Risk of Acute Anterior Uveitis in Ankylosing Spondylitis According to the Type of Tumor Necrosis Factor-Alpha Inhibitor and History of Uveitis: A Nationwide Population-Based Study
Abstract
:1. Introduction
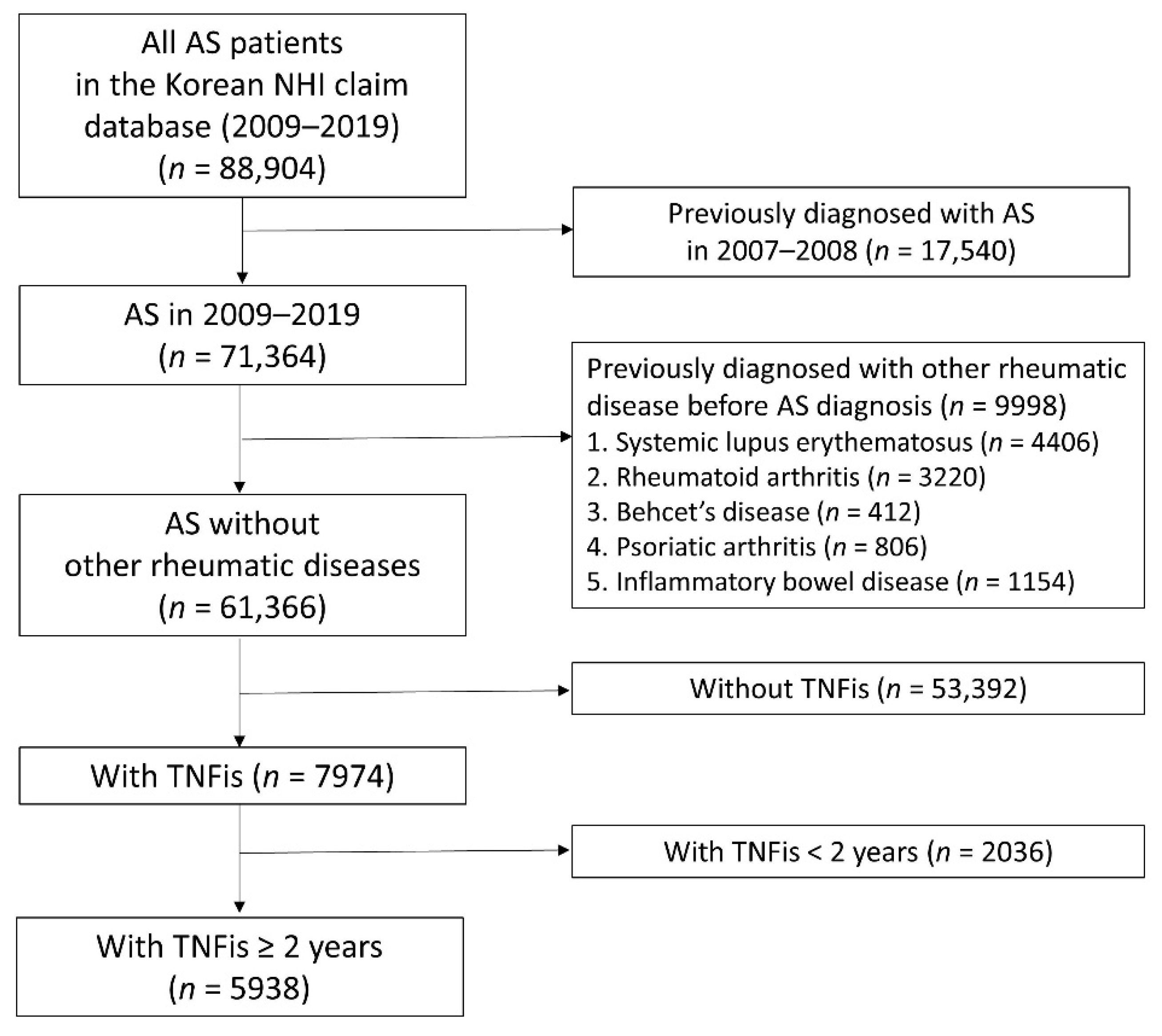
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures and Outcomes
2.3. Statistical Analysis
3. Results
3.1. Study Population
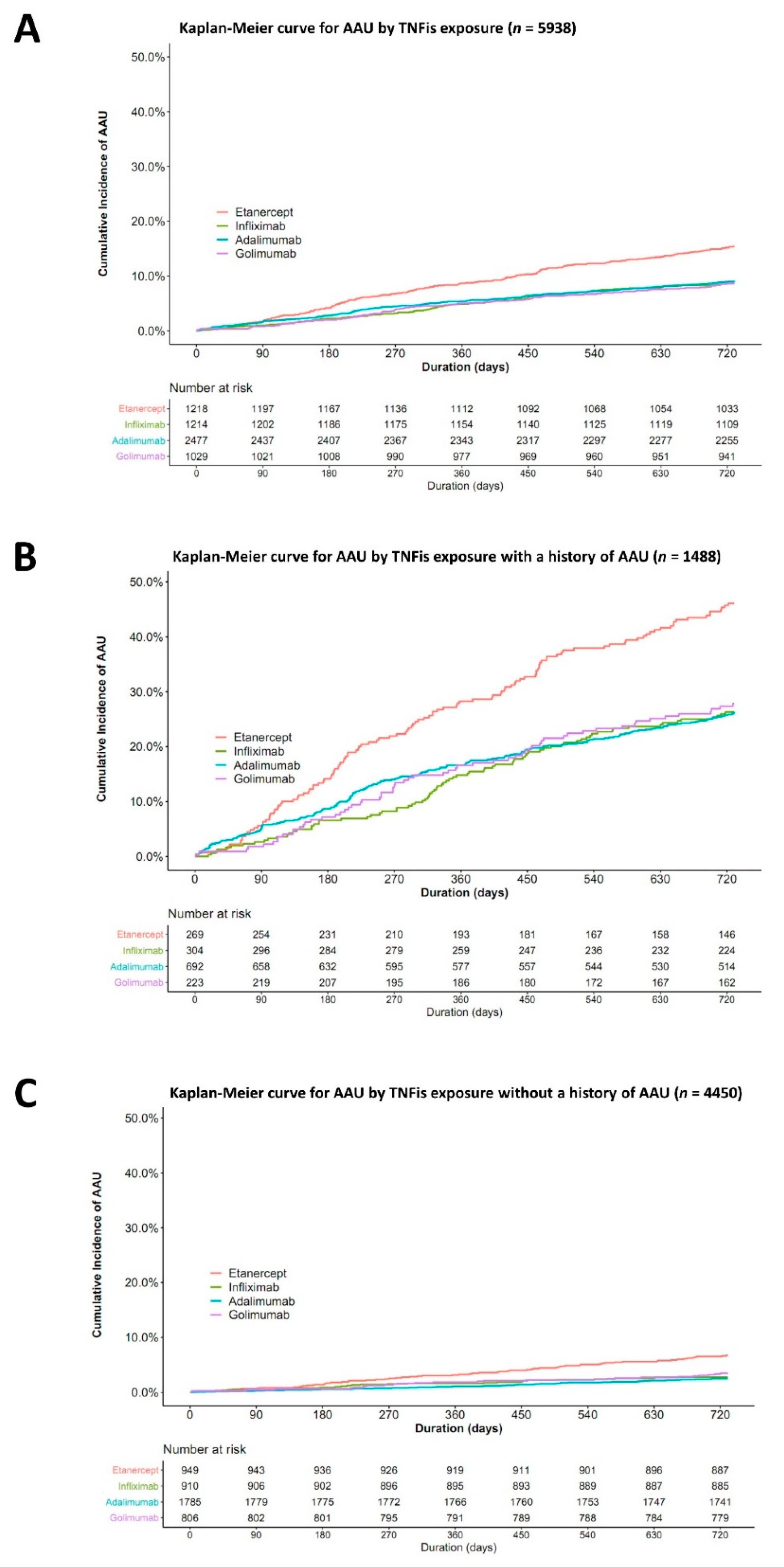
3.2. The Risk of AAU According to the Type of TNFis
3.3. Incidence of All AAU Events within 2 Years after the Initiation of TNF Inhibitors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taurog, J.D.; Chhabra, A.; Colbert, R.A. Ankylosing Spondylitis and Axial Spondyloarthritis. N. Engl. J. Med. 2016, 374, 2563–2574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stolwijk, C.; van Tubergen, A.; Castillo-Ortiz, J.D.; Boonen, A. Prevalence of extra-articular manifestations in patients with ankylosing spondylitis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabiani, C.; Vitale, A.; Orlando, I.; Capozzoli, M.; Fusco, F.; Rana, F.; Franceschini, R.; Sota, J.; Frediani, B.; Galeazzi, M.; et al. Impact of Uveitis on Quality of Life: A Prospective Study from a Tertiary Referral Rheumatology-Ophthalmology Collaborative Uveitis Center in Italy. Isr. Med. Assoc. J. 2017, 19, 478–483. [Google Scholar] [PubMed]
- Monaco, C.; Nanchahal, J.; Taylor, P.; Feldmann, M. Anti-TNF therapy: Past, present and future. Int. Immunol. 2014, 27, 55–62. [Google Scholar] [CrossRef]
- Braun, J.; Baraliakos, X.; Listing, J.; Sieper, J. Decreased incidence of anterior uveitis in patients with ankylosing spondylitis treated with the anti-tumor necrosis factor agents infliximab and etanercept. Arthritis Rheum. 2005, 52, 2447–2451. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Rødevand, E.; Holck, P.; Vanhoof, J.; Kron, M.; Kary, S.; Kupper, H. Adalimumab effectively reduces the rate of anterior uveitis flares in patients with active ankylosing spondylitis: Results of a prospective open-label study. Ann. Rheum. Dis. 2009, 68, 696–701. [Google Scholar] [CrossRef] [Green Version]
