Vasodilator Strain Stress Echocardiography in Suspected Coronary Microvascular Angina
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
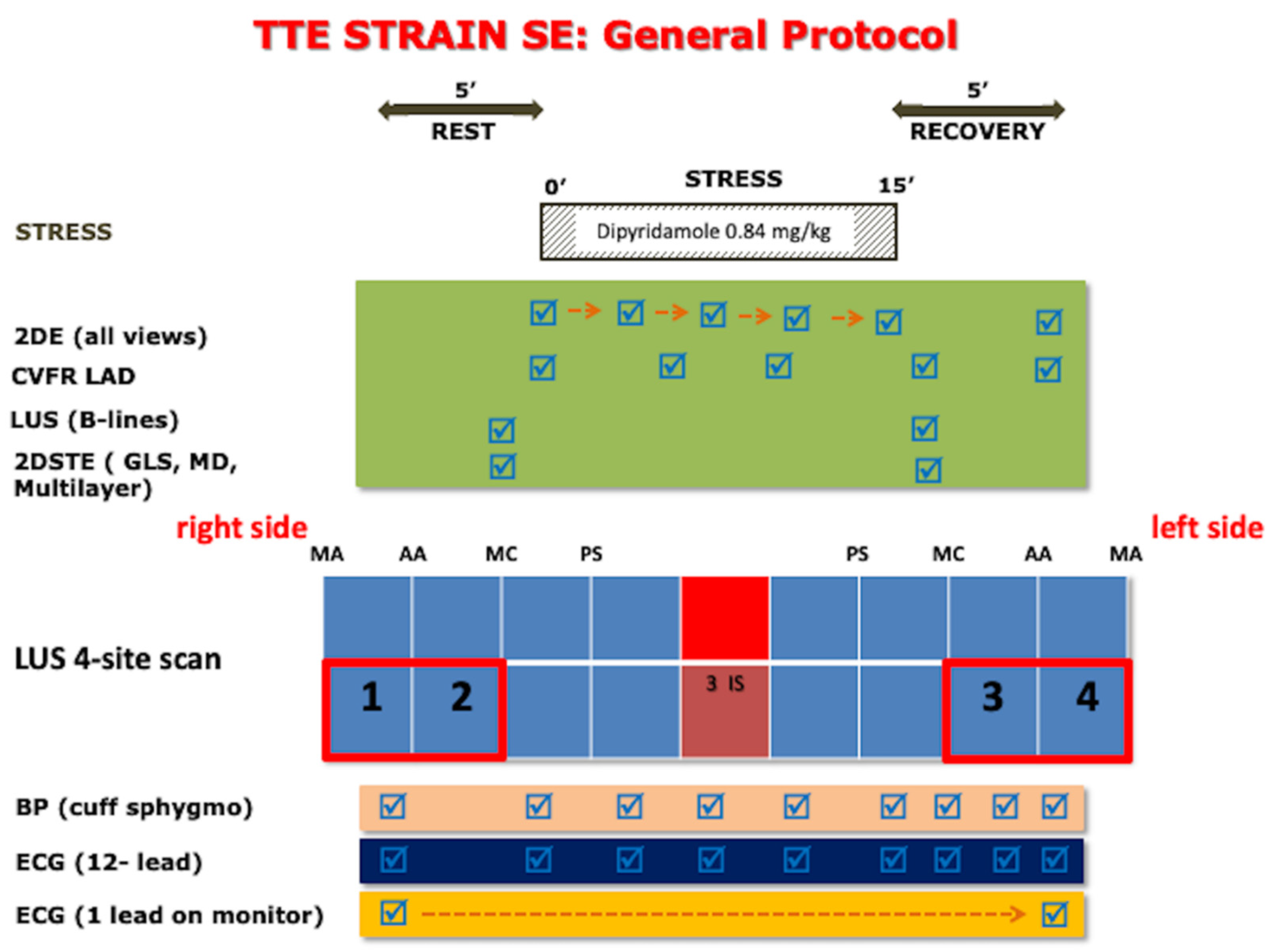
2.2. Rest and Stress Echocardiography
2.3. Statistical Analysis
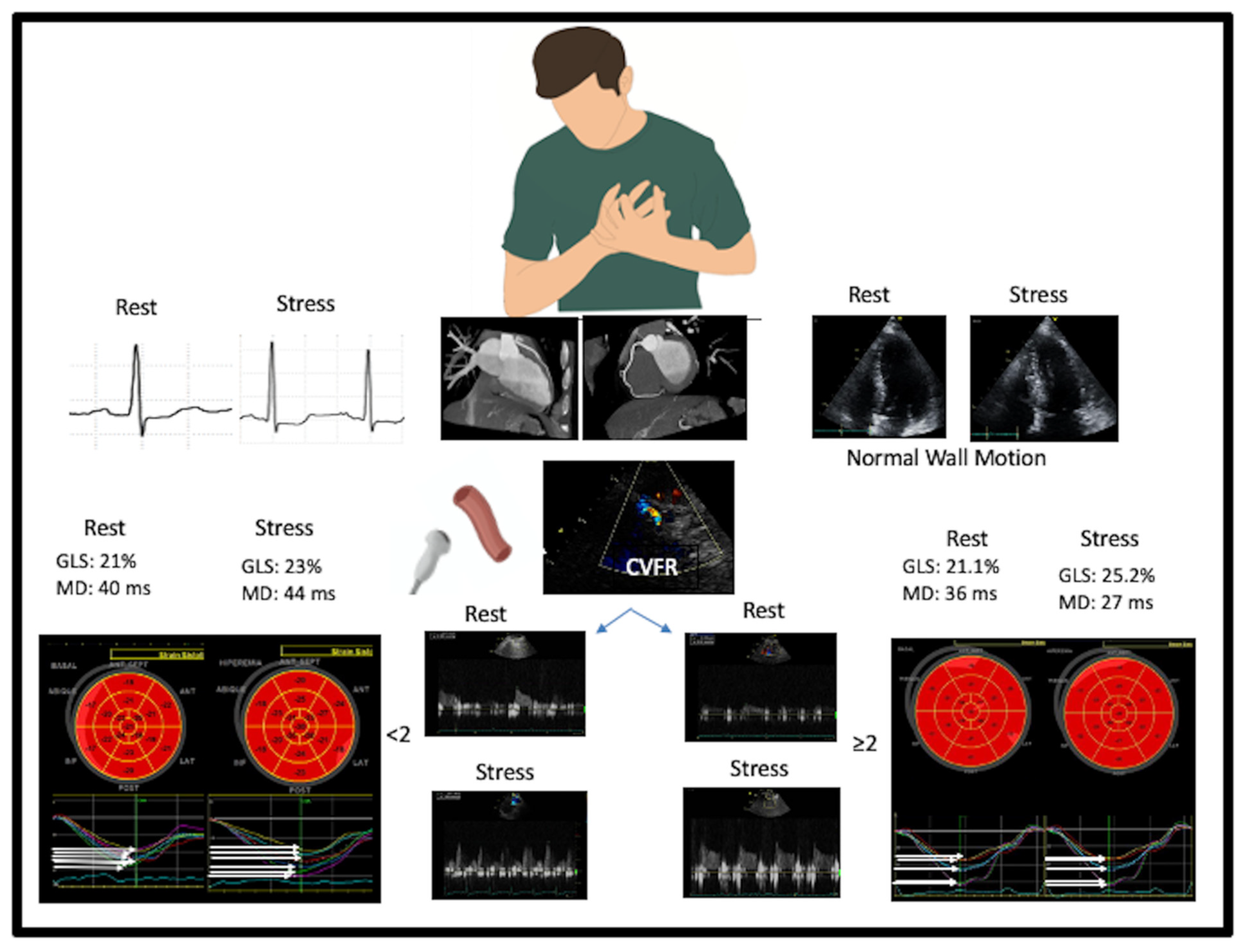
3. Results
Feasibility and Reproducibility Analysis
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Merz, C.N.B.; Pepine, C.J.; Walsh, M.N.; Fleg, J.L.; Camici, P.G.; Chilian, W.M.; Clayton, J.A.; Cooper, L.S.; Crea, F.; Di Carli, M.; et al. Ischemia and No Obstructive Coronary Artery Disease (INOCA). Circulation 2017, 135, 1075–1092. [Google Scholar] [CrossRef] [PubMed]
- Jespersen, L.; Hvelplund, A.; Abildstrøm, S.Z.; Pedersen, F.; Galatius, S.; Madsen, J.K.; Jørgensen, E.; Kelbaek, H.; Prescott, E. Stable angina pectoris with no obstructive coronary artery disease is associated with increased risks of major adverse cardiovascular events. Eur. Heart J. 2011, 33, 734–744. [Google Scholar] [CrossRef] [PubMed]
- Gulati, M.; Cooper-DeHoff, R.M.; McClure, C.; Johnson, B.D.; Shaw, L.J.; Handberg, E.; Zineh, I.; Kelsey, S.F.; Arnsdorf, M.F.; Black, H.R.; et al. Adverse Cardiovascular Outcomes in Women with Nonobstructive Coronary Artery Disease. Arch. Intern. Med. 2009, 169, 843–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sara, J.D.; Widmer, R.J.; Matsuzawa, Y.; Lennon, R.J.; Lerman, L.O.; Lerman, A. Prevalence of Coronary Microvascular Dysfunction Among Patients with Chest Pain and Nonobstructive Coronary Artery Disease. JACC Cardiovasc. Interv. 2015, 8, 1445–1453. [Google Scholar] [CrossRef]
- Sucato, V.; Novo, G.; Saladino, A.; Evola, S.; Galassi, A.R. Coronary microvascular dysfunction. Minerva Cardioangiol. 2020, 68, 153–163. [Google Scholar] [CrossRef]
- Ciampi, Q.; Zagatina, A.; Cortigiani, L.; Gaibazzi, N.; Daros, C.B.; Zhuravskaya, N.; Wierzbowska-Drabik, K.; Kasprzak, J.D.; de Castro e Silva Pretto, J.L.; D’Andrea, A.; et al. Functional, Anatomical, and Prognostic Correlates of Coronary Flow Velocity Reserve During Stress Echocardiography. J. Am. Coll. Cardiol. 2019, 74, 2278–2291. [Google Scholar] [CrossRef]
