Genetic Factors of Idiopathic Gigantomastia: Clinical Implications of Aromatase and Progesterone Receptor Polymorphisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Measurements
Genetic Study
2.3. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dancey, A.; Khan, M.; Dawson, J.; Peart, F. Gigantomastia—A classification and review of the literature. J. Plast. Reconstr. Aesthetic Surg. 2008, 61, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Taylor, C.J.; Chester, D.L. Bilateral breast reduction surgery in elderly women: A retrospective review of outcomes. J. Plast. Reconstr. Aesthetic Surg. 2012, 65, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Netscher, D.T.; Meade, R.A.; Goodman, C.M.; Brehm, B.J.; Friedman, J.D.; Thornby, J. Physical and psychosocial symptoms among 88 volunteer subjects compared to patients seeking plastic surgery procedures to the breast. Plast. Reconstr. Surg. 2000, 105, 2366–2373. [Google Scholar] [CrossRef] [PubMed]
- Jud, S.M.; Brendle-Behnisch, A.; Hack, C.C.; Preuss, C.; Arkudas, A.; Horch, R.E.; Beckmann, M.W.; Lux, M.P. Macromastia: An economic burden? A disease cost analysis based on real-world data in Germany. Arch. Gynecol. Obstet. 2021, 303, 521–531. [Google Scholar] [CrossRef] [PubMed]
- Govrin-Yehudain, J.; Kogan, L.; Cohen, H.I.; Falik-Zaccai, T.C. Familial juvenile hypertrophy of the breast. J. Adolesc. Health 2004, 35, 151–155. [Google Scholar] [CrossRef]
- Latham, K.; Fernandez, S.; Iteld, L.; Panthaki, Z.; Armstrong, M.B.; Thaller, S. Pediatric breast deformity. J. Craniofacial Surg. 2006, 17, 454–467. [Google Scholar] [CrossRef]
- Morimoto, T.; Komaki, K.; Mori, T.; Sasa, M.; Miki, H.; Inoue, H.; Monden, Y.; Nakanishi, H. Juvenile gigantomastia: Report of a case. Surg. Today 1993, 23, 260–264. [Google Scholar] [CrossRef]
- O’Hare, P.M.; Frieden, I.J. Virginal breast hypertrophy. Pediatric Dermatol. 2000, 17, 277–281. [Google Scholar] [CrossRef]
- Jabs, A.D.; Frantz, A.G.; Smith-Vaniz, A.; Hugo, N.E. Mammary hypertrophy is not associated with increased estrogen receptors. Plast. Reconstr. Surg. 1990, 86, 64–66. [Google Scholar] [CrossRef]
- Gliosci, A.; Presutti, F. Virginal gigantomastia: Validity of combined surgical and hormonal treatments. Aesthetic Plast. Surg. 1993, 17, 61–65. [Google Scholar] [CrossRef]
- Kasielska-Trojan, A.; Danilewicz, M.; Strużyna, J.; Bugaj, M.; Antoszewski, B. The role of oestrogen and progesterone receptors in gigantomastia. Arch. Med. Sci. 2020, 33, 403–408. [Google Scholar] [CrossRef]
- Kasielska-Trojan, A.; Danilewicz, M.; Sitek, A.; Antoszewski, B. Body size measurements, digit ratio (2D:4D) and oestrogen and progesterone receptors' expressions in juvenile gigantomastia. J. Pediatr. Endocrinol. Metab. 2020, 33, 403–408. [Google Scholar] [CrossRef]
