Myeloid-Derived Suppressor Cells (MDSC) in the Umbilical Cord Blood: Biological Significance and Possible Therapeutic Applications
Abstract
:1. Introduction: Myeloid-Derived Suppressor Cells
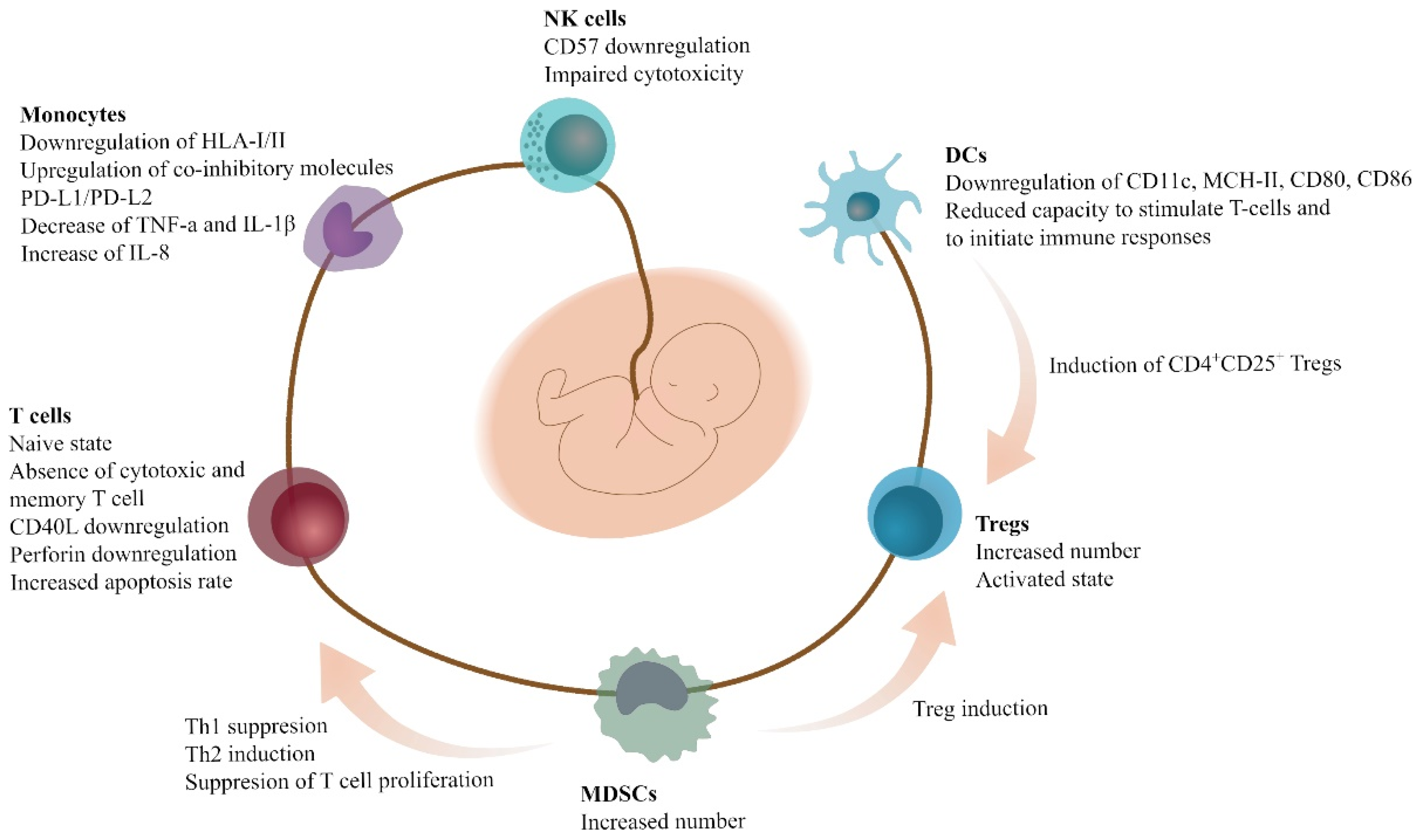
2. Immune Cells and MDSCs in Pregnancy, Fetal-Maternal Cross-Talk, and Neonatal Period
3. Immune Cells and MDSCs in the Umbilical Cord Blood (UCB)
3.1. In Vitro and Ex Vivo Expansion of UCB-MDSCs
3.2. Applications of UCB-MDSCs: Experimental and Clinical
4. Unpublished Data from the Public Cord Blood Bank of Crete
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bronte, V.; Brandau, S.; Chen, S.-H.; Colombo, M.P.; Frey, A.B.; Greten, T.F.; Mandruzzato, S.; Murray, P.J.; Ochoa, A.; Ostrand-Rosenberg, S.; et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat. Commun. 2016, 7, 12150. [Google Scholar] [CrossRef] [Green Version]
- Young, M.R.; Newby, M.; Wepsic, H.T. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res. 1987, 47, 100–105. [Google Scholar]
- Gabrilovich, D.I.; Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 2009, 9, 162–174. [Google Scholar] [CrossRef]
- Arocena, A.R.; Onofrio, L.I.; Pellegrini, A.V.; Silva, E.A.C.; Paroli, A.; Cano, R.C.; Aoki, M.P.; Gea, S. Myeloid-derived suppressor cells are key players in the resolution of inflammation during a model of acute infection. Eur. J. Immunol. 2013, 44, 184–194. [Google Scholar] [CrossRef] [Green Version]
- Gabrilovich, D.I. MDSCs. Cancer Immunol. Res. 2018, 5, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Seman, B.G.; Robinson, C.M. The Enigma of Low-Density Granulocytes in Humans: Complexities in the Characterization and Function of LDGs during Disease. Pathogens 2021, 10, 1091. [Google Scholar] [CrossRef]
- Vanhaver, C.; van der Bruggen, P.; Bruger, A. MDSC in Mice and Men: Mechanisms of Immunosuppression in Cancer. J. Clin. Med. 2021, 10, 2872. [Google Scholar] [CrossRef]
- Millrud, C.R.; Bergenfelz, C.; Leandersson, K. On the origin of myeloid-derived suppressor cells. Oncotarget 2016, 8, 3649–3665. [Google Scholar] [CrossRef] [Green Version]
- Bunt, S.K.; Yang, L.; Sinha, P.; Clements, V.K.; Leips, J.; Ostrand-Rosenberg, S. Reduced Inflammation in the Tumor Microenvironment Delays the Accumulation of Myeloid-Derived Suppressor Cells and Limits Tumor Progression. Cancer Res. 2007, 67, 10019–10026. [Google Scholar] [CrossRef] [Green Version]
- Gabitass, R.F.; Annels, N.E.; Stocken, D.D.; Pandha, H.A.; Middleton, G.W. Elevated myeloid-derived suppressor cells in pancreatic, esophageal and gastric cancer are an independent prognostic factor and are associated with significant elevation of the Th2 cytokine interleukin-13. Cancer Immunol. Immunother. 2011, 60, 1419–1430. [Google Scholar] [CrossRef] [Green Version]