- Picano, E.; Pellikka, P.A. Stress echo applications beyond coronary artery disease. Eur. Heart J. 2014, 35, 1033–1040. [Google Scholar] [CrossRef] [Green Version]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: The Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef]
- Kunadian, V.; Chieffo, A.; Camici, P.G.; Berry, C.; Escaned, J.; Maas, A.H.E.M.; Prescott, E.; Karam, N.; Appelman, Y.; Fraccaro, C.; et al. An EAPCI Expert Consensus Document on Ischaemia with Non-Obstructive Coronary Arteries in Collaboration with European Society of Cardiology Working Group on Coronary Pathophysiology & Microcirculation Endorsed by Coronary Vasomotor Disorders International Study Group. Eur. Heart J. 2020, 41, 3504–3520. [Google Scholar] [CrossRef]
- Lang, R.M.; Badano, L.P.; Mor-Avi, V.; Afilalo, J.; Armstrong, A.; Ernande, L.; Flachskampf, F.A.; Foster, E.; Goldstein, S.A.; Kuznetsova, T.; et al. Recommendations for Cardiac Chamber Quantification by Echocardiography in Adults: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2015, 16, 233–271. [Google Scholar] [CrossRef]
- Sicari, R.; Nihoyannopoulos, P.; Evangelista, A.; Kasprzak, J.; Lancellotti, P.; Poldermans, D.; Voigt, J.-U.; Zamorano, J.L.; on behalf of the European Association of Echocardiography. Stress Echocardiography Expert Consensus Statement—Executive Summary: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur. Heart J. 2008, 30, 278–289. [Google Scholar] [CrossRef] [Green Version]
- Pellikka, P.A.; Arruda-Olson, A.; Chaudhry, F.A.; Chen, M.H.; Marshall, J.E.; Porter, T.R.; Sawada, S.G. Guidelines for Performance, Interpretation, and Application of Stress Echocardiography in Ischemic Heart Disease: From the American Society of Echocardiography. J. Am. Soc. Echocardiogr. 2020, 33, 1–41.e8. [Google Scholar] [CrossRef] [Green Version]
- Scali, M.C.; Zagatina, A.; Simova, I.; Zhuravskaya, N.; Ciampi, Q.; Paterni, M.; Marzilli, M.; Carpeggiani, C.; Picano, E.; Citro, R.; et al. B-lines with Lung Ultrasound: The Optimal Scan Technique at Rest and During Stress. Ultrasound Med. Biol. 2017, 43, 2558–2566. [Google Scholar] [CrossRef]