- Das, L.; Rai, A.; Vaiphei, K.; Garg, A.; Mohsina, S.; Bhansali, A.; Dutta, P.; Tripathy, S. Idiopathic gigantomastia: Newer mechanistic insights implicating the paracrine milieu. Endocrine 2019, 66, 166–177. [Google Scholar] [CrossRef]
- Hu, X.; Jiang, L.; Tang, C.; Ju, Y.; Jiu, L.; Wei, Y.; Guo, L.; Zhao, Y. Association of three single nucleotide polymorphisms of ESR1 with breast cancer susceptibility: A meta-analysis. J. Biomed. Res. 2017, 31, 213–225. [Google Scholar]
- Li, T.; Zhao, J.; Yang, J.; Ma, X.; Dai, Q.; Huang, H.; Wang, L.; Liu, P. A Meta-Analysis of the Association between ESR1 Genetic Variants and the Risk of Breast Cancer. PLoS ONE 2016, 11, e0153314. [Google Scholar] [CrossRef]
- Flote, V.G.; Furberg, A.S.; McTiernan, A.; Frydenberg, H.; Ursin, G.; Iversen, A.; Lofteroed, T.; Ellison, P.T.; Wist, E.A.; Egeland, T.; et al. Gene variations in oestrogen pathways, CYP19A1, daily 17β-estradiol and mammographic density phenotypes in premenopausal women. Breast Cancer Res. 2014, 16, 499. [Google Scholar] [CrossRef] [Green Version]
- Kasielska-Trojan, A.; Mikołajczyk, M.; Antoszewski, B. BreastIdea Volume Estimator: A New Tool for Breast Volume Estimation-Presentation and Validation for Women. Plast. Reconstr. Surg. 2020, 146, 744e–748e. [Google Scholar] [CrossRef]
- Horita, N.; Kaneko, T. Genetic model selection for a case-control study and a meta-analysis. Meta Gene 2015, 5, 1–8. [Google Scholar] [CrossRef]
- Kusano, A.S.; Trichopoulos, D.; Terry, K.L.; Chen, W.Y.; Willett, W.C.; Michels, K.B. A prospective study of breast size and premenopausal breast cancer incidence. Int. J. Cancer 2006, 118, 2031–2034. [Google Scholar] [CrossRef]
- Eriksson, N.; Benton, G.M.; Do, C.B.; Kiefer, A.K.; Mountain, J.L.; Hinds, D.A.; Francke, U.; Tung, J.Y. Genetic variants associated with breast size also influence breast cancer risk. BMC Med. Genet. 2012, 13, 53. [Google Scholar] [CrossRef] [Green Version]
- Romano, A.; Delvoux, B.; Fischer, D.C.; Groothuis, P. The PROGINS polymorphism of the human progesterone receptor diminishes the response to progesterone. J. Mol. Endocrinol. 2007, 38, 331–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rockwell, L.C.; Rowe, E.J.; Arnson, K.; Jackson, F.; Froment, A.; Ndumbe, P.; Seck, B.; Jackson, R.; Lorenz, J.G. Worldwide distribution of allelic variation at the progesterone receptor locus and the incidence of female reproductive cancers. Am. J. Hum. Biol. 2012, 24, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Ghali, R.M.; Al-Mutawa, M.A.; Ebrahim, B.H.; Jrah, H.H.; Zaied, S.; Bhiri, H.; Hmila, F.; Mahjoub, T.; Almawi, W.Y. Progesterone Receptor (PGR) Gene Variants Associated with Breast Cancer and Associated Features: A Case-Control Study. Pathol. Oncol. Res. 2020, 26, 141–147. [Google Scholar] [CrossRef]