- Wendling, D.; Joshi, A.; Reilly, P.; Jalundhwala, Y.J.; Mittal, M.; Bao, Y. Comparing the risk of developing uveitis in patients initiating anti-tumor necrosis factor therapy for ankylosing spondylitis: An analysis of a large US claims database. Curr. Med. Res. Opin. 2014, 30, 2515–2521. [Google Scholar] [CrossRef]
- Wu, D.; Guo, Y.-Y.; Xu, N.-N.; Zhao, S.; Hou, L.-X.; Jiao, T.; Zhang, N. Efficacy of anti–tumor necrosis factor therapy for extra-articular manifestations in patients with ankylosing spondylitis: A meta–analysis. BMC Musculoskelet. Disord. 2015, 16, 19. [Google Scholar] [CrossRef] [Green Version]
- Lie, E.; Lindström, U.; Zverkova-Sandström, T.; Olsen, I.C.; Forsblad-d’Elia, H.; Askling, J.; Kapetanovic, M.C.; Kristensen, L.E.; Jacobsson, L.T.H. Tumour necrosis factor inhibitor treatment and occurrence of anterior uveitis in ankylosing spondylitis: Results from the Swedish biologics register. Ann. Rheum. Dis. 2017, 76, 1515–1521. [Google Scholar] [CrossRef]
- Khoury, G.; Morel, J.; Combe, B.; Lukas, C. Occurrence of anterior uveitis in patients with spondyloarthritis treated with tumor necrosis factor inhibitors: Comparing the soluble receptor to monoclonal antibodies in a large observational cohort. Arthritis Res. Ther. 2020, 22, 94. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.A.; Yoon, S.; Kim, L.Y.; Kim, D.S. Towards Actualizing the Value Potential of Korea Health Insurance Review and Assessment (HIRA) Data as a Resource for Health Research: Strengths, Limitations, Applications, and Strategies for Optimal Use of HIRA Data. J. Korean Med. Sci. 2017, 32, 718–728. [Google Scholar] [CrossRef] [PubMed]
- Park, E.H.; Lee, J.S.; Kim, Y.J.; Lee, S.M.; Jun, J.K.; Lee, E.B.; Kim, Y.G. Pregnancy outcomes in Korean women with ankylosing spondylitis. Korean J. Intern. Med. 2021, 36, 721–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Linden, S.; Valkenburg, H.A.; Cats, A. Evaluation of diagnostic criteria for ankylosing spondylitis. A proposal for modification of the New York criteria. Arthritis Rheum. 1984, 27, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Standardization of Uveitis Nomenclature for Reporting Clinical Data. Results of the First International Workshop. Am. J. Ophthalmol. 2005, 140, 509–516. [Google Scholar] [CrossRef]