- Scali, M.C.; Zagatina, A.; Ciampi, Q.; Cortigiani, L.; D’Andrea, A.; Daros, C.B.; Zhuravskaya, N.; Kasprzak, J.D.; Wierzbowska-Drabik, K.; Pretto, J.L.D.C.E.S.; et al. Lung Ultrasound and Pulmonary Congestion During Stress Echocardiography. JACC Cardiovasc. Imaging 2020, 13, 2085–2095. [Google Scholar] [CrossRef]
- Cortigiani, L.; Huqi, A.; Ciampi, Q.; Bombardini, T.; Bovenzi, F.; Picano, E. Integration of Wall Motion, Coronary Flow Velocity, and Left Ventricular Contractile Reserve in a Single Test: Prognostic Value of Vasodilator Stress Echocardiography in Patients with Diabetes. J. Am. Soc. Echocardiogr. 2018, 31, 692–701. [Google Scholar] [CrossRef]
- Ciampi, Q.; Zagatina, A.; Cortigiani, L.; Wierzbowska-Drabik, K.; Kasprzak, J.D.; Haberka, M.; Djordjevic-Dikic, A.; Beleslin, B.; Boshchenko, A.; Ryabova, T.; et al. Prognostic value of stress echocardiography assessed by the ABCDE protocol. Eur. Heart J. 2021, 42, 3869–3878. [Google Scholar] [CrossRef]
- Ciampi, Q.; Picano, E.; Paterni, M.; Daros, C.B.; Simova, I.; Pretto, J.L.D.C.E.S.; Scali, M.C.; Gaibazzi, N.; Severino, S.; Djordjevic-Dikic, A.; et al. Quality control of regional wall motion analysis in stress Echo 2020. Int. J. Cardiol. 2017, 249, 479–485. [Google Scholar] [CrossRef]
- Scali, M.C.; Ciampi, Q.; Picano, E.; Bossone, E.; Ferrara, F.; Citro, R.; Colonna, P.; Costantino, M.F.; Cortigiani, L.; Andrea, A.D.; et al. Quality control of B-lines analysis in stress Echo 2020. Cardiovasc. Ultrasound 2018, 16, 20. [Google Scholar] [CrossRef] [Green Version]
- Carpeggiani, C.; Ciampi, Q.; Paterni, M.; De Nes, M.; Zagatina, A.; Simova, I.; Djordievic-Dikic, A.; Citro, R.; Colonna, P.; Picano, E. Multi-step Web-based Training: The Road to Stress Echo 2020. Rev. Argent. Cardiol. 2018, 86, 385–390. [Google Scholar] [CrossRef]
- Mada, R.O.; Lysyansky, P.; Daraban, A.M.; Duchenne, J.; Voigt, J.-U. How to Define End-Diastole and End-Systole? JACC Cardiovasc. Imaging 2015, 8, 148–157. [Google Scholar] [CrossRef] [Green Version]