- Pooley, K.A.; Healey, C.S.; Smith, P.L.; Pharoah, P.D.; Thompson, D.; Tee, L.; West, J.; Jordan, C.; Easton, D.F.; Ponder, B.A.; et al. Association of the progesterone receptor gene with breast cancer risk: A single-nucleotide polymorphism tagging approach. Cancer Epidemiol. Biomark. Prev. 2006, 15, 675–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lundin, E.; Wirgin, I.; Lukanova, A.; Afanasyeva, Y.; Krogh, V.; Axelsson, T.; Hemminki, K.; Clendenen, T.V.; Arslan, A.A.; Ohlson, N.; et al. Selected polymorphisms in sex hormone-related genes, circulating sex hormones and risk of endometrial cancer. Cancer Epidemiol. 2012, 36, 445–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodman, M.T.; Lurie, G.; Thompson, P.J.; McDuffie, K.E.; Carney, M.E. Association of two common single-nucleotide polymorphisms in the CYP19A1 locus and ovarian cancer risk. Endocr. -Relat. Cancer 2008, 15, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Setiawan, V.W.; Doherty, J.A.; Shu, X.O.; Akbari, M.R.; Chen, C.; De Vivo, I.; DeMichele, A.; Garcia-Closas, M.; Goodman, M.T.; Haiman, C.A.; et al. Two estrogen-related variants in CYP19A1 and endometrial cancer risk: A pooled analysis in the Epidemiology of Endometrial Cancer Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 242–247. [Google Scholar] [CrossRef] [Green Version]
- Golmohammadzadeh, G.; Mohammadpour, A.; Ahangar, N.; Shokrzadeh, M. Polymorphisms in Phase I (CYP450) Genes CYP1A1 (rs4646421), CYP1B1 (rs1056836), CYP19A1 (rs749292) and CYP2C8 (rs1058930) and Their Relation to Risk of Breast Cancer: A Case-Control Study in Mazandaran Province in North of Iran. Open Access Maced. J. Med. Sci. 2019, 7, 2488–2496. [Google Scholar] [CrossRef] [Green Version]
- Czajka-Oraniec, I.; Zgliczynski, W.; Kurylowicz, A.; Mikula, M.; Ostrowski, J. Association between gynecomastia and aromatase (CYP19) polymorphisms. Eur. J. Endocrinol. 2008, 158, 721–727. [Google Scholar] [CrossRef]
| Characteristic | Women with Gigantomastia n = 60 Mean ± SD |
|---|---|
| Age (years) | 39.6 ± 11.12 |
| Age of gigantomastia onset (years) | 14.6 ± 3.5 |
| Breast volume (right/left) (cm3) * | 897/871 |
| Age of menarche (years) | 12.8 ± 1.5 |
| Length of the cycle (days) | 28.7 ± 2 |
| BMI (kg/m2) | 25.8 ± 3.1 |
| WHR ** | 0.89 ± 0.07 |
| Family history for gigantomastia (yes) | 37 |
| Original Data | Second Step | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Controls n = 64 (%) | Gigantomastia n = 60 (%) | OR | (95% CI) | p-Value | (OR1; OR2) * | Model | ||||