- Ahmadi, M.; Mohammadi, M.; Ali-Hassanzadeh, M.; Zare, M.; Gharesi-Fard, B. MDSCs in pregnancy: Critical players for a balanced immune system at the feto-maternal interface. Cell. Immunol. 2019, 346, 103990. [Google Scholar] [CrossRef] [PubMed]
- Umansky, V.; Blattner, C.; Gebhardt, C.; Utikal, J. The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression. Vaccines 2016, 4, 36. [Google Scholar] [CrossRef] [PubMed]
- Mauti, L.A.; Le Bitoux, M.-A.; Baumer, K.; Stehle, J.-C.; Golshayan, D.; Provero, P.; Stamenkovic, I. Myeloid-derived suppressor cells are implicated in regulating permissiveness for tumor metastasis during mouse gestation. J. Clin. Investig. 2011, 121, 2794–2807. [Google Scholar] [CrossRef]
- Poschke, I.; Mao, Y.; Adamson, L.; Salazar-Onfray, F.; Masucci, G.; Kiessling, R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol. Immunother. 2011, 61, 827–838. [Google Scholar] [CrossRef] [Green Version]
- Fleming, V.; Hu, X.; Weber, R.; Nagibin, V.; Groth, C.; Altevogt, P.; Utikal, J.; Umansky, V. Targeting Myeloid-Derived Suppressor Cells to Bypass Tumor-Induced Immunosuppression. Front. Immunol. 2018, 9, 398. [Google Scholar] [CrossRef]
- Tebartz, C.; Horst, S.A.; Sparwasser, T.; Huehn, J.; Beineke, A.; Peters, G.; Medina, E. A Major Role for Myeloid-Derived Suppressor Cells and a Minor Role for Regulatory T Cells in Immunosuppression during Staphylococcus aureus Infection Christina. J. Immunol. 2015, 194, 1100–1111. [Google Scholar] [CrossRef] [Green Version]
- Tsukamoto, H.; Nishikata, R.; Senju, S.; Nishimura, Y. Myeloid-Derived Suppressor Cells Attenuate T H 1 Development through IL-6 Production to Promote Tumor Progression. Cancer Immunol. Res. 2013, 1, 64–76. [Google Scholar] [CrossRef] [Green Version]
- Condamine, T.; Gabrilovich, D.I. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. 2011, 32, 19–25. [Google Scholar] [CrossRef] [Green Version]
- De Sanctis, F.; Solito, S.; Ugel, S.; Molon, B.; Bronte, V.; Marigo, I. MDSCs in cancer: Conceiving new prognostic and therapeutic targets. Biochim. Biophys. Acta 2016, 1865, 35–48. [Google Scholar] [CrossRef]
- Solito, S.; Pinton, L.; Mandruzzato, S. In Brief: Myeloid-derived suppressor cells in cancer. J. Pathol. 2017, 242, 7–9. [Google Scholar] [CrossRef] [Green Version]
- Pastuła, A.; Marcinkiewicz, J. Myeloid-derived suppressor cells: A double-edged sword? Int. J. Exp. Pathol. 2011, 92, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Budhwar, S.; Verma, P.; Verma, R.; Rai, S.; Singh, K. The Yin and Yang of Myeloid Derived Suppressor Cells. Front. Immunol. 2018, 9, 2776. [Google Scholar] [CrossRef] [PubMed]
- Pawelec, G.; Verschoor, C.P.; Ostrand-Rosenberg, S. Myeloid-Derived Suppressor Cells: Not Only in Tumor Immunity. Front. Immunol. 2019, 10, 1099. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, S.; Yang, C.; Rong, R. The Crosstalk between Myeloid Derived Suppressor Cells and Immune Cells: To Establish Immune Tolerance in Transplantation. J. Immunol. Res. 2016, 2016, 4986797. [Google Scholar] [CrossRef] [Green Version]
