Neural Substrates of Transcutaneous Spinal Cord Stimulation: Neuromodulation across Multiple Segments of the Spinal Cord
Abstract
:1. Introduction
2. Historical Perspective
3. Properties of Transcutaneous Spinal Cord Stimulation (tSCS)
3.1. Parameters of tSCS
3.2. Current Flow Involved in tSCS
3.3. Transcutaneous SCS Carrier Frequency Is Important for Reducing Discomfort, but Its Role in Restoring Motor Function Remains Unclear
3.4. Mechanisms of tSCS Recruitment
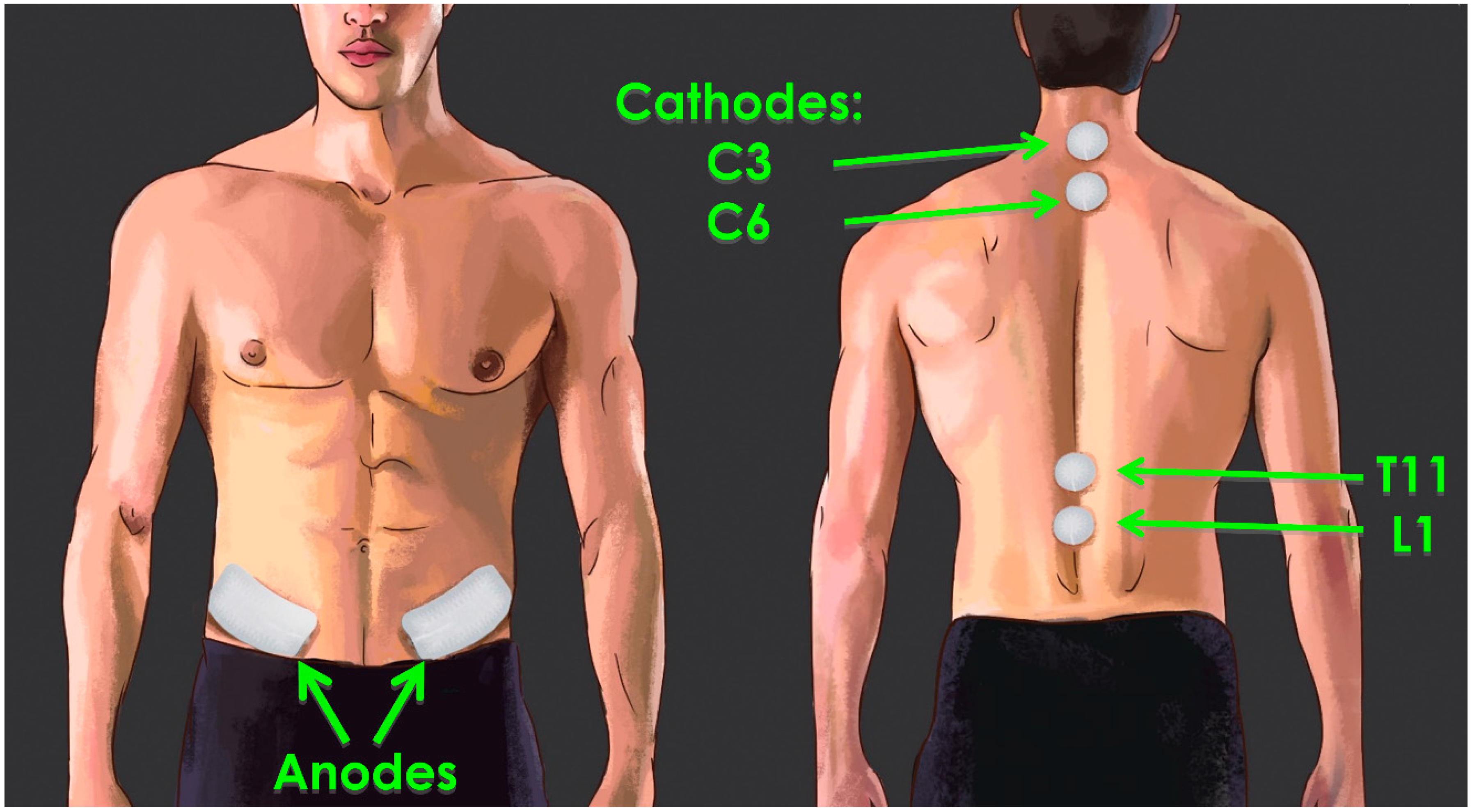
4. Transcutaneous SCS Alters Excitability across Multiple Levels of the Spinal Cord
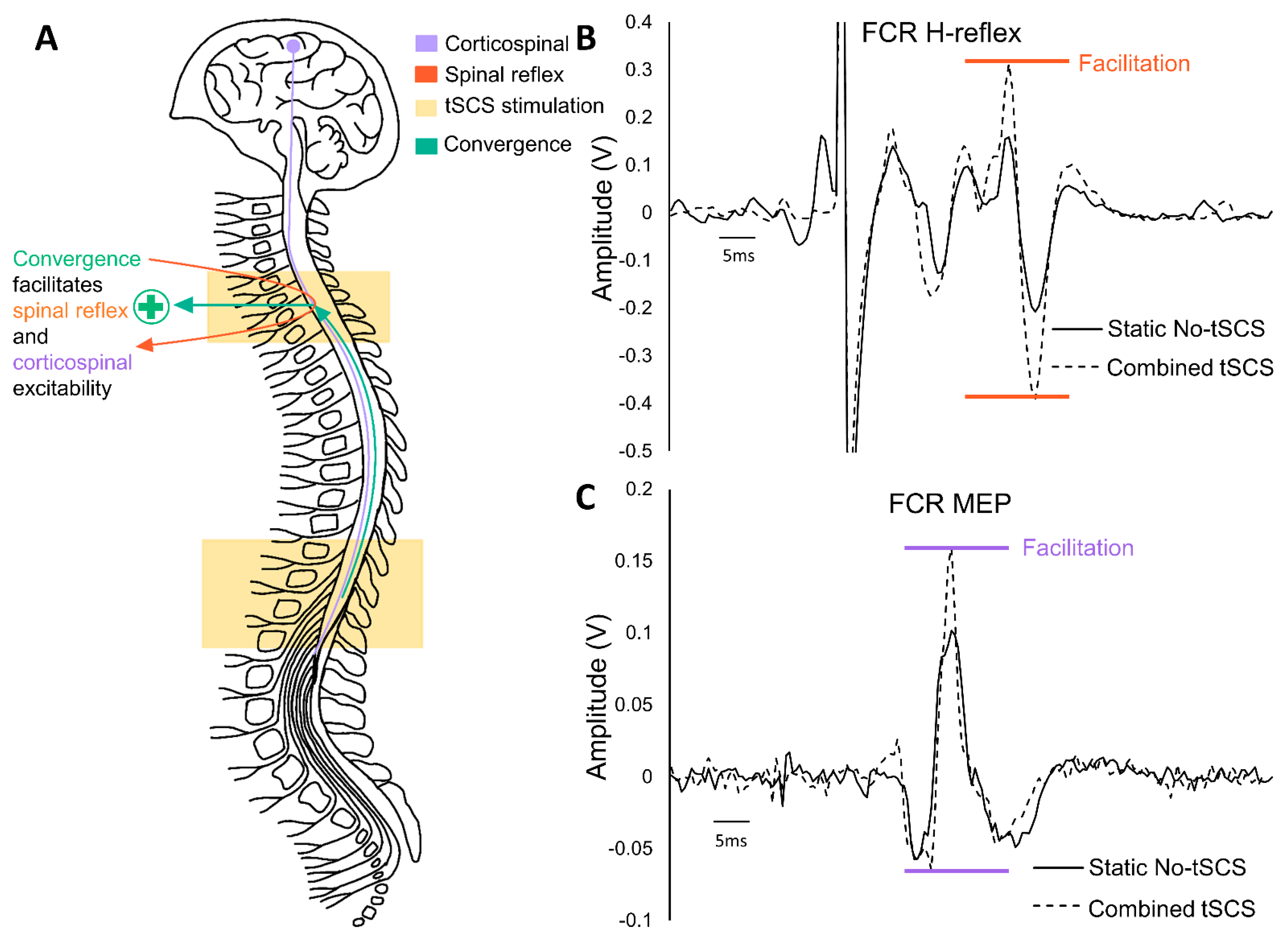
5. Multiple Sites of tSCS Converge to Facilitate Alterations in Excitability
6. Is There a Role for tSCS to Facilitate Cervicolumbar Coupling to Improve Walking?
7. Trunk Stability Improvements with tSCS
8. Previously Developed Rehabilitative Approaches Are Enhanced through tSCS
9. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Balykin, M.V.; Yakupov, R.N.; Mashin, V.V.; Kotova, E.Y.; Balykin, Y.M.; Gerasimenko, Y.P. The influence of non-invasive electrical stimulation of the spinal cord on the locomotor function of patients presenting with movement disorders of central genesis. Vopr. Kurortol. Fizioter. Lech. Fiz. Kult. 2017, 94, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Inanici, F.; Samejima, S.; Gad, P.; Edgerton, V.R.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous electrical spinal stimulation promotes long-term recovery of upper extremity function in chronic tetraplegia. IEEE Trans. Neural Syst. Rehabil. Eng. 2018, 26, 1272–1278. [Google Scholar] [CrossRef] [PubMed]
- Harkema, S.; Gerasimenko, Y.; Hodes, J.; Burdick, J.; Angeli, C.; Chen, Y.; Ferreira, C.; Willhite, A.; Rejc, E.; Grossman, R.; et al. Effect of Epidural stimulation of the lumbosacral spinal cord on voluntary movement, standing, and assisted stepping after motor complete paraplegia: A case study. Lancet 2011, 377, 1938–1947. [Google Scholar] [CrossRef] [Green Version]
- Angeli, C.A.; Boakye, M.; Morton, R.A.; Vogt, J.; Benton, K.; Chen, Y.; Ferreira, C.K.; Harkema, S.J. Recovery of over-ground walking after chronic motor complete spinal cord injury. N. Engl. J. Med. 2018, 379, 1244–1250. [Google Scholar] [CrossRef] [PubMed]
- Fregni, F.; Grecco, L.; Li, S.; Michel, S.; Castillo-Saavedra, L.; Mourdoukoutas, A.; Bikson, M. Transcutaneous spinal stimulation as a therapeutic strategy for spinal cord injury: State of the art. J. Neurorestoratol. 2015, 3, 73–82. [Google Scholar] [CrossRef] [Green Version]
- Dimitrijević, M.R. Residual motor functions in spinal cord injury. Adv. Neurol. 1988, 47, 138–155. [Google Scholar]
- Courtine, G.; Roy, R.R.; Raven, J.; Hodgson, J.; Mckay, H.; Yang, H.; Zhong, H.; Tuszynski, M.H.; Edgerton, V.R. Performance of locomotion and foot grasping following a unilateral thoracic corticospinal tract lesion in monkeys (Macaca mulatta). Brain 2005, 128, 2338–2358. [Google Scholar] [CrossRef] [Green Version]
