Crosstalk between Irisin Levels, Liver Fibrogenesis and Liver Damage in Non-Obese, Non-Diabetic Individuals with Non-Alcoholic Fatty Liver Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Analytical Determinations
2.3. Histology
2.4. Statistical Analysis
3. Results
3.1. Histological Features Versus Circulating Irisin and Biomarkers of Fibrogenesis
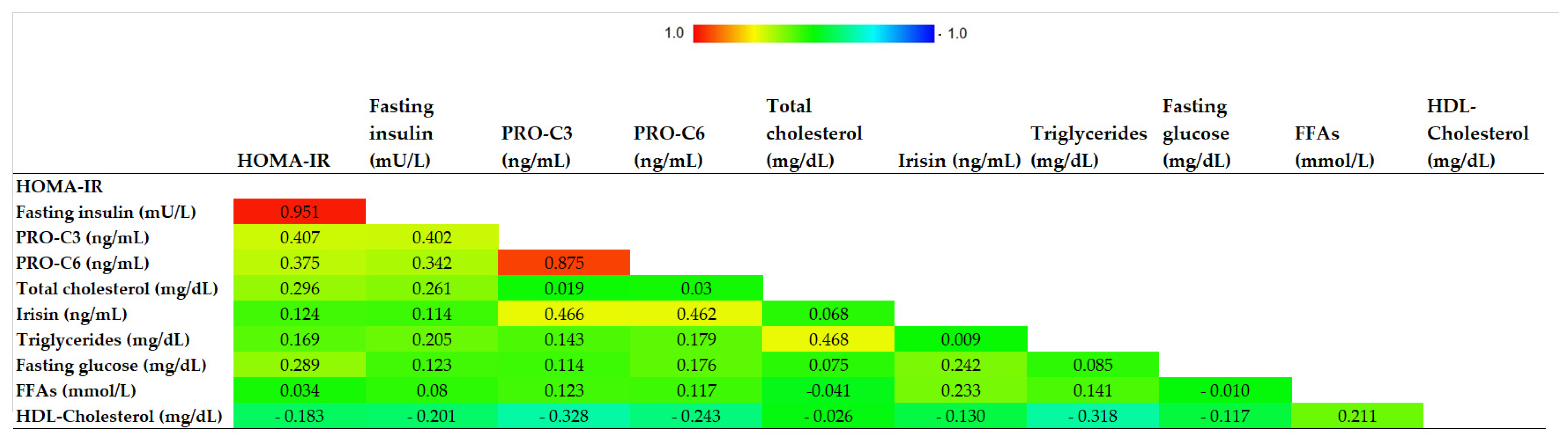
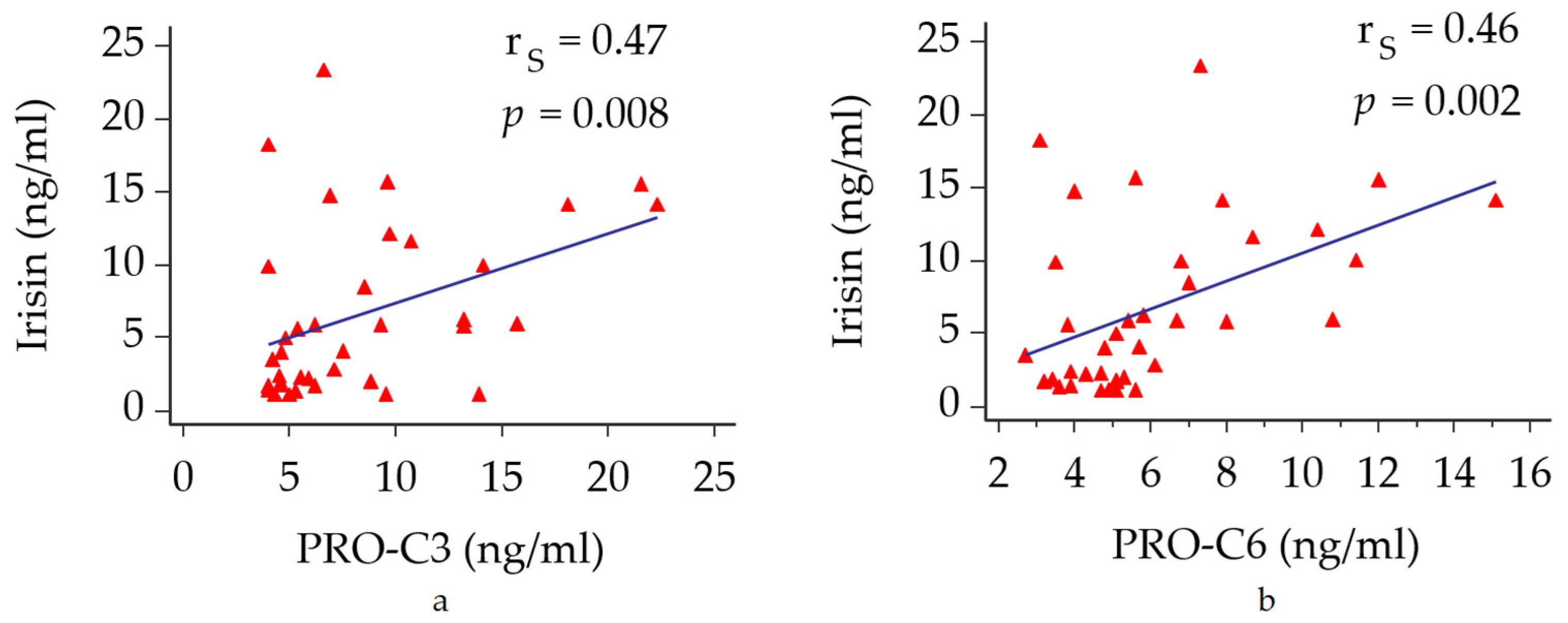
3.2. Circulating Irisin Versus Metabolic Profile and Biomarkers of Fibrogenesis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Hagström, H.; Nasr, P.; Ekstedt, M.; Hammar, U.; Stål, P.; Hultcrantz, R.; Kechagias, S. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J. Hepatol. 2017, 67, 1265–1273. [Google Scholar] [CrossRef]
- Armandi, A.; Rosso, C.; Caviglia, G.P.; Bugianesi, E. Insulin Resistance across the Spectrum of Nonalcoholic Fatty Liver Disease. Metabolites 2021, 11, 155. [Google Scholar] [CrossRef] [PubMed]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012, 11, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Huh, J.Y.; Dincer, F.; Mesfum, E.; Mantzoros, C.S. Irisin stimulates muscle growth-related genes and regulates adipocyte differentiation and metabolism in humans. Int. J. Obes. 2014, 38, 1538–1544. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Chen, P.; Jin, H.; Xie, X.; Gao, T.; Yang, L.; Yu, X. Circulating levels of irisin in middle-aged first-degree relatives of type 2 diabetes mellitus—Correlation with pancreatic β-cell function. Diabetol. Metab. Syndr. 2014, 5, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, T.; Shi, C.; Gao, R.; Sun, H.; Xiong, X.; Ding, L.; Chen, Q.; Li, Y.H.; Wang, J.J.; Kang, Y.M.; et al. Irisin inhibits hepatic gluconeogenesis and increases glycogen synthesis via the PI3K/Akt pathway in type 2 diabetic mice and hepatocytes. Clin. Sci. 2015, 129, 839–850. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Yu, R.; Liu, S.; Huwatibieke, B.; Li, Z.; Zhang, W. Irisin Inhibits Hepatic Cholesterol Synthesis via AMPK-SREBP2 Signaling. EBioMedicine 2016, 6, 139–148. [Google Scholar] [CrossRef] [Green Version]
- Batirel, S.; Bozaykut, P.; Altundag, E.M.; Ozer, N.K.; Mantzoros, C.S. The effect of Irisin on antioxidant system in liver. Free Radic. Biol. Med. 2014, 75, S16. [Google Scholar] [CrossRef]
