Hedden, T.; Sperling, R.A. The parahippocampal gyrus links the default-mode cortical network with the medial temporal lobe memory system. Hum. Brain Mapp. 2014, 35, 1061–1073. [Google Scholar] [CrossRef] [Green Version]
- Putcha, D.; Brickhouse, M.; O’Keefe, K.; Sullivan, C.; Rentz, D.; Marshall, G.; Dickerson, B.; Sperling, R. Hippocampal hyperactivation associated with cortical thinning in Alzheimer’s disease signature regions in non-demented elderly adults. J. Neurosci. 2011, 31, 17680–17688. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, Z.; Du, Z.; Qi, X.; Shu, H.; Liu, D.; Su, F.; Ye, Q.; Liu, X.; Zhou, Z.; et al. Impaired parahippocampal gyrus-orbitofrontal cortex circuit associated with visuospatial memory deficit as a potential biomarker and interventional approach for Alzheimer disease. Neurosci Bull. 2020, 36, 831–844. [Google Scholar] [CrossRef]
- Zamoscik, V.; Huffziger, S.; Ebner-Priemer, U.; Kuehner, C.; Kirsch, P. Increased involvement of the parahippocampal gyri in a sad mood predicts future depressive symptoms. Soc. Cogn. Affect. Neurosci. 2014, 9, 2034–2040. [Google Scholar] [CrossRef] [Green Version]
- Milne, A.M.; MacQueen, G.M.; Hall, G.B. Abnormal hippocampal activation in patients with extensive history of major depression: An fMRI study. J. Psychiatry Neurosci. 2012, 37, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Ma, X.; Liu, J.; Liu, T.; Ma, L.; Wang, W.; Shi, S.; Wang, Y.; Gong, Q.; Wang, M. Altered resting-state functional activity in medication-naive patients with first-episode major depression disorder vs. healthy control: A quantitative meta-analysis. Front. Behav. Neurosci. 2019, 13, 89. [Google Scholar] [CrossRef] [Green Version]
- Arimura, D.; Shinohara, K.; Takahashi, Y.; Sugimura, Y.K.; Sugimoto, M.; Tsurugizawa, T.; Marumo, K.; Kato, F. Primary role of the amygdala in spontaneous inflammatory pain—Associated activation of pain networks—A chemogenetic manganese-enhanced MRI approach. Front. Neural Circuits. 2019, 13, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Oathes, D.; Hush, J. Perturbed connectivity of the amygdala and its subregions with the central executive and default mode networks in chronic pain. Pain 2016, 157, 1970–1978. [Google Scholar] [CrossRef] [PubMed]
- Simons, L.E.; Moulton, E.A.; Linnman, C.; Carpino, E.; Becerra, L.; Borsook, D. The human amygdala and pain: Evidence from neuroimaging. Hum. Brain Mapp. 2014, 35, 527–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Ettinger-Veenstra, H.; Lundberg, P.; Alföldi, P.; Södermark, M.; Graven-Nielsen, T.; Sjörs, A.; Engström, M.; Gerdle, B. Chronic widespread pain patients show disrupted cortical connectivity in default mode and salience networks, modulated by pain sensitivity. J. Pain Res. 2019, 12, 1743–1755. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pospelova, M.; Kasumova, A.A.; Fionik, O.; Alekseeva, T.M.; Samochernykh, K.A.; Krasnikova, V. Possibilities of using the transcranial magnetic stimulation method in the treatment of chronic pain syndromes. Mod. Prob. Sci. Educ. 2021, 87. [Google Scholar] [CrossRef]
- Delanian, S. Is radiation-induced arteriopathy in long-term breast cancer survivors an underdiagnosed situation?: Critical and pragmatic review of available literature. Radiother. Oncol. 2021, 157, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Schwitzer, J.A.; Dekker, P.; Kanuri, A.; Tirrell, A.; Sher, S.R. Post-mastectomy radiation arteritis in a patient presenting with upper extremity claudication. AME Case Rep. 2019, 3, 49. [Google Scholar] [CrossRef]
- Nascimben Matheus, C.; Caldeira de Oliveira Guirro, E. Change in blood flow velocity demonstrated by Doppler ultrasound in upper limb after axillary dissection surgery for the treatment of breast cancer. Breast Cancer Res. Treat. 2011, 127, 697–704. [Google Scholar] [CrossRef]
- Stouten-Kemperman, M.M.; de Ruiter, M.B.; Koppelmans, V.; Boogerd, W.; Reneman, L.; Schagen, S.B. Neurotoxicity in breast cancer survivors ≥10 years post-treatment is dependent on treatment type. Brain Imaging Behav. 2015, 9, 275–284. [Google Scholar] [CrossRef]
- Miao, H.; Chen, X.; Yan, Y.; He, X.; Hu, S.; Kong, J.; Wu, M.; Wei, Y.; Zhou, Y.; Wang, L.; et al. Functional connectivity change of brain default mode network in breast cancer patients after chemotherapy. Neuroradiology 2016, 58, 921–928. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, X.D.; Zheng, G.; Zhang, L.J. Chemotherapy-induced brain changes in breast cancer survivors: Evaluation with multimodality magnetic resonance imaging. Brain Imaging Behav. 2019, 13, 1799–1814. [Google Scholar] [CrossRef] [PubMed]