- Friedli, L.; Rosenzweig, E.S.; Barraud, Q.; Schubert, M.; Dominici, N.; Awai, L.; Nielson, J.L.; Musienko, P.; Nout-Lomas, Y.; Zhong, H.; et al. Pronounced species divergence in corticospinal tract reorganization and functional recovery after lateralized spinal cord injury favors primates. Sci. Transl. Med. 2015, 7, 302ra134. [Google Scholar] [CrossRef] [Green Version]
- Rosenzweig, E.S.; Courtine, G.; Jindrich, D.L.; Brock, J.H.; Ferguson, A.R.; Strand, S.C.; Nout, Y.S.; Roy, R.R.; Miller, D.M.; Beattie, M.S.; et al. Extensive spontaneous plasticity of corticospinal projections after primate spinal cord injury. Nat. Neurosci. 2010, 13, 1505–1512. [Google Scholar] [CrossRef]
- Courtine, G.; Song, B.; Roy, R.R.; Zhong, H.; Herrmann, J.E.; Ao, Y.; Qi, J.; Edgerton, V.R.; Sofroniew, M.V. Recovery of supraspinal control of stepping via indirect propriospinal relay connections after spinal cord injury. Nat. Med. 2008, 14, 69–74. [Google Scholar] [CrossRef]
- Gerasimenko, Y.; Musienko, P.; Bogacheva, I.; Moshonkina, T.; Savochin, A.; Lavrov, I.; Roy, R.R.; Edgerton, V.R. Propriospinal bypass of the serotonergic system that can facilitate stepping. J. Neurosci. 2009, 29, 5681–5689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asboth, L.; Friedli, L.; Beauparlant, J.; Martinez-Gonzalez, C.; Anil, S.; Rey, E.; Baud, L.; Pidpruzhnykova, G.; Anderson, M.A.; Shkorbatova, P.; et al. Cortico-reticulo-spinal circuit reorganization enables functional recovery after severe spinal cord contusion. Nat. Neurosci. 2018, 21, 576–588. [Google Scholar] [CrossRef] [PubMed]
- Mayr, W.; Krenn, M.; Dimitrijevic, M.R. Epidural and transcutaneous spinal electrical stimulation for restoration of movement after incomplete and complete spinal cord injury. Curr. Opin. Neurol. 2016, 29, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Formento, E.; Minassian, K.; Wagner, F.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Rowald, A.; Bloch, J.; Micera, S.; Capogrosso, M.; Courtine, G. Electrical spinal cord stimulation must preserve proprioception to enable locomotion in humans with spinal cord injury. Nat. Neurosci. 2018, 21, 1728–1741. [Google Scholar] [CrossRef]
- Herman, R.; He, J.; D’Luzansky, S.; Willis, W.; Dilli, S. Spinal cord stimulation facilitates functional walking in a chronic, incomplete spinal cord injured. Spinal Cord 2002, 40, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Wagner, F.B.; Mignardot, J.B.; Le Goff-Mignardot, C.G.; Demesmaeker, R.; Komi, S.; Capogrosso, M.; Rowald, A.; Seáñez, I.; Caban, M.; Pirondini, E.; et al. Targeted neurotechnology restores walking in humans with spinal cord injury. Nature 2018, 563, 65–93. [Google Scholar] [CrossRef]
- Kou, J.; Cai, M.; Xie, F.; Wang, Y.; Wang, N.; Xu, M. Complex Electrical Stimulation Systems in Motor Function Rehabilitation after Spinal Cord Injury. Hindawi 2021, 2021, 2214762. [Google Scholar] [CrossRef]