- Cortigiani, L.; Carpeggiani, C.; Landi, P.; Raciti, M.; Bovenzi, F.; Picano, E. Usefulness of Blunted Heart Rate Reserve as an Imaging-Independent Prognostic Predictor During Dipyridamole Stress Echocardiography. Am. J. Cardiol. 2019, 124, 972–977. [Google Scholar] [CrossRef]
- Hearse, D.J. Myocardial ischaemia: Can we agree on a definition for the 21st century? Cardiovasc. Res. 1994, 28, 1737–1744. [Google Scholar] [CrossRef]
- Ross, J. Assessment of ischemic regional myocardial dysfunction and its reversibility. Circulation 1986, 74, 1186–1190. [Google Scholar] [CrossRef] [Green Version]
- Hanekom, L.; Cho, G.-Y.; Leano, R.; Jeffriess, L.; Marwick, T.H. Comparison of two-dimensional speckle and tissue Doppler strain measurement during dobutamine stress echocardiography: An angiographic correlation. Eur. Heart J. 2007, 28, 1765–1772. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.; Sitges, M.; Pham, P.N.; Tran, D.T.; Delgado, V.; Bertini, M.; Nucifora, G.; Vidaic, J.; Allman, C.; Holman, E.R.; et al. Incremental value of 2-dimensional speckle tracking strain imaging to wall motion analysis for detection of coronary artery disease in patients undergoing dobutamine stress echocardiography. Am. Heart J. 2009, 158, 836–844. [Google Scholar] [CrossRef]
- Aggeli, C.; Lagoudakou, S.; Felekos, I.; Panagopoulou, V.; Kastellanos, S.; Toutouzas, K.; Roussakis, G.; Tousoulis, D. Two-dimensional speckle tracking for the assessment of coronary artery disease during dobutamine stress echo: Clinical tool or merely research method. Cardiovasc. Ultrasound 2015, 13, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yü, Y.; Villarraga, H.R.; Saleh, H.K.; Cha, S.S.; Pellikka, P.A. Can ischemia and dyssynchrony be detected during early stages of dobutamine stress echocardiography by 2-dimensional speckle tracking echocardiography? Int. J. Cardiovasc. Imaging 2012, 29, 95–102. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Alamgir, M.F.; Nikitin, N.P.; Fraser, A.G.; Clark, A.L.; Cleland, J.G. The effect of pharmacological stress on intraventricular dyssynchrony in left ventricular systolic dysfunction. Eur. J. Heart Fail. 2008, 10, 412–420. [Google Scholar] [CrossRef]