- Zhang, H.-J.; Zhang, X.-F.; Ma, Z.-M.; Pan, L.-L.; Chen, Z.; Han, H.-W.; Han, C.-K.; Zhuang, X.-J.; Lu, Y.; Li, X.-J.; et al. Irisin is inversely associated with intrahepatic triglyceride contents in obese adults. J. Hepatol. 2013, 59, 557–562. [Google Scholar] [CrossRef]
- Metwally, M.; Bayoumi, A.; Romero-Gomez, M.; Thabet, K.; John, M.; Adams, L.A.; Huo, X.; Aller, R.; García-Monzón, C.; Arias-Loste, M.T.; et al. A polymorphism in the Irisin-encoding gene (FNDC5) associates with hepatic steatosis by differential miRNA binding to the 3′UTR. J. Hepatol. 2019, 70, 494–500. [Google Scholar] [CrossRef]
- Petta, S.; Valenti, L.; Svegliati-Baroni, G.; Ruscica, M.; Pipitone, M.R.; Dongiovanni, P.; Rychlicki, C.; Ferri, N.; Cammà, C.; Fracanzani, A.L.; et al. Fibronectin Type III Domain-Containing Protein 5 rs3480 A > G Polymorphism, Irisin, and Liver Fibrosis in Patients with Nonalcoholic Fatty Liver Disease. J. Clin. Endocrinol. Metab. 2017, 102, 2660–2669. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.; Ke, Y.; Wu, F.; Liu, S.; Ji, C.; Zhu, X.; Zhang, Y. Circulating Irisin Levels in Patients with Nonalcoholic Fatty Liver Disease: A Systematic Review and Meta-Analysis. Gastroenterol. Res. Pract. 2020, 2020, 8818191. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bril, F.; Leeming, D.J.; Karsdal, M.A.; Kalavalapalli, S.; Barb, D.; Lai, J.; Rabe, M.; Cusi, K. Use of Plasma Fragments of Propeptides of Type III, V, and VI Procollagen for the Detection of Liver Fibrosis in Type 2 Diabetes. Diabetes Care 2019, 42, 1348–1351. [Google Scholar] [CrossRef]
- Caussy, C.; Bhargava, M.; Villesen, I.F.; Gudmann, N.S.; Leeming, D.J.; Karsdal, M.A.; Faulkner, C.; Bao, D.; Liu, A.; Lo, M.; et al. Collagen Formation Assessed by N-Terminal Propeptide of Type 3 Procollagen Is a Heritable Trait and Is Associated with Liver Fibrosis Assessed by Magnetic Resonance Elastography. Hepatology 2019, 70, 127–141. [Google Scholar] [CrossRef]
- Kleiner, D.E.; Brunt, E.M.; Van Natta, M.; Behling, C.; Contos, M.J.; Cummings, O.W.; Ferrell, L.D.; Liu, Y.-C.; Torbenson, M.S.; Unalp-Arida, A.; et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 2005, 41, 1313–1321. [Google Scholar] [CrossRef]