- Gill, M.L.; Grahn, P.J.; Calvert, J.S.; Linde, M.B.; Lavrov, I.A.; Strommen, J.A.; Beck, L.A.; Sayenko, D.G.; Van Straaten, M.G.; Drubach, D.I.; et al. Neuromodulation of lumbosacral spinal networks enables independent stepping after complete paraplegia. Nat. Med. 2018, 24, 1677–1682. [Google Scholar] [CrossRef]
- Courtine, G.; Gerasimenko, Y.; Van Den Brand, R.; Yew, A.; Zhong, H.; Song, B.; Ao, Y.; Ichiyama, R.M.; Lavrov, I.; Roy, R.R.; et al. Transformation of nonfunctional spinal circuits into functional states after the loss of brain input. Nat. Neurosci. 2009, 12, 1333–1342. [Google Scholar] [CrossRef]
- Angeli, C.A.; Edgerton, V.R.; Gerasimenko, Y.P.; Harkema, S.J. Altering spinal cord excitability enables voluntary movements after chronic complete paralysis in humans. Brain 2014, 137, 1394–1409. [Google Scholar] [CrossRef] [Green Version]
- García, A.M.; Serrano-Muñoz, D.; Taylor, J.; Avendaño-Coy, J.; Gómez-Soriano, J. Transcutaneous Spinal Cord Stimulation and Motor Rehabilitation in Spinal Cord Injury: A Systematic Review. Neurorehabil. Neural Repair 2020, 34, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Gerasimenko, Y.; Gorodnichev, R.; Moshonkina, T.; Sayenko, D.; Gad, P.; Edgerton, V.R. Transcutaneous electrical spinal-cord stimulation in humans HHS Public Access. Ann. Phys. Rehabil. Med. 2015, 58, 225–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin, R. Utility and Feasibility of Transcutaneous Spinal Cord Stimulation for Patients with Incomplete SCI in Therapeutic Settings: A Review of Topic. Front. Rehabil. Sci. 2021, 2, 724003. [Google Scholar] [CrossRef]
- Hofstoetter, U.S.; Freundl, B.; Binder, H.; Minassian, K. Common neural structures activated by epidural and transcutaneous lumbar spinal cord stimulation: Elicitation of posterior root-muscle reflexes. PLoS ONE 2018, 13, e0192013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ladenbauer, J.; Minassian, K.; Hofstoetter, U.S.; Dimitrijevic, M.R.; Rattay, F. Stimulation of the human lumbar spinal cord with implanted and surface electrodes: A computer simulation study. IEEE Trans. Neural Syst. Rehabil. Eng. 2010, 18, 637–645. [Google Scholar] [CrossRef]
- Danner, S.M.; Hofstoetter, U.S.; Ladenbauer, J.; Rattay, F.; Minassian, K. Can the Human Lumbar Posterior Columns Be Stimulated by Transcutaneous Spinal Cord Stimulation? A Modeling Study. Artif. Organs 2011, 35, 257–262. [Google Scholar] [CrossRef]
- Gad, P.; Lee, S.; Terrafranca, N.; Zhong, H.; Turner, A.; Gerasimenko, Y.; Edgerton, V.R. Non-invasive activation of cervical spinal networks after severe paralysis. J. Neurotrauma 2018, 35, 2145–2158. [Google Scholar] [CrossRef]
- Zheng, Y.; Hu, X. Elicited upper limb motions through transcutaneous cervical spinal cord stimulation. J. Neural Eng. 2020, 17, 036001. [Google Scholar] [CrossRef]