- Michelsen, M.M.; Pena, A.; Mygind, N.D.; Bech, J.; Gustafsson, I.; Kastrup, J.; Hansen, H.S.; Høst, N.; Hansen, P.R.; Prescott, E. Coronary microvascular dysfunction and myocardial contractile reserve in women with angina and no obstructive coronary artery disease. Echocardiography 2018, 35, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Ikonomidis, I.; Tzortzis, S.; Paraskevaidis, I.; Triantafyllidi, H.; Papadopoulos, C.; Papadakis, I.; Trivilou, P.; Parissis, J.; Anastasiou-Nana, M.; Lekakis, J. Association of abnormal coronary microcirculatory function with impaired response of longitudinal left ventricular function during adenosine stress echocardiography in untreated hypertensive patients. Eur. Heart J. Cardiovasc. Imaging 2012, 13, 1030–1040. [Google Scholar] [CrossRef] [Green Version]
- Lafitte, S.; Bordachar, P.; Lafitte, M.; Garrigue, S.; Reuter, S.; Reant, P.; Serri, K.; Lebouffos, V.; Berrhouet, M.; Jais, P.; et al. Dynamic Ventricular Dyssynchrony: An Exercise-Echocardiography Study. J. Am. Coll. Cardiol. 2006, 47, 2253–2259. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez-Zanella, H.; Haugaa, K.; Boccalini, F.; Secco, E.; Edvardsen, T.; Badano, L.P.; Muraru, D. Physiological Determinants of Left Ventricular Mechanical Dispersion. JACC: Cardiovasc. Imaging 2018, 11, 650–651. [Google Scholar] [CrossRef] [PubMed]
- Schnell, F.; Matelot, D.; Daudin, M.; Kervio, G.; Mabo, P.; Carré, F.; Donal, E. Mechanical Dispersion by Strain Echocardiography: A Novel Tool to Diagnose Hypertrophic Cardiomyopathy in Athletes. J. Am. Soc. Echocardiogr. 2017, 30, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Tzortzis, S.; Ikonomidis, I.; Triantafyllidi, H.; Trivilou, P.; Pavlidis, G.; Katsanos, S.; Katogiannis, K.; Birba, D.; Thymis, J.; Makavos, G.; et al. Optimal Blood Pressure Control Improves Left Ventricular Torsional Deformation and Vascular Function in Newly Diagnosed Hypertensives: A 3-Year Follow-up Study. J. Cardiovasc. Transl. Res. 2020, 13, 814–825. [Google Scholar] [CrossRef]