- Ostrand-Rosenberg, S. Myeloid derived-suppressor cells: Their role in cancer and obesity. Curr. Opin. Immunol. 2018, 51, 68–75. [Google Scholar] [CrossRef]
- Köstlin-Gille, N.; Gille, C. Myeloid-Derived Suppressor Cells in Pregnancy and the Neonatal Period. Front. Immunol. 2020, 11, 584712. [Google Scholar] [CrossRef]
- Wegmann, T.G.; Lin, H.; Guilbert, L.; Mosmann, T.R. Biderectional cytokine interactions in the maternal-fetal relationship: Is successful pregnancy a TH2 phenomenon ? Immunol Today. 1993, 14, 5–8. [Google Scholar] [CrossRef]
- Zhao, A.; Xu, H.; Kang, X.; Zhao, A.; Lu, L. New insights into myeloid-derived suppressor cells and their roles in feto-maternal immune cross-talk. J. Reprod. Immunol. 2016, 113, 35–41. [Google Scholar] [CrossRef]
- Crncic, T.B.; Laskarin, G.; Juretic, K.; Strbo, N.; Dupor, J.; Srsen, S.; Randic, L.; Bouteiller, P.L.; Tabiasco, J.; Rukavina, D. Perforin and Fas/FasL Cytolytic Pathways at the Maternal—Fetal Interface. Am. J. Reprod. Immunol. 2005, 54, 241–248. [Google Scholar] [CrossRef]
- Raghupathy, R. Th 1-type immunity is incompatible with successful pregnancy. Immunol. Today 1997, 18, 478–482. [Google Scholar] [CrossRef]
- Tripathi, S.; Guleria, I. Biomarkers in Fetomaternal Tolerance. Clin. Lab. Med. 2018, 39, 145–156. [Google Scholar] [CrossRef] [PubMed]
- Robinson, D.P.; Klein, S.L. Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm. Behav. 2012, 62, 263–271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mor, G.; Cardenas, I.; Abrahams, V.; Guller, S. Inflammation and pregnancy: The role of the immune system at the implantation site. Ann. N. Y. Acad. Sci. 2011, 1221, 80–87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Köstlin, N.; Kugel, H.; Spring, B.; Leiber, A.; Marmé, A.; Henes, M.; Rieber, N.; Hartl, D.; Poets, C.F.; Gille, C. Granulocytic myeloid derived suppressor cells expand in human pregnancy and modulate T-cell responses. Eur. J. Immunol. 2014, 44, 2582–2591. [Google Scholar] [CrossRef]
- Nair, R.R.; Sinha, P.; Khanna, A.; Singh, K. Reduced Myeloid-derived Suppressor Cells in the Blood and Endometrium is Associated with Early Miscarriage. Am. J. Reprod. Immunol. 2014, 73, 479–486. [Google Scholar] [CrossRef]
- Sykes, L.; MacIntyre, D.A.; Yap, X.J.; Teoh, T.G.; Bennett, P.R. The Th1:Th2 Dichotomy of Pregnancy and Preterm Labour. Mediat. Inflamm. 2012, 2012, 967629. [Google Scholar] [CrossRef] [Green Version]