- Inanici, F.; Brighton, L.N.; Samejima, S.; Hofstetter, C.P.; Moritz, C.T. Transcutaneous Spinal Cord Stimulation Restores Hand and Arm Function after Spinal Cord Injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 310–319. [Google Scholar] [CrossRef]
- Gerasimenko, Y.P.; Lu, D.C.; Modaber, M.; Zdunowski, S.; Gad, P.; Sayenko, D.G.; Morikawa, E.; Haakana, P.; Ferguson, A.R.; Roy, R.R.; et al. Noninvasive Reactivation of Motor Descending Control after Paralysis. J. Neurotrauma 2015, 32, 1968–1980. [Google Scholar] [CrossRef] [Green Version]
- Solopova, I.A.; Sukhotina, I.A.; Zhvansky, D.S.; Ikoeva, G.A.; Vissarionov, S.V.; Baindurashvili, A.G.; Edgerton, V.R.; Gerasimenko, Y.P.; Moshonkina, T.R. Effects of spinal cord stimulation on motor functions in children with cerebral palsy. Neurosci. Lett. 2017, 639, 192–198. [Google Scholar] [CrossRef] [PubMed]
- McHugh, L.V.; Miller, A.A.; Leech, K.A.; Salorio, C.; Martin, R.H. Feasibility and utility of transcutaneous spinal cord stimulation combined with walking-based therapy for people with motor incomplete spinal cord injury. Spinal Cord Ser. Cases 2020, 6, 104. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; Freundl, B.; Danner, S.M.; Krenn, M.J.; Mayr, W. Transcutaneous Spinal Cord Stimulation Induces Temporary Attenuation of Spasticity in Individuals with Spinal Cord Injury. J. Neurotrauma 2020, 493, 481–493. [Google Scholar] [CrossRef] [PubMed]
- Madhavan, S.; Hofstoetter, U.; Estes, S.P.; Iddings, J.A.; Field-Fote, E.C. Priming neural circuits to Modulate spinal reflex excitability. Front. Neurol. 2017, 8, 17. [Google Scholar] [CrossRef] [Green Version]
- Cogiamanian, F.; Ardolino, G.; Vergari, M.; Ferrucci, R.; Ciocca, M.; Scelzo, E.; Barbieri, S.; Priori, A. Transcutaneous Spinal Direct Current Stimulation. Front. Psychiatry 2012, 3, 63. [Google Scholar] [CrossRef] [Green Version]
- Powell, E.S.; Carrico, C.; Salyers, E.; Westgate, P.M.; Sawaki, L. The effect of transcutaneous spinal direct current stimulation on corticospinal excitability in chronic incomplete spinal cord injury. NeuroRehabilitation 2018, 43, 125–134. [Google Scholar] [CrossRef] [Green Version]
- Taylor, C.; McHugh, C.; Mockler, D.; Minogue, C.; Reilly, R.B.; Fleming, N. Transcutaneous spinal cord stimulation and motor responses in individuals with spinal cord injury: A methodological review. PLoS ONE 2021, 16, e0260166. [Google Scholar] [CrossRef]
- Mushahwar, V.K.; Horch, K.W. Proposed specifications for a lumbar spinal cord electrode array for control of lower extremities in paraplegia. IEEE Trans. Rehabil. Eng. 1997, 5, 237–243. [Google Scholar] [CrossRef]
- Mushahwar, V.K.; Jacobs, P.L.; Normann, R.A.; Triolo, R.J.; Kleitman, N. New functional electrical stimulation approaches to standing and walking. J. Neural Eng. 2007, 4, S181–S197. [Google Scholar] [CrossRef]
- Shealy, C.N.; Mortimer, J.T.; Reswick, J.B. Electrical Inhibition of Pain by Stimulation of the Dorsal Columns: Preliminary Clinical Report. Available online: https://pubmed.ncbi.nlm.nih.gov/4952225/ (accessed on 19 November 2020).