- Charlson, M.E.; Pompei, P.; Ales, K.L.; MacKenzie, C.R. A new method of classifying prognostic comorbidity in longitudinal studies: Development and validation. J. Chronic Dis. 1987, 40, 373–383. [Google Scholar] [CrossRef]
- De Parisot, A.; Kodjikian, L.; Errera, M.-H.; Sedira, N.; Heron, E.; Pérard, L.; Cornut, P.-L.; Schneider, C.; Rivière, S.; Ollé, P.; et al. Randomized Controlled Trial Evaluating a Standardized Strategy for Uveitis Etiologic Diagnosis (ULISSE). Am. J. Ophthalmol. 2017, 178, 176–185. [Google Scholar] [CrossRef]
- Canoui-Poitrine, F.; Lekpa, F.K.; Farrenq, V.; Boissinot, V.; Hacquard-Bouder, C.; Comet, D.; Bastuji-Garin, S.; Thibout, E.; Claudepierre, P. Prevalence and factors associated with uveitis in spondylarthritis patients in France: Results from an observational survey. Arthritis Care Res. 2012, 64, 919–924. [Google Scholar] [CrossRef]
- Maxwell, L.J.; Zochling, J.; Boonen, A.; Singh, J.A.; Veras, M.M.S.; Tanjong Ghogomu, E.; Benkhalti Jandu, M.; Tugwell, P.; Wells, G.A. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst. Rev. 2015, 4, CD005468. [Google Scholar] [CrossRef]
- De Vos, A.F.; Van Haren, M.A.C.; Verhagen, C.; Hoekzema, R.; Kijlstra, A. Systemic anti-tumor necrosis factor antibody treatment exacerbates endotoxin-induced uveitis in the rat. Exp. Eye Res. 1995, 61, 667–675. [Google Scholar] [CrossRef]
- Kakkassery, V.; Mergler, S.; Pleyer, U. Anti-TNF-alpha treatment: A possible promoter in endogenous uveitis? Observational report on six patients: Occurrence of uveitis following etanercept treatment. Curr. Eye Res. 2010, 35, 751–756. [Google Scholar] [CrossRef]
- Kim, M.; Won, J.Y.; Choi, S.Y.; Ju, J.H.; Park, Y.H. Anti-TNFalpha Treatment for HLA-B27-Positive Ankylosing Spondylitis-Related Uveitis. Am. J. Ophthalmol. 2016, 170, 32–40. [Google Scholar] [CrossRef]
- Guignard, S.; Gossec, L.; Salliot, C.; Ruyssen-Witrand, A.; Luc, M.; Duclos, M.; Dougados, M. Efficacy of tumour necrosis factor blockers in reducing uveitis flares in patients with spondylarthropathy: A retrospective study. Ann. Rheum. Dis. 2006, 65, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, C.; Vitale, A.; Lopalco, G.; Iannone, F.; Frediani, B.; Cantarini, L. Different roles of TNF inhibitors in acute anterior uveitis associated with ankylosing spondylitis: State of the art. Clin. Rheumatol. 