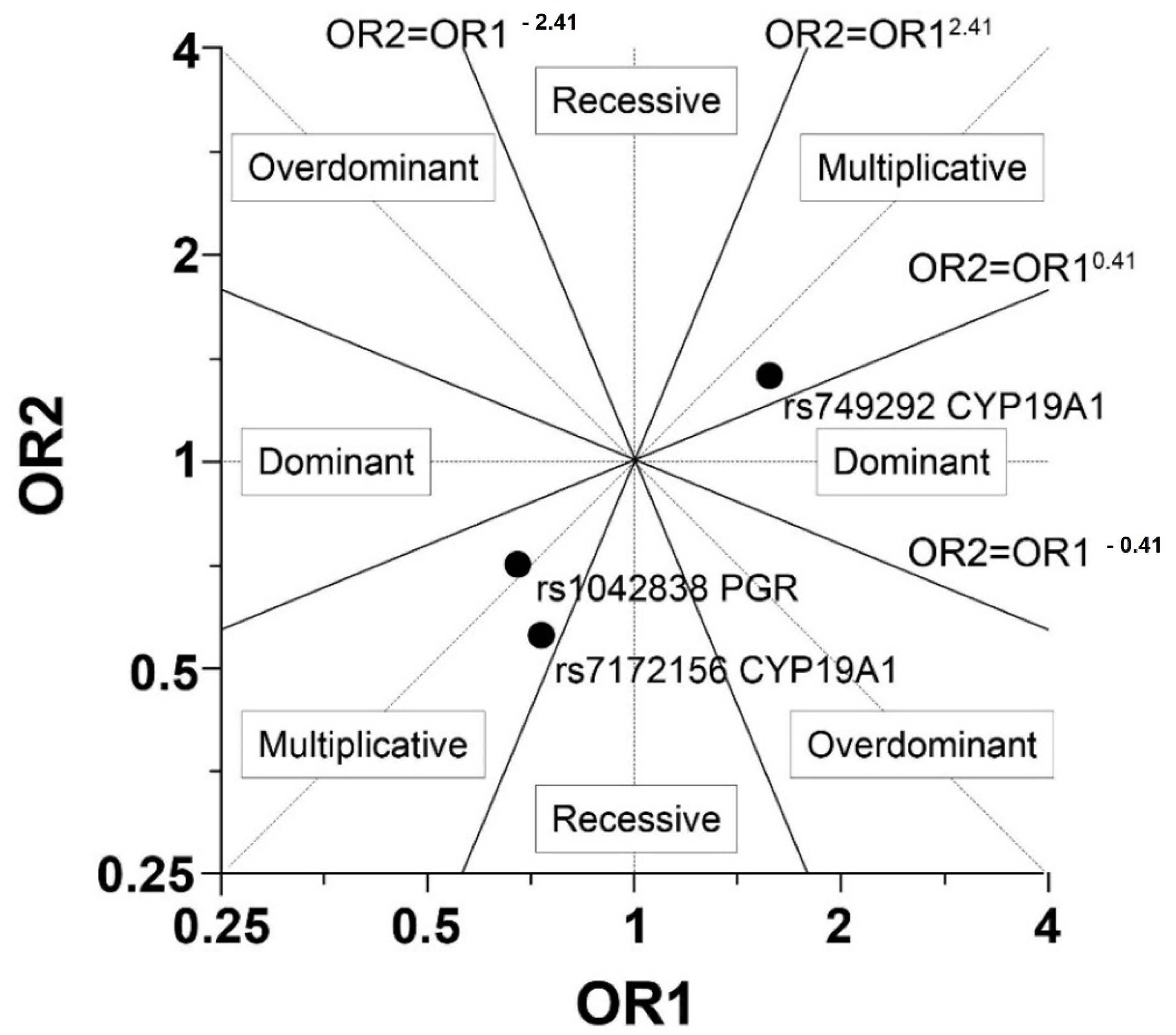
| rs7172156 CYP19A1 | AA | 6 | (9.38) | 9 | (15) | 1.29 | (0.38; 4.36) | 0.685 | (0.78; 0.64) | Mu |
| AG | 18 | (28.13) | 21 | (35) | 1.00 (ref) | |||||
| GG | 40 | (62.5) | 30 | (50) | 0.64 | (0.29; 1.42) | 0.274 | |||
| rs749292 CYP19A1 | AA | 23 | (35.94) | 14 | (23.33) | 0.61 | (0.26; 1.42) | 0.246 | (1.64; 1.45) | Mu |
| AG | 30 | (46.88) | 30 | (50) | 1.00 (ref) | |||||
| GG | 11 | (17.19) | 16 | (26.67) | 1.45 | (0.57; 3.68) | 0.426 | |||
| rs1042838 PGR | AA | 2 | (3.13) | 3 | (5) | 1.40 | (0.20; 9.86) | 0.734 | (0.71; 0.82) | Mu |
| AC | 14 | (21.88) | 15 | (25) | 1.00 (ref) | |||||
| CC | 48 | (75) | 42 | (70) | 0.82 | (0.35; 1.91) | 0.637 | |||
| Multiplicative Model SNP | Logistic Regression | |||
|---|---|---|---|---|
| OR | (95% CI) | p-Value | ||
| rs7172156 CYP19A1 | AA/AG/GG | 0.69 | (0.41; 1.15) | 0.154 |
| rs749292 CYP19A1 | AA/AG/GG | 1.55 | (0.93; 2.59) | 0.09 |
| rs1042838 PGR | AA/AC/CC | 0.96 | (0.73; 1.26) | 0.771 |
| Mean Breast Volume * | WHR ** | |||||
|---|---|---|---|---|---|---|
| SNP Variant | n | Mean ± SD | p-Value ^ | n | Mean ± SD | p-Value ^ |
| rs7172156_CYP19A1 | ||||||
| AA | 7 | 791.21 ± 349.95 | 0.392 | 8 | 0.88 ± 0.09 | 0.716 |
| AG | 13 | 955.04 ± 169.82 | 13 | 0.91 ± 0.04 | ||
| GG | 20 | 870.90 ± 272.71 | 22 | 0.89 ± 0.08 | ||
| rs1042838_PGR | ||||||
| AA | 3 | 109.17 ± 378.80 | 0.248 | 3 | 0.90 ± 0.06 | 0.028 |
| AC | 10 | 822.15 ± 267.21 | 10 | 0.84 ± 0.06 | ||
| CC | 27 | 882.33 ± 240.45 | 30 | 0.91 ± 0.07 | ||
| rs749292_CYP19A1 | ||||||
| AA | 11 | 858.64 ± 237.12 | 0.778 | 12 | 0.90 ± 0.07 | 0.879 |
| AG | 17 | 918.88 ± 267.22 | 18 | 0.89 ± 0.07 | ||
| GG | 12 | 858.83 ± 283.34 | 13 | 0.89 ± 0.07 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kasielska-Trojan, A.; Pietrusiński, M.; Bugaj-Tobiasz, M.; Strużyna, J.; Borowiec, M.; Antoszewski, B. Genetic Factors of Idiopathic Gigantomastia: Clinical Implications of Aromatase and Progesterone Receptor Polymorphisms. J. Clin. Med. 2022, 11, 642. https://doi.org/10.3390/jcm11030642
Kasielska-Trojan A, Pietrusiński M, Bugaj-Tobiasz M, Strużyna J, Borowiec M, Antoszewski B. Genetic Factors of Idiopathic Gigantomastia: Clinical Implications of Aromatase and Progesterone Receptor Polymorphisms. Journal of Clinical Medicine. 2022; 11(3):642. https://doi.org/10.3390/jcm11030642
Chicago/Turabian StyleKasielska-Trojan, Anna, Michał Pietrusiński, Magdalena Bugaj-Tobiasz, Jerzy Strużyna, Maciej Borowiec, and Bogusław Antoszewski. 2022. "Genetic Factors of Idiopathic Gigantomastia: Clinical Implications of Aromatase and Progesterone Receptor Polymorphisms" Journal of Clinical Medicine 11, no. 3: 642. https://doi.org/10.3390/jcm11030642