- Cook, A.W.; Weinstein, S.P. Chronic dorsal column stimulation in multiple sclerosis. Preliminary report. N. Y. State J. Med. 1973, 73, 2868–2872. [Google Scholar]
- Richardson, R.R.; Cerullo, L.J.; McLone, D.G.; Gutierrez, F.A.; Lewis, V. Percutaneous epidural neurostimulation in modulation of paraplegic spasticity—Six case reports. Acta Neurochir. 1979, 49, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.R.; McLone, D.G. Percutaneous epidural neurostimulation for paraplegic spasticity. Surg. Neurol. 1978, 9, 153–155. [Google Scholar] [PubMed]
- Pinter, M.M.; Gerstenbrand, F.; Dimitrijevic, M.R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 3. Control Of spasticity. Spinal Cord 2000, 38, 524–531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, O.; Forssberg, H.; Grillner, S.; Lindquist, M. Phasic gain control of the transmission in cutaneous reflex pathways to motoneurones during “fictive” locomotion. Brain Res 1978, 149, 503–507. [Google Scholar] [CrossRef]
- Grillner, S.; Zangger, P. On the central generation of locomotion in the low spinal cat. Exp. Brain Res. 1979, 34, 241–261. [Google Scholar] [CrossRef]
- Huang, H.; He, J.; Herman, R.; Carhart, M.R. Modulation effects of epidural spinal cord stimulation on muscle activities during walking. IEEE Trans. Neural Syst. Rehabil. Eng. 2006, 14, 14–23. [Google Scholar] [CrossRef]
- Carhart, M.R.; He, J.; Herman, R.; D’Luzansky, S.; Willis, W.T. Epidural Spinal-Cord Stimulation Facilitates Recovery of Functional Walking Following Incomplete Spinal-Cord Injury. IEEE Trans. Neural Syst. Rehabil. Eng. 2004, 12, 32–42. [Google Scholar] [CrossRef]
- Rattay, F.; Minassian, K.; Dimitrijevic, M.R. Epidural electrical stimulation of posterior structures of the human lumbosacral cord: 2. Quantitative analysis by computer modeling. Spinal Cord 2000, 38, 473–489. [Google Scholar] [CrossRef] [Green Version]
- De Noordhout, A.M.; Rothwell, J.C.; Thompson, P.D.; Marsden, C.D. Percutaneous electrical stimulation of lumbosacral roots in man. Neurosurg. Psychiatry 1988, 51, 174–181. [Google Scholar] [CrossRef] [Green Version]
- Shapkova, E. Spinal locomotor capability revealed by electrical stimulation of the lumbar enlargement in paraplegic patients. Prog. Mot. Control 2004, 3, 253–289. [Google Scholar]
- Minassian, K.; Persy, I.; Rattay, F.; Dimitrijevic, M.; Hofer, C.; Kern, H. Posterior root–muscle reflexes elicited by transcutaneous stimulation of the human lumbosacral cord. Muscle Nerve 2007, 35, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.; Hofer, C.; Kern, H.; Danner, S.; Mayr, W.; Dimitrijevic, M.; Minassian, K. Effects of transcutaneous spinal cord stimulation on voluntary locomotor activity in an incomplete spinal cord injured individual. Biomed. Tech. 2013, 58, 2–3. [Google Scholar] [CrossRef] [PubMed]
- Hofstoetter, U.S.; McKay, W.B.; Tansey, K.E.; Mayr, W.; Kern, H.; Minassian, K. Modification of spasticity by transcutaneous spinal cord stimulation in individuals with incomplete spinal cord injury. J. Spinal Cord Med. 2014, 37, 202–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, A.R.; Robertson, V.J. Sensory, motor, and pain thresholds for stimulation with medium frequency alternating current. Arch. Phys. Med. Rehabil. 1998, 79, 273–278. [Google Scholar] [CrossRef]
- Manson, G.A.; Calvert, J.S.; Ling, J.; Tychhon, B.; Ali, A.; Sayenko, D.G. The relationship between maximum tolerance and motor activation during transcutaneous spinal stimulation is unaffected by the carrier frequency or vibration. Physiol. Rep. 2020, 8, e14397. [Google Scholar] [CrossRef]
- Jensen, M.P.; Brownstone, R.M. Mechanisms of spinal cord stimulation for the treatment of pain: Still in the dark after 50 years. Eur. J. Pain (United Kingdom) 2019, 23, 652–659. [Google Scholar] [CrossRef] [Green Version]
- Burke, D. Clinical uses of H reflexes of upper and lower limb muscles. Clin. Neurophysiol. Pract. 2016, 1, 9. [Google Scholar] [CrossRef] [Green Version]
- Lavrov, I.; Gerasimenko, Y.P.; Ichiyama, R.M.; Courtine, G.; Zhong, H.; Roy, R.R.; Edgerton, V.R. Plasticity of spinal cord reflexes after a complete transection in adult rats: Relationship to stepping ability. J. Neurophysiol. 2006, 96, 1699–1710. [Google Scholar] [CrossRef]