2016, 35, 2631–2638. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.; Backhouse, O.C. Does etanercept induce uveitis? Br. J. Ophthalmol. 2003, 87, 925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raffeiner, B.; Ometto, F.; Bernardi, L.; Botsios, C.; Punzi, L. Inefficacy or paradoxical effect? Uveitis in ankylosing spondylitis treated with etanercept. Case Rep. Med. 2014, 2014, 471319. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.Y.; Lee, M.; Lee, C.S. Uveitis occurrence in patients with ankylosing spondylitis according to the type of tumour necrosis factor inhibitor: A cohort study of 175 patients. Clin. Exp. Rheumatol. 2020, 38, 1132–1137. [Google Scholar]
- Furst, D.E.; Wallis, R.; Broder, M.; Beenhouwer, D.O. Tumor Necrosis Factor Antagonists: Different Kinetics and/or Mechanisms of Action May Explain Differences in the Risk for Developing Granulomatous Infection. Semin. Arthritis Rheum. 2006, 36, 159–167. [Google Scholar] [CrossRef]
- Nicolela Susanna, F.; Pavesio, C. A review of ocular adverse events of biological anti-TNF drugs. J. Ophthalmic Inflamm. Infect. 2020, 10, 11. [Google Scholar] [CrossRef]
- Scallon, B.; Cai, A.; Solowski, N.; Rosenberg, A.; Song, X.Y.; Shealy, D.; Wagner, C. Binding and functional comparisons of two types of tumor necrosis factor antagonists. J. Pharmacol. Exp. Ther. 2002, 301, 418–426. [Google Scholar] [CrossRef] [Green Version]
| Total (n = 5938) | ADA (n = 2477) | ETN (n = 1218) | IFX (n = 1214) | GOL (n = 1029) | p-Value | |
|---|---|---|---|---|---|---|
| Treatment duration, year | 4.7 ± 2.2 | 4.8 ± 2.3 | 4.8 ± 2.3 | 5.3 ± 2.3 | 3.6 ± 1.3 | <0.001 |
| Age, year | 37.2 ± 13.1 | 36.8 ± 13.2 | 37.6 ± 14.8 | 37.0 ± 13.1 | 38.0 ± 13.6 | 0.083 |
| Age group, year | 0.143 | |||||
| <30 | 1983 (33.4) | 839 (33.9) | 409 (33.6) | 405 (33.4) | 330 (32.1) | |
| 30–39 | 1537 (25.9) | 654 (26.4) | 286 (23.5) | 325 (26.8) | 272 (26.4) | |
| 40–49 | 1239 (20.9) | 522 (21.1) | 254 (20.9) | 251 (20.7) | 212 (20.6) | |