- Kurdiova, T.; Balaz, M.; Vician, M.; Maderova, D.; Vlcek, M.; Valkovič, L.; Srbecky, M.; Imrich, R.; Kyselovicova, O.; Belan, V.; et al. Effects of obesity, diabetes and exercise on Fndc5 gene expression and irisin release in human skeletal muscle and adipose tissue: In vivo and in vitro studies. J. Physiol. 2014, 592, 1091–1107. [Google Scholar] [CrossRef]
- Mo, L.; Shen, J.; Liu, Q.; Zhang, Y.; Kuang, J.; Pu, S.; Cheng, S.; Zou, M.; Jiang, W.; Jiang, C.; et al. Irisin Is Regulated by CAR in Liver and Is a Mediator of Hepatic Glucose and Lipid Metabolism. Mol. Endocrinol. 2016, 30, 533–542. [Google Scholar] [CrossRef] [Green Version]
- Kukla, M.; Skladany, L.; Menżyk, T.; Derra, A.; Stygar, D.; Skonieczna, M.; Hudy, D.; Nabrdalik, K.; Gumprecht, J.; Marlicz, W.; et al. Irisin in Liver Cirrhosis. J. Clin. Med. 2020, 9, 3158. [Google Scholar] [CrossRef]
- Zhao, M.; Zhou, X.; Yuan, C.; Li, R.; Ma, Y.; Tang, X. Association between serum irisin concentrations and sarcopenia in patients with liver cirrhosis: A cross-sectional study. Sci. Rep. 2020, 10, 16093. [Google Scholar] [CrossRef]
- Moreno-Navarrete, J.M.; Ortega, F.; Serrano, M.; Guerra, E.; Pardo, G.; Tinahones, F. Irisin is expressed and produced by human muscle and adipose tissue in association with obesity and insulin resistance. J. Clin. Endocrinol. Metab. 2013, 98, E769–E778. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Cai, X.; Yin, H.; Zügel, M.; Sun, Z.; Steinacker, J.M.; Schumann, U. Association between circulating irisin and insulin resistance in non-diabetic adults: A meta-analysis. Metabolism 2016, 65, 825–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moreno, M.; Moreno-Navarrete, J.M.; Serrano, M.; Ortega, F.; Delgado, E.; Sanchez-Ragnarsson, C.; Valdés, S.; Botas, P.; Ricart, W.; Fernández-Real, J.M. Circulating irisin levels are positively associated with metabolic risk factors in sedentary subjects. PLoS ONE 2015, 10, e0124100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aronis, K.N.; Moreno, M.; Polyzos, S.A.; Moreno-Navarrete, J.M.; Ricart, W.; Delgado, E.; de La Hera, J.; Sahin-Efe, A.; Chamberland, J.P.; Berman, R.; et al. Circulating irisin levels and coronary heart disease: Association with future acute coronary syndrome and major adverse cardiovascular events. Int. J. Obes. 2015, 39, 156–161. [Google Scholar] [CrossRef]