- Wu, G.; Ringkamp, M.; Murinson, B.B.; Pogatzki, E.M.; Hartke, T.V.; Weerahandi, H.M.; Campbell, J.N.; Griffin, J.W.; Meyer, R.A. Degeneration of myelinated efferent fibers induces spontaneous activity in uninjured C-fiber afferents. J. Neurosci. 2002, 22, 7746–7753. [Google Scholar] [CrossRef]
- Ahmed, S.; Yearwood, T.; De Ridder, D.; Vanneste, S. Burst and high frequency stimulation: Underlying mechanism of action. Expert Rev. Med. Devices 2018, 15, 61–70. [Google Scholar] [CrossRef]
- Arle, J.E.; Mei, L.; Carlson, K.W.; Shils, J.L. High-Frequency Stimulation of Dorsal Column Axons: Potential Underlying Mechanism of Paresthesia-Free Neuropathic Pain Relief. Neuromodulation 2016, 19, 385–397. [Google Scholar] [CrossRef] [PubMed]
- North, J.M.; Hong, K.S.J.; Cho, P.Y. Clinical Outcomes of 1 kHz Subperception Spinal Cord Stimulation in Implanted Patients with Failed Paresthesia-Based Stimulation: Results of a Prospective Randomized Controlled Trial. Neuromodulation Technol. Neural Interface 2016, 19, 731–737. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, K.; Richter, H.; Christo, P.J.; Williams, K.; Guan, Y. Spinal Cord Stimulation for Treating Chronic Pain: Reviewing Preclinical and Clinical Data on Paresthesia-Free High-Frequency Therapy. Neuromodulation 2018, 21, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Benavides, F.D.; Jo, H.J.; Lundell, H.; Edgerton, V.R.; Gerasimenko, Y.; Perez, M.A. Cortical and subcortical effects of transcutaneous spinal cord stimulation in humans with tetraplegia. J. Neurosci. 2020, 40, 2633–2643. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Rath, M.; Ferguson, A.R.; Burdick, J.W.; Havton, L.A.; Edgerton, V.R.; Gerasimenko, Y.P. Self-assisted standing enabled by non-invasive spinal stimulation after spinal cord injury. J. Neurotrauma 2019, 36, 1435–1450. [Google Scholar] [CrossRef]
- Milosevic, M.; Masugi, Y.; Sasaki, A.; Sayenko, D.G.; Nakazawa, K. On the reflex mechanisms of cervical transcutaneous spinal cord stimulation in human subjects. J. Neurophysiol. 2019, 121, 1672–1679. [Google Scholar] [CrossRef]
- Sayenko, D.G.; Angeli, C.; Harkema, S.J.; Reggie Edgerton, V.; Gerasimenko, Y.P. Neuromodulation of evoked muscle potentials induced by epidural spinal-cord stimulation in paralyzed individuals. J. Neurophysiol. 2014, 111, 1088–1099. [Google Scholar] [CrossRef]
- Parhizi, B.; Barss, T.S.; Mushahwar, V.K. Simultaneous Cervical and Lumbar Spinal Cord Stimulation Induces Facilitation of both Spinal and Corticospinal Circuitry in Humans. Front. Neurosci. 2021, 15, 379. [Google Scholar] [CrossRef]
- Al’joboori, Y.; Hannah, R.; Lenham, F.; Borgas, P.; Kremers, C.J.P.; Bunday, K.L.; Rothwell, J.; Duffell, L.D. The Immediate and Short-Term Effects of Transcutaneous Spinal Cord Stimulation and Peripheral Nerve Stimulation on Corticospinal Excitability. Front. Neurosci. 2021, 15, 749042. [Google Scholar] [CrossRef]
- Sasaki, A.; de Freitas, R.M.; Sayenko, D.G.; Masugi, Y.; Nomura, T.; Nakazawa, K.; Milosevic, M. Low-intensity and short-duration continuous cervical transcutaneous spinal cord stimulation intervention does not prime the corticospinal and spinal reflex pathways in able-bodied subjects. J. Clin. Med. 2021, 10, 3633. [Google Scholar] [CrossRef]
- Macefield, G.; Gandevia, S.C.; Burke, D. Conduction velocities of muscle and cutaneous afferents in the upper and lower limbs of human subjects. Brain 1989, 112, 1519–1532. [Google Scholar] [CrossRef] [PubMed]
- Rijken, N.H.M.; Vonhögen, L.H.; Duysens, J.; Keijsers, N.L.W. The effect of spinal cord stimulation (SCS) on static balance and gait. Neuromodulation 2013, 16, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Dubuisson, D. Effect of dorsal-column stimulation on gelatinosa and marginal neurons of cat spinal cord. J. Neurosurg. 1989, 70, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Zehr, E.P.; Stein, R.B. What functions do reflexes serve during human locomotion? Prog. Neurobiol. 1999, 58, 185–205. [Google Scholar] [CrossRef]
- Zehr, E.P.; Barss, T.S.; Dragert, K.; Frigon, A.; Vasudevan, E.V.; Haridas, C.; Hundza, S.; Kaupp, C.; Klarner, T.; Klimstra, M.; et al. Neuromechanical interactions between the limbs during human locomotion: An evolutionary perspective with translation to rehabilitation. Exp. Brain Res. 2016, 234, 3059–3081. [Google Scholar] [CrossRef] [Green Version]