| 50–59 | 793 (13.4) | 326 (13.2) | 170 (14.0) | 161 (13.3) | 136 (13.2) | |
| 60< | 386 (6.5) | 136 (5.5) | 99 (8.2) | 72 (5.9) | 79 (7.6) | |
| Sex | 0.041 | |||||
| Male | 4610 (77.6) | 1959 (79.1) | 953 (78.2) | 915 (75.4) | 783 (76.1) | |
| Female | 1328 (22.4) | 518 (20.9) | 265 (21.8) | 299 (24.6) | 246 (23.9) | |
| Previous AAU history | 1488 (25.1) | 692 (27.9) | 269 (22.1) | 304 (25.0) | 223 (21.7) | <0.001 |
| Charlson comorbidity index | 0.53 ± 0.97 | 0.52 ± 0.93 | 0.61 ± 1.11 | 0.47 ± 0.84 | 0.57 ± 1.04 | 0.002 |
| 0 | 3939 (66.3) | 1648 (66.5) | 786 (64.5) | 825 (68.0) | 680 (66.1) | 0.001 |
| 1 to 2 | 1734 (29.2) | 730 (29.5) | 363 (29.8) | 356 (29.3) | 285 (27.7) | |
| 3 to 4 | 265 (4.5) | 99 (4.0) | 69 (5.7) | 33 (2.7) | 64 (6.2) | |
| Comorbidities (1 year prior to index date) | ||||||
| Dyslipidemia | 756 (12.7) | 307 (12.4) | 170 (14.0) | 154 (12.7) | 125 (12.1) | 0.528 |
| Hypertension | 702 (11.8) | 298 (12.0) | 145 (11.9) | 132 (10.9) | 127 (12.3) | 0.699 |
| Diabetes mellitus | 305 (5.1) | 127 (5.1) | 72 (5.9) | 53 (4.4) | 53 (5.2) | 0.394 |
| COPD | 226 (3.8) | 94 (3.8) | 55 (4.5) | 41 (3.4) | 36 (3.5) | 0.466 |
| Ischemic heart disease | 118 (2.0) | 54 (2.2) | 27 (2.2) | 18 (1.5) | 19 (1.8) | 0.477 |
| Psoriasis | 61 (1.0) | 26 (1.0) | 9 (0.7) | 14 (1.2) | 12 (1.2) | 0.707 |
| Stroke | 47 (0.8) | 19 (0.8) | 14 (1.1) | 6 (0.5) | 8 (0.8) | 0.337 |
| Renal failure | 25 (0.4) | 7 (0.3) | 10 (0.8) | 1 (0.1) | 7 (0.7) | 0.013 |
| Asthma | 21 (0.4) | 10 (0.4) | 7 (0.6) | 0 (0.0) | 4 (0.4) | 0.102 |
| Total (n = 5938) | ADA (n = 2477) | ETN (n = 1218) | IFX (n = 1214) | GOL (n = 1029) | p-Value | |
|---|---|---|---|---|---|---|
| Use of corticosteroid (≥90 days) | 2993 (50.4) | 1254 (50.6) | 611 (50.2) | 668 (55.0) | 460 (44.7) | <0.001 |
| Use of NSAIDs (≥0.7 PDC) | 2424 (40.8) | 939 (37.9) | 533 (43.8) | 535 (44.1) | 417 (40.5) | <0.001 |
| Immune modulating agents | 5117 (86.2) | 2214 (89.4) | 1016 (83.4) | 1082 (89.1) | 805 (78.2) | <0.001 |
| Sulfasalazine | 4792 (80.7) | 2082 (84.1) | 934 (76.7) | 1011 (83.3) | 765 (74.3) | <0.001 |
| Methotrexate | 1998 (33.6) | 872 (35.2) | 422 (34.6) | 449 (37.0) | 255 (24.8) | <0.001 |
| Cyclosporine | 192 (3.2) | 83 (3.4) | 34 (2.8) | 45 (3.7) | 30 (2.9) | 0.558 |