- Chang, J.S.; Kim, T.H.; Nguyen, T.T.; Park, K.-S.; Kim, N.; Kong, I.D. Circulating irisin levels as a predictive biomarker for sarcopenia: A cross-sectional community-based study. Geriatr. Gerontol. Int. 2017, 17, 2266–2273. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.Y.; Kim, S.; Park, J.W.; Lee, N.S.; Hwang, S.Y.; Huh, J.Y.; Hong, H.C.; Yoo, H.J.; Baik, S.H.; Youn, B.S.; et al. Implication of Circulating Irisin Levels with Brown Adipose Tissue and Sarcopenia in Humans. J. Clin. Endocrinol. Metab. 2014, 99, 2778–2785. [Google Scholar] [CrossRef] [Green Version]
| Variables | All (n = 41) | F0/F1 (n = 26) | F ≥ 2 (n = 15) | p Value |
|---|---|---|---|---|
| Age (years), median (95% CI) | 45 (41–51) | 44 (38–48) | 51 (38–64) | 0.068 |
| BMI (kg/m2), median (95% CI) | 25.7 (24.6–26.6) | 25.6 (23.6–27.6) | 25.7 (20.1–26.4) | 0.705 |
| Male/Female gender, n (%) | 33/8 (80.4/19.6) | 24/4 (85.7/14.3) | 9/4 (69.2/30.8) | 0.221 |
| AST (IU/L), median (95% CI) | 31 (28–36) | 31 (26-35) | 31 (25-58) | 0.424 |
| ALT (IU/L), median (95% CI) | 48 (41–67) | 47 (41–70) | 53 (27–99) | 0.801 |
| Platelets (×109/L), median (95% CI) | 230 (216–261) | 230 (206–270) | 218 (201–283) | 0.889 |
| Insulin (mU/L), median (95% CI) | 10.2 (9–11.8) | 9.7 (8.1–10.5) | 12.2 (10.1–17) | 0.004 |
| Glucose (mg/dL), median (95% CI) | 94 (90–98) | 92 (90–97) | 97 (89–121) | 0.165 |
| HOMA-IR | 2.5 (2.08–2.73) | 2.15 (1.71–2.4) | 2.92 (2.26–3.6) | 0.012 |
| HOMA-IR ≥ 2.5, n (%) | 16 (39) | 7 (25) | 9 (69) | 0.008 |
| Total-Chol (mg/dL), median (95% CI) | 184 (177–200) | 184 (175–201) | 190 (178–213) | 0.268 |
| HDL-Chol (mg/dL), median (95% CI) | 46 (42–49) | 47 (41–50) | 44 (39–51) | 0.492 |
| Triglycerides (mg/dL), median (95% CI) | 100 (86–117) | 93 (79–118) | 116 (90–178) | 0.179 |
| FFAs (mmol/L), mean (sd) | 0.627 ± 0.225 | 0.61 ± 0.24 | 0.66 ± 0.2 | 0.449 |
| Irisin (ng/mL), median (95% CI) | 5.8 (2.87–5.96) | 2.42 (1.73–5.95) | 5.96 (4.42–9.19) | 0.033 |
| PRO-C3 (ng/mL), median (95% CI) | 8.65 (6.32–9.64) | 6.2 (4.9–8.9) | 9.5 (7.7–13.6) | 0.016 |