- Beekhuizen, K.S.; Field-Fote, E.C. Massed practice versus massed practice with stimulation: Effects on upper extremity function and cortical plasticity in individuals with incomplete cervical spinal cord injury. Neurorehabil. Neural Repair 2005, 19, 33–45. [Google Scholar] [CrossRef]
- Duysens, J.; Pearson, K.G. The role of cutaneous afferents from the distal hindlimb in the regulation of the step cycle of thalamic cats. Exp. Brain Res. 1976, 24, 245–255. [Google Scholar] [CrossRef]
- Vallbo, Å.B.; Hagbarth, K.-E. Activity from Skin Percutaneously Mechanoreceptors in Awake Human Recorded Subjects. Exp. Neurol. 1968, 289, 270–289. [Google Scholar] [CrossRef]
- Guiho, T.; Baker, S.N.; Jackson, A. Epidural and transcutaneous spinal cord stimulation facilitates descending inputs to upper-limb motoneurons in monkeys. J. Neural Eng. 2021, 18, 046011. [Google Scholar] [CrossRef]
- Kaneko, N.; Sasaki, A.; Masugi, Y.; Nakazawa, K. The Effects of Paired Associative Stimulation with Transcutaneous Spinal Cord Stimulation on Corticospinal Excitability in Multiple Lower-limb Muscles. Neuroscience 2021, 476, 45–59. [Google Scholar] [CrossRef]
- Barss, T.S.; Parhizi, B.; Mushahwar, V.K. Transcutaneous spinal cord stimulation of the cervical cord modulates lumbar networks. J. Neurophysiol. 2020, 123, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ferris, D.P.; Huang, H.J.; Kao, P.C. Moving the arms to activate the legs. Exerc. Sport Sci. Rev. 2006, 34, 113–120. [Google Scholar] [CrossRef] [PubMed]
- Hundza, S.R.; Zehr, E.P. Suppression of soleus H-reflex amplitude is graded with frequency of rhythmic arm cycling. Exp. Brain Res. 2009, 193, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Parhizi, B.; Assh, J.; Alvarado, L.; Ogilvie, R.; Chong, S.L.; Mushahwar, V.K. Effect of cervicolumbar coupling on spinal reflexes during cycling after incomplete spinal cord injury. J. Neurophysiol. 2018, 120, 3172–3186. [Google Scholar] [CrossRef] [Green Version]
- Dietz, V. Do human bipeds use quadrupedal coordination? Trends Neurosci. 2002, 25, 462–467. [Google Scholar] [CrossRef]
- Zehr, E.P.; Hundza, S.R.; Vasudevan, E. V The quadrupedal nature of human bipedal locomotion. Exerc. Sport Sci. Rev. 2009, 37, 102–108. [Google Scholar] [CrossRef]
- Frigon, A.; Collins, D.F.; Zehr, E.P. Effect of rhythmic arm movement on reflexes in the legs: Modulation of soleus H-reflexes and somatosensory conditioning. J. Neurophysiol. 2004, 91, 1516–1523. [Google Scholar] [CrossRef] [Green Version]
- Frigon, A. The neural control of interlimb coordination during mammalian locomotion. J. Neurophysiol. 2017, 117, 2224–2241. [Google Scholar] [CrossRef]
- de Ruiter, G.C.; Hundza, S.R.; Zehr, E.P. Phase-dependent modulation of soleus H-reflex amplitude induced by rhythmic arm cycling. Neurosci. Lett. 2010, 475, 7–11. [Google Scholar] [CrossRef]
- Gorodnichev, R.M.; Pivovarova, E.A.; Pukhov, A.; Moiseev, S.A.; Savokhin, A.A.; Moshonkina, T.R.; Shcherbakova, N.A.; Kilimnik, V.A.; Selionov, V.A.; Kozlovskaia, I.B.; et al. Transcutaneous electrical stimulation of the spinal cord: Non-invasive tool for activation of locomotor circuitry in human. Fiziol. Cheloveka 2012, 38, 46–56. [Google Scholar]
- Gerasimenko, Y.; Gorodnichev, R.; Puhov, A.; Moshonkina, T.; Savochin, A.; Selionov, V.; Roy, R.R.; Lu, D.C.; Edgerton, V.R. Initiation and modulation of locomotor circuitry output with multisite transcutaneous electrical stimulation of the spinal cord in noninjured humans. J. Neurophysiol. 2015, 113, 834–842. [Google Scholar] [CrossRef] [PubMed]
- Sayenko, D.G.; Atkinson, D.A.; Floyd, T.C.; Gorodnichev, R.M.; Moshonkina, T.R.; Harkema, S.J.; Edgerton, V.R.; Gerasimenko, Y.P. Effects of paired transcutaneous electrical stimulation delivered at single and dual sites over lumbosacral spinal cord. Neurosci. Lett. 2015, 609, 229–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothwell, J. Control of Human Voluntary Movement, 2nd ed.; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Yamaguchi, T. Descending pathways eliciting forelimb stepping in the lateral funiculus: Experimental studies with stimulation and lesion of the cervical cord in decerebrate cats. Brain Res. 1986, 379, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Juvin, L.; Simmers, J.; Morin, D. Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J. Neurosci. 2005, 25, 6025–6035. [Google Scholar] [CrossRef]