| Azathioprine | 84 (1.4) | 26 (1.0) | 16 (1.3) | 35 (2.9) | 7 (0.7) | <0.001 |
| Mycophenolate mofetil | 12 (0.2) | 6 (0.2) | 1 (0.1) | 5 (0.4) | 0 (0.0) | 0.122 |
| Cyclophosphamide | 2 (0.0) | 0 (0.0) | 0 (0.0) | 2 (0.2) | 0 (0.0) | 0.051 |
| Total | AAU Cases | Sum of py | IR (/100 py) | 95% CI | HR | 95% CI | p-Value | aHR * | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | 5938 | 609 | 11203 | 5.4 | 5.0–5.9 | ||||||
| Adalimumab | 2477 | 226 | 4696 | 4.8 | 4.2–5.5 | Ref | Ref | ||||
| Etanercept | 1218 | 188 | 2232 | 8.4 | 7.3–9.7 | 1.75 | 1.44–2.12 | <0.001 | 1.77 | 1.46–2.14 | <0.001 |
| Infliximab | 1214 | 105 | 2312 | 4.5 | 3.7–5.5 | 0.94 | 0.75–1.19 | 0.620 | 0.92 | 0.73–1.16 | 0.495 |
| Golimumab | 1029 | 90 | 1962 | 4.6 | 3.7–5.6 | 0.95 | 0.75–1.22 | 0.698 | 0.98 | 0.76–1.25 | 0.846 |
| AAU history (+) | 1488 | 448 | 2469 | 18.1 | 16.5–19.9 | ||||||
| Adalimumab | 692 | 182 | 1170 | 15.6 | 13.4–18.0 | Ref | Ref | ||||
| Etanercept | 269 | 124 | 398 | 31.2 | 25.9–37.2 | 1.99 | 1.58–2.50 | <0.001 | 1.99 | 1.58–2.50 | <0.001 |
| Infliximab | 304 | 80 | 523 | 15.3 | 12.1–19.0 | 0.98 | 0.75–1.27 | 0.876 | 0.97 | 0.74–1.26 | 0.807 |
| Golimumab | 223 | 62 | 379 | 16.4 | 12.6–21.0 | 1.05 | 0.79–1.40 | 0.739 | 1.07 | 0.80–1.43 | 0.647 |
| AAU history (−) | 4450 | 161 | 8733 | 1.8 | 1.6–2.2 | ||||||
| Adalimumab | 1785 | 44 | 3526 | 1.2 | 0.9–1.7 | Ref | Ref | ||||
| Etanercept | 949 | 64 | 1834 | 3.5 | 2.7–4.5 | 2.80 | 1.91–4.11 | <0.001 | 2.82 | 1.92–4.13 | <0.001 |
| Infliximab | 910 | 25 | 1789 | 1.4 | 0.9–2.1 | 1.12 | 0.69–1.83 | 0.651 | 1.10 | 0.67–1.79 | 0.709 |
| Golimumab | 806 | 28 | 1584 | 1.8 | 1.2–2.6 | 1.42 | 0.88–2.28 | 0.150 | 1.45 | 0.90–2.32 | 0.128 |
| AAU Cases | Sum of py | IR (/100 py) | 95% CI | IRR | 95% CI | p-Value | IRR * | 95% CI | p-Value | |
|---|---|---|---|---|---|---|---|---|---|---|
| Total | ||||||||||
| Adalimumab | 389 | 5612 | 6.8 | 5.9–7.8 | Ref | Ref | ||||
| Etanercept | 355 | 2760 | 12.4 | 10.7–14.3 | 1.82 | 1.49–2.22 | <0.001 | 1.78 | 1.46–2.18 | <0.001 |
| Infliximab | 168 | 2620 | 6.2 | 5.0–7.6 | 0.91 | 0.70–1.17 | 0.456 | 0.92 | 0.72–1.18 | 0.525 |