- Kang, X.; Zhang, X.; Liu, Z.; Xu, H.; Wang, T.; He, L.; Zhao, A. Granulocytic myeloid-derived suppressor cells maintain feto-maternal tolerance by inducing Foxp3 expression in CD4+CD25-T cells by activation of the TGF- b/b-catenin pathway. Mol. Hum. Reprod. 2016, 22, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Zeng, W.; Tian, F.; Zhang, S.; Wu, F.; Qin, X.; Zhang, Y.; Lin, Y. Myeloid-derived suppressor cells depletion may cause pregnancy loss via upregulating the cytotoxicity of decidual natural killer cells. Am. J. Reprod. Immunol. 2019, 81, e13099. [Google Scholar] [CrossRef]
- Giudice, L.C. Genes associated with embryonic attachment and implantation and the role of progesterone. J. Reprod. Med. 1999, 44, 165–171. [Google Scholar]
- Pan, T.; Liu, Y.; Zhong, L.M.; Shi, M.H.; Duan, X.B.; Wu, K.; Yang, Q.; Liu, C.; Wei, J.Y.; Ma, X.R.; et al. Myeloid-derived suppressor cells are essential for maintaining feto-maternal immunotolerance via STAT3 signaling in mice. J. Leukoc. Biol. 2016, 100, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Pan, T.; Zhong, L.; Wu, S.; Cao, Y.; Yang, Q.; Cai, Z.; Cai, X.; Zhao, W.; Ma, N.; Zhang, W.; et al. 17β-Oestradiol enhances the expansion and activation of myeloid-derived suppressor cells via signal transducer and activator of transcription (STAT)-3 signalling in human pregnancy. Clin. Exp. Immunol. 2016, 86–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blencowe, H.; Cousens, S. Review: Addressing the challenge of neonatal mortality. Trop. Med. Int. Health 2013, 18, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Gantt, S.; Gervassi, A.; Jaspan, H.; Horton, H. The Role of Myeloid-Derived Suppressor Cells in Immune Ontogeny. Front. Immunol. 2014, 5, 387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, O. Innate immunity of the newborn: Basic mechanisms and clinical correlates. Nat. Rev. Immunol. 2007, 7, 379–390. [Google Scholar] [CrossRef] [PubMed]
- Philbin, V.J.; Levy, O. Developmental Biology of the Innate Immune Response: Implications for Neonatal and Infant Vaccine Development. Pediatr. Res. 2009, 65, 98R–105R. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilson, C.B.; Lewis, D.B. Basis and Implications of Selectively Diminished Cytokine Production in Neonatal Susceptibility to Infection. Clin. Infect. Dis. 1990, 12, S410–S420. [Google Scholar] [CrossRef]
- Kollman, T.R.; Levy, O.; Montgomery, R.R.; Goriely, S. Innate Immune Sensing by Toll-like Receptors in Newborns and the Elderly. Immunity 2012, 37, 771–783. [Google Scholar] [CrossRef] [Green Version]
- Maródi, L. Innate cellular immune responses in newborns. Clin. Immunol. 2006, 118, 137–144. [Google Scholar] [CrossRef]
- Zaghouani, H.; Hoeman, C.M.; Adkins, B. Neonatal immunity: Faulty T-helpers and the shortcomings of dendritic cells. Trends Immunol. 2009, 30, 585–591. [Google Scholar] [CrossRef] [Green Version]
- Rieber, N.; Gille, C.; Köstlin, N.; Schäfer, I.; Spring, B.; Ost, M.; Spieles, H.; Kugel, H.A.; Pfeiffer, M.; Heininger, V.; et al. Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin. Exp