- Juvin, L.; Le Gal, J.-P.; Simmers, J.; Morin, D. Cervicolumbar coordination in mammalian quadrupedal locomotion: Role of spinal thoracic circuitry and limb sensory inputs. J. Neurosci. 2012, 32, 953–965. [Google Scholar] [CrossRef] [Green Version]
- Zhou, R.; Alvarado, L.; Ogilvie, R.; Chong, S.L.; Shaw, O.; Mushahwar, V.K. Non-gait-specific intervention for the rehabilitation of walking after SCI: Role of the arms. J. Neurophysiol. 2018, 119, 2194–2211. [Google Scholar] [CrossRef]
- Klarner, T.; Barss, T.S.; Sun, Y.; Kaupp, C.; Loadman, P.M.; Zehr, E.P. Exploiting Interlimb Arm and Leg Connections for Walking Rehabilitation: A Training Intervention in Stroke. Neural Plast. 2016, 2016, 1517968. [Google Scholar] [CrossRef] [Green Version]
- Klarner, T.; Barss, T.; Sun, Y.; Kaupp, C.; Loadman, P.; Zehr, E. Long-Term Plasticity in Reflex Excitability Induced by Five Weeks of Arm and Leg Cycling Training after Stroke. Brain Sci. 2016, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Kaupp, C.; Pearcey, G.E.P.; Klarner, T.; Sun, Y.; Cullen, H.; Barss, T.S.; Zehr, E.P. Rhythmic arm cycling training improves walking and neurophysiological integrity in chronic stroke: The arms can give legs a helping hand in rehabilitation. J. Neurophysiol. 2018, 119, 1095–1112. [Google Scholar] [CrossRef] [Green Version]
- Robertson, J.V.G.; Roby-Brami, A. The trunk as a part of the kinematic chain for reaching movements in healthy subjects and hemiparetic patients. Brain Res. 2011, 1382, 137–146. [Google Scholar] [CrossRef]
- Cetisli Korkmaz, N.; Can Akman, T.; Kilavuz Oren, G.; Bir, L.S. Trunk control: The essence for upper limb functionality in patients with multiple sclerosis. Mult. Scler. Relat. Disord. 2018, 24, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Rath, M.; Vette, A.H.; Ramasubramaniam, S.; Li, K.; Burdick, J.; Edgerton, V.R.; Gerasimenko, Y.P.; Sayenko, D.G. Trunk Stability Enabled by Noninvasive Spinal Electrical Stimulation after Spinal Cord Injury. J. Neurotrauma 2018, 35, 2540–2553. [Google Scholar] [CrossRef] [PubMed]
- Gad, P.; Gerasimenko, Y.; Zdunowski, S.; Turner, A.; Sayenko, D.; Lu, D.C.; Edgerton, V.R. Weight bearing over-ground stepping in an exoskeleton with non-invasive spinal cord neuromodulation after motor complete paraplegia. Front. Neurosci. 2017, 11, 333. [Google Scholar] [CrossRef]
- Estes, S.; Zarkou, A.; Hope, J.M.; Suri, C.; Field-Fote, E.C. Combined transcutaneous spinal stimulation and locomotor training to improve walking function and reduce spasticity in subacute spinal cord injury: A randomized study of clinical feasibility and efficacy. J. Clin. Med. 2021, 10, 1167. [Google Scholar] [CrossRef]
- Wu, Y.K.; Levine, J.M.; Wecht, J.R.; Maher, M.T.; LiMonta, J.M.; Saeed, S.; Santiago, T.M.; Bailey, E.; Kastuar, S.; Guber, K.S.; et al. Posteroanterior cervical transcutaneous spinal stimulation targets ventral and dorsal nerve roots. Clin. Neurophysiol. 2020, 131, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Parhizi, B.; Barss, T.S.; Mushahwar, V.K. Alteration of H-reflex and MEP amplitude in the flexor carpi radialis muscle with transcutaneous spinal cord stimulation during static and leg cycling tasks in neurologically intact male and female humans. Front. Neurosci. 2021, 15, 379. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barss, T.S.; Parhizi, B.; Porter, J.; Mushahwar, V.K. Neural Substrates of Transcutaneous Spinal Cord Stimulation: Neuromodulation across Multiple Segments of the Spinal Cord. J. Clin. Med. 2022, 11, 639. https://doi.org/10.3390/jcm11030639
Barss TS, Parhizi B, Porter J, Mushahwar VK. Neural Substrates of Transcutaneous Spinal Cord Stimulation: Neuromodulation across Multiple Segments of the Spinal Cord. Journal of Clinical Medicine. 2022; 11(3):639. https://doi.org/10.3390/jcm11030639
Chicago/Turabian StyleBarss, Trevor S., Behdad Parhizi, Jane Porter, and Vivian K. Mushahwar. 2022. "Neural Substrates of Transcutaneous Spinal Cord Stimulation: Neuromodulation across Multiple Segments of the Spinal Cord" Journal of Clinical Medicine 11, no. 3: 639. https://doi.org/10.3390/jcm11030639
APA StyleBarss, T. S., Parhizi, B., Porter, J., & Mushahwar, V. K. (2022). Neural Substrates of Transcutaneous Spinal Cord Stimulation: Neuromodulation across Multiple Segments of the Spinal Cord. Journal of Clinical Medicine, 11(3), 639. https://doi.org/10.3390/jcm11030639