| Golimumab | 145 | 2232 | 6.5 | 5.3–8.0 | 0.96 | 0.75–1.22 | 0.712 | 0.92 | 0.72–1.18 | 0.512 |
| AAU history (+) | ||||||||||
| Adalimumab | 313 | 1549 | 19.9 | 17.2–22.9 | Ref | Ref | ||||
| Etanercept | 230 | 602 | 37.6 | 32.5–43.6 | 1.89 | 1.54–2.33 | <0.001 | 1.86 | 1.51–2.29 | <0.001 |
| Infliximab | 132 | 652 | 19.8 | 16.0–24.5 | 1.00 | 0.77–1.29 | 0.985 | 1.00 | 0.78–1.30 | 0.986 |
| Golimumab | 106 | 488 | 21.4 | 17.2–26.8 | 1.08 | 0.83–1.40 | 0.568 | 1.05 | 0.81–1.36 | 0.702 |
| AAU history (−) | ||||||||||
| Adalimumab | 76 | 4063 | 1.9 | 1.4–2.5 | Ref | Ref | ||||
| Etanercept | 125 | 2159 | 5.5 | 4.3–7.1 | 2.98 | 2.04–4.37 | <0.001 | 2.92 | 2.00–4.26 | <0.001 |
| Infliximab | 36 | 1969 | 1.8 | 1.2–2.6 | 0.95 | 0.57–1.58 | 0.852 | 0.97 | 0.59–1.60 | 0.920 |
| Golimumab | 39 | 1745 | 2.3 | 1.6–3.3 | 1.22 | 0.76–1.95 | 0.417 | 1.16 | 0.72–1.87 | 0.554 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, S.M.; Kim, M.; Kim, Y.-J.; Lee, Y.; Kim, Y.-G. Risk of Acute Anterior Uveitis in Ankylosing Spondylitis According to the Type of Tumor Necrosis Factor-Alpha Inhibitor and History of Uveitis: A Nationwide Population-Based Study. J. Clin. Med. 2022, 11, 631. https://doi.org/10.3390/jcm11030631
Ahn SM, Kim M, Kim Y-J, Lee Y, Kim Y-G. Risk of Acute Anterior Uveitis in Ankylosing Spondylitis According to the Type of Tumor Necrosis Factor-Alpha Inhibitor and History of Uveitis: A Nationwide Population-Based Study. Journal of Clinical Medicine. 2022; 11(3):631. https://doi.org/10.3390/jcm11030631
Chicago/Turabian StyleAhn, Soo Min, Minju Kim, Ye-Jee Kim, Yusun Lee, and Yong-Gil Kim. 2022. "Risk of Acute Anterior Uveitis in Ankylosing Spondylitis According to the Type of Tumor Necrosis Factor-Alpha Inhibitor and History of Uveitis: A Nationwide Population-Based Study" Journal of Clinical Medicine 11, no. 3: 631. https://doi.org/10.3390/jcm11030631
APA StyleAhn, S. M., Kim, M., Kim, Y.-J., Lee, Y., & Kim, Y.-G. (2022). Risk of Acute Anterior Uveitis in Ankylosing Spondylitis According to the Type of Tumor Necrosis Factor-Alpha Inhibitor and History of Uveitis: A Nationwide Population-Based Study. Journal of Clinical Medicine, 11(3), 631. https://doi.org/10.3390/jcm11030631