- Leung, M.C.H.; Meredith, I.T.; Cameron, J. Aortic stiffness affects the coronary blood flow response to percutaneous coronary intervention. Am. J. Physiol. Circ. Physiol. 2006, 290, H624–H630. [Google Scholar] [CrossRef]
- Sicari, R.; Rigo, F.; Cortigiani, L.; Gherardi, S.; Galderisi, M.; Picano, E. Additive Prognostic Value of Coronary Flow Reserve in Patients with Chest Pain Syndrome and Normal or Near-Normal Coronary Arteries. Am. J. Cardiol. 2009, 103, 626–631. [Google Scholar] [CrossRef] [Green Version]



| General Population | CFVR ≤ 2.0 | CFVR > 2.0 | ||
|---|---|---|---|---|
| n = 130 | n = 35 | n = 95 | p | |
| Age (years) | 63 ± 12 | 68 ± 11 | 62 ± 12 | 0.012 |
| Female (%) | 67 (51.4) | 18 (51.4) | 49 (51.6) | 0.988 |
| Height (cm) | 161 ± 9 | 159 ± 10 | 161 ± 8 | 0.073 |
| Weight (kg) | 72.7 ± 13.8 | 69.6 ± 14.1 | 73.5 ± 13 | 0.505 |
| BMI (kg/m2) | 28.1 ± 5.2 | 27.7 ± 6.4 | 28.3 ± 4.6 | 0.251 |
| BSA (m2) | 1.79 ± 0.2 | 1.75 ± 0.21 | 1.8 ± 0.2 | 0.122 |
| Indication of stress test (%) | 0.298 | |||
| Dyspnea as the primary symptom | 12 (9.2) | 5 (14.3) | 7 (7.4) | |
| Atypical chest pain | 76 (58.5) | 20 (57.1) | 56 (59) | |
| Typical chest pain | 25 (19.2) | 4 (11.4) | 21 (22.1) | |
| High clinical risk | 15 (11.5) | 6 (17.1) | 9 (9.5) | |
| Re-stratification | 2 (1.5) | 0 | 2 (2.1) | |
| LBBB (%) | 8 (6.2) | 2 (5.7) | 6 (6.3) | 0.631 |
| Hypertension (%) | 92 (70.7) | 28 (80) | 64 (86.4) | 0.195 |
| Diabetes (%) | 41 (31.5) | 12 (34.3) | 29 (30.5) | 0.677 |
| Smoker (%) | 0.165 | |||
| Current | 28 (21.5) | 4 (11.4) | 24 (25.3) | |
| Former | 32 (24.6) | 8 (22.8) | 24 (25.3) | |
| Dyslipidemia (%) | 60 (46.1) | 13 (37.1) | 47 (49.5) | 0.211 |
| Dialysis (%) | 3 (2.3) | 2 (5.7) | 1 (1.1) | 0.176 |
| COPD (%) | 13 (10) | 2 (5.7) | 11 (11.6) | 0.512 |
| General Population | CFVR < 2.0 | CFVR ≥ 2.0 | ||
|---|---|---|---|---|
| n = 130 | n = 35 | n = 95 | p | |
| Beta blocker (%) | 59 (45.4) | 14 (40) | 45 (47.4) | 0.454 |
| ACE inhibitors (%) | 54 (42.5) | 15 (42.8) | 39 (41.1) | 0.853 |
| ARA-2 inhibitors (%) | 38 (29.2) | 13 (37.2) | 25 (26.3) | 0.278 |
| ARB´s (%) | 5 (3.8) | 13 (37.1) | 25 (26.3) | 0.722 |