- Kesler, S.R.; Rao, A.; Blayney, D.W.; Oakley-Girvan, I.A.; Karuturi, M.; Palesh, O. Predicting long-term cognitive outcome following breast cancer with pre-treatment resting state fMRI and random forest machine learning. Front. Hum. Neurosci. 2017, 15, 555. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Syndrome | Number of Patients with the Syndrome | Number of Patients without the Syndrome |
|---|---|---|
| Lymphedema | 23 | 23 |
| Postmastectomy pain syndrome | 24 | 22 |
| Vestibulocerebellar ataxia | 18 | 28 |
| Depression | 19 | 27 |
| Target Region | Side | T | Beta | p-unc |
|---|---|---|---|---|
| Parietal Operculum | Left | 2.43 | 0.11 | 0.018390 |
| Precentral Gyrus | Left | −2.20 | −0.11 | 0.032464 |
| Parietal Operculum | Right | 2.15 | 2.15 | 0.036042 |
| Fusiform Gyrus (Temp-Occ) | Right | −2.04 | −2.04 | 0.046336 |
| Target Region | Side | T | Beta | p-unc |
|---|---|---|---|---|
| Lateral Occipital Cortex | Left | −2.74 | −0.23 | 0.012076 |
| Cerebellum | Left | 2.73 | 0.21 | 0.012222 |
| Occipital Pole | Right | −2.69 | −0.18 | 0.013510 |
| Middle Temporal Gyrus | Left | −2.66 | −0.28 | 0.014181 |
| Thalamus | Right | 2.57 | 0.20 | 0.017496 |
| Inferior Frontal Gyrus | Left | −2.49 | −0.24 | 0.020691 |
| Middle Frontal Gyrus | Left | −2.46 | −0.23 | 0.022441 |
| Thalamus | Left | 2.33 | 0.16 | 0.029463 |
| Cerebellum | Right | −2.22 | −0.18 | 0.036737 |
| Fusiform Gyrus (Temp) | Left | −2.12 | −0.15 | 0.045543 |
| Target Region | Side | T | Beta | p-unc |
|---|---|---|---|---|
| Cerebellum | Left | 3.34 | 0.24 | 0.003469 |
| Inferior Frontal Gyrus | Right | −3.32 | −0.26 | 0.003615 |
| Inferior Temporal Gyrus | Right | −3.02 | −0.21 | 0.007069 |
| Salience network (SMG) | Right | −2.88 | −0.29 | 0.009688 |
| Occipital Pole | Left | 2.36 | 0.18 | 0.029258 |
| Dorsal Attention. FEF | −2.25 | −0.17 | 0.036340 | |
| Amygdala | Right | −2.13 | −0.14 | 0.046265 |
| Target Region | Side | T | Beta | p-unc |
|---|---|---|---|---|
| Caudate | Right | 3.14 | 0.28 | 0.003531 |
| Lateral Occipital Cortex | Left | −2.51 | −0.25 | 0.016856 |
| Fusiform Gyrus (Temp) | Right | −2.38 | −0.19 | 0.023308 |
| Heschl’s Gyrus | Right | −2.36 | −0.19 | 0.024008 |
| Fusiform Gyrus (Temp-Occ) | Left | −2.23 | −0.17 | 0.032363 |
| Cerebellum | Left | −2.16 | −0.16 | 0.037966 |
| Lateral Occipital Cortex | Right | −2.04 | −0.19 | 0.049005 |
| Target Region | Side | T | Beta | p-unc |
|---|---|---|---|---|
| Dorsal Attention. FEF | 3.39 | −0.18 | 0.002925 | |
| Cuneal Cortex | Left | −2.99 | −0.21 | 0.007221 |
| Parahippocampal gyrus | Left | −2.77 | −0.16 | 0.011720 |
| Planum Polare | Right | −2.46 | −0.16 | 0.023100 |
| Fusiform Gyrus (Temp) | Right | −2.37 | 0.13 | 0.028196 |
| Parahippocampal Gyrus | Right | 2.11 | −0.14 | 0.047445 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukkieva, T.; Pospelova, M.; Efimtsev, A.; Fionik, O.; Alekseeva, T.; Samochernych, K.; Gorbunova, E.; Krasnikova, V.; Makhanova, A.; Levchuk, A.; et al. Functional Network Connectivity Reveals the Brain Functional Alterations in Breast Cancer Survivors. J. Clin. Med. 2022, 11, 617. https://doi.org/10.3390/jcm11030617
Bukkieva T, Pospelova M, Efimtsev A, Fionik O, Alekseeva T, Samochernych K, Gorbunova E, Krasnikova V, Makhanova A, Levchuk A, et al. Functional Network Connectivity Reveals the Brain Functional Alterations in Breast Cancer Survivors. Journal of Clinical Medicine. 2022; 11(3):617. https://doi.org/10.3390/jcm11030617
Chicago/Turabian StyleBukkieva, Tatyana, Maria Pospelova, Aleksandr Efimtsev, Olga Fionik, Tatyana Alekseeva, Konstantin Samochernych, Elena Gorbunova, Varvara Krasnikova, Albina Makhanova, Anatoliy Levchuk, and et al. 2022. "Functional Network Connectivity Reveals the Brain Functional Alterations in Breast Cancer Survivors" Journal of Clinical Medicine 11, no. 3: 617. https://doi.org/10.3390/jcm11030617
APA StyleBukkieva, T., Pospelova, M., Efimtsev, A., Fionik, O., Alekseeva, T., Samochernych, K., Gorbunova, E., Krasnikova, V., Makhanova, A., Levchuk, A., Trufanov, G., Combs, S., & Shevtsov, M. (2022). Functional Network Connectivity Reveals the Brain Functional Alterations in Breast Cancer Survivors. Journal of Clinical Medicine, 11(3), 617. https://doi.org/10.3390/jcm11030617