| PRO-C6 (ng/mL), median (95% CI) | 5.6 (5.1–6.74) | 5.1 (4.2–5.4) | 6.6 (5.6–7.9) | 0.013 |
| Histological Features | All (n = 41) | F0/F1 (n = 26) | F ≥ 2 (n = 15) | p Value |
|---|---|---|---|---|
| Hepatic steatosis (%), median (95% CI) | 25 (10–40) | 22 (10–40) | 32 (15–45) | 0.583 |
| Lobular Inflammation (0/1/2), n (%) | 8/31/2 (19/76/5) | 6/20/- (23/77/-) | 2/11/2 (13/74/13) | 0.005 |
| Ballooning (0/1/2), n (%) | 4/21/16 (10/51/39) | 3/14/9 (11/54/35) | 1/7/7 (6/47/47) | 0.091 |
| NASH, n (%) | 31 (76) | 19 (73) | 12 (80) | 0.623 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Armandi, A.; Rosso, C.; Nicolosi, A.; Caviglia, G.P.; Abate, M.L.; Olivero, A.; D’Amato, D.; Vernero, M.; Gaggini, M.; Saracco, G.M.; et al. Crosstalk between Irisin Levels, Liver Fibrogenesis and Liver Damage in Non-Obese, Non-Diabetic Individuals with Non-Alcoholic Fatty Liver Disease. J. Clin. Med. 2022, 11, 635. https://doi.org/10.3390/jcm11030635
Armandi A, Rosso C, Nicolosi A, Caviglia GP, Abate ML, Olivero A, D’Amato D, Vernero M, Gaggini M, Saracco GM, et al. Crosstalk between Irisin Levels, Liver Fibrogenesis and Liver Damage in Non-Obese, Non-Diabetic Individuals with Non-Alcoholic Fatty Liver Disease. Journal of Clinical Medicine. 2022; 11(3):635. https://doi.org/10.3390/jcm11030635
Chicago/Turabian StyleArmandi, Angelo, Chiara Rosso, Aurora Nicolosi, Gian Paolo Caviglia, Maria Lorena Abate, Antonella Olivero, Daphne D’Amato, Marta Vernero, Melania Gaggini, Giorgio Maria Saracco, and et al. 2022. "Crosstalk between Irisin Levels, Liver Fibrogenesis and Liver Damage in Non-Obese, Non-Diabetic Individuals with Non-Alcoholic Fatty Liver Disease" Journal of Clinical Medicine 11, no. 3: 635. https://doi.org/10.3390/jcm11030635
APA StyleArmandi, A., Rosso, C., Nicolosi, A., Caviglia, G. P., Abate, M. L., Olivero, A., D’Amato, D., Vernero, M., Gaggini, M., Saracco, G. M., Ribaldone, D. G., Leeming, D. J., Gastaldelli, A., & Bugianesi, E. (2022). Crosstalk between Irisin Levels, Liver Fibrogenesis and Liver Damage in Non-Obese, Non-Diabetic Individuals with Non-Alcoholic Fatty Liver Disease. Journal of Clinical Medicine, 11(3), 635. https://doi.org/10.3390/jcm11030635