| Diuretics (%) | 23 (17.7) | 6 (17.1) | 17 (17.9) | 0.921 |
| Digitalis (%) | 1 (0.8) | 0 | 1 (1.1) | 0.542 |
| Calcium antagonist (%) | 31 (23.8) | 14 (40) | 17 (17.9) | 0.009 |
| Nitrate (%) | 8 (6.2) | 3 (8.6) | 5 (5.3) | 0.486 |
| Aspirin (%) | 79 (60.8) | 22 (62.8) | 57 (60) | 0.841 |
| Antiplatelet agent (%) | 20 (15.4) | 4 (11.4) | 16 (16.8) | 0.448 |
| Antidiabetic drugs (%) | 27 (20.8) | 8 (22.9) | 19 (20) | 0.722 |
| Insulin (%) | 12 (9.2) | 3 (8.6) | 9 (9.5) | 0.875 |
| Anticoagulant (%) | 12 (9.2) | 2 (5.7) | 10 (10.5) | 0.512 |
| Statins (%) | 69 (53.1) | 16 (45.7) | 53 (55.8) | 0.307 |
| General Population | CFVR < 2.0 | CFVR ≥ 2.0 | ||
|---|---|---|---|---|
| n = 130 | n = 35 | n = 95 | p | |
| HR (bpm) | 67 ± 13 | 68 ± 13 | 66 ± 12 | 0.482 |
| SBP (mmHg) | 131 ± 22 | 141 ± 26 | 127 ± 19 | 0.002 |
| DBP (mmHg) | 71 ± 15 | 77 ± 19 | 69 ± 13 | 0.005 |
| iLVEDV (ml/m2) | 55 ± 16 | 57 ± 22 | 54 ± 14 | 0.403 |
| iLVESV (ml/m2) | 21.7 ± 9.8 | 23 ± 13 | 31 ± 8 | 0.206 |
| LVEF 3D (%) | 61 ± 8 | 59 ± 9 | 61 ± 7 | 0.458 |
| LV force index (mmHg/mL/m2) | 6.7 (5.2–7.9) | 6.9 (5.1–9.2) | 6.6 (5.1–7.7) | 0.332 |
| B lines | 0 (0–5) | 0 (0–10) | 0 (0–5) | 0.251 |
| GLS (%) | 20.2 ± 3.3 | 20.1 ± 3.8 | 20.2 ± 3 | 0.971 |
| Epicardial LS (%) | 17.9 ± 3 | 17.9 ± 2 | 17.9 ± 3 | 0.915 |
| Mesocardial LS (%) | 20.3 ± 3.3 | 20 ± 3.7 | 20.4 ± 3.2 | 0.526 |
| Endocardial LS (%) | 23.5 ± 3.8 | 23.5 ± 4.6 | 23.3 ± 4.6 | 0.746 |
| Delta endo-epi (%) | 5.3 ± 1.6 | 5.6 ± 2 | 5.4 ± 1.4 | 0.557 |
| MD (ms) | 49.5 ± 15.5 | 53.8 ± 18 | 47.9 ± 14.3 | 0.056 |
| General Population | CFVR < 2.0 | CFVR ≥ 2.0 | ||
|---|---|---|---|---|
| n = 130 | n = 35 | n = 95 | p | |
| HR (bpm) | 85 ± 14 | 82 ± 13 | 86 ± 14 | 0.183 |
| SBP (mmHg) | 129 ± 20 | 137 ± 18 | 125 ± 20 | 0.002 |
| DBP (mmHg) | 68 ± 14 | 70 ± 14 | 67 ± 14 | 0.397 |
| iLVEDV (ml/m2) | 56.1 ± 16 | 56.9 ± 19 | 55.8 ± 15 | 0.735 |
| iLVESV (ml/m2) | 17 ± 8.7 | 18.7 ± 12 | 16.3 ± 6.7 | 0.166 |
| LVEF 3D (%) | 70 ± 9 | 68 ± 10 | 70 ± 8 | 0.301 |
| LV force index (mmHg/mL/m2) | 8.2 (6.4–10.8) | 8.6 (6.2–11.8) | 8.2 (6.5–10.3) | 0.653 |
| B lines | 0 (0–13) | 1 (0–13) | 0 (0–7) | 0.199 |
| GLS (%) | 22.9 ± 3.8 | 21 ± 4.5 | 23.6 ± 3.3 | 0.007 |
| MD (ms) | 45.2 ± 17.7 | 53.9 ± 15 | 41.9 ± 17.6 | 0.0005 |
| Epicardial LS (%) | 20.1 ± 3.5 | 18.2 ± 4 | 20.7 ± 3.5 | 0.0002 |
| Mesocardial LS (%) | 23 ± 4.1 | 20.9 ± 4.9 | 23.8 ± 3.5 | 0.0004 |
| Endocardial LS (%) | 26.3 ± 4.7 | 24.1 ± 5.4 | 27.1 ± 3.6 | 0.001 |
| Delta endo-epi | 6.2 ± 2.2 | 5.9 ± 2.3 | 6.4 ± 2.1 | 0.262 |
| LV contractile reserve | 1.33 ± 0.42 | 1.33 ± 0.4 | 1.33 ± 0.43 | 0.844 |
| LV Synchrony Delta | 6 (4.7–16) | 3 (−8–8.1) | 7 (−1–18.8) | 0.035 |
| Strain Stress-Rest Delta | 2.9 (0.6–4.8) | 0.4 (−2–3.4) | 3.3 (1–3–5.3) | <0.0001 |
| Strain Reserve | 1.14 ± 0.16 | 1.01 (0.91–1.16) | 1.17 (1.06–1.28) | <0.0001 |
| Endo-Epi Reserve | 1.13 (0.94–1.45) | 1.0 (0.89–1.32) | 1.2 (0.96–1.47) | 0.047 |
| HR delta | 18 (10–26) | 13 (7–23) | 19(11–29) | 0.041 |
| HRR | 1.27 (1.13–1.45) | 1.24 (1.11–1.38) | 1.29 (1.17–1.47) | 0.044 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodriguez-Zanella, H.; Arbucci, R.; Fritche-Salazar, J.F.; Ortiz-Leon, X.A.; Tuttolomondo, D.; Lowenstein, D.H.; Wierzbowska-Drabik, K.; Ciampi, Q.; Kasprzak, J.D.; Gaibazzi, N.; et al. Vasodilator Strain Stress Echocardiography in Suspected Coronary Microvascular Angina. J. Clin. Med. 2022, 11, 711. https://doi.org/10.3390/jcm11030711
Rodriguez-Zanella H, Arbucci R, Fritche-Salazar JF, Ortiz-Leon XA, Tuttolomondo D, Lowenstein DH, Wierzbowska-Drabik K, Ciampi Q, Kasprzak JD, Gaibazzi N, et al. Vasodilator Strain Stress Echocardiography in Suspected Coronary Microvascular Angina. Journal of Clinical Medicine. 2022; 11(3):711. https://doi.org/10.3390/jcm11030711
Chicago/Turabian StyleRodriguez-Zanella, Hugo, Rosina Arbucci, Juan Francisco Fritche-Salazar, Xochitl Arely Ortiz-Leon, Domenico Tuttolomondo, Diego Haber Lowenstein, Karina Wierzbowska-Drabik, Quirino Ciampi, Jarosław D. Kasprzak, Nicola Gaibazzi, and et al. 2022. "Vasodilator Strain Stress Echocardiography in Suspected Coronary Microvascular Angina" Journal of Clinical Medicine 11, no. 3: 711. https://doi.org/10.3390/jcm11030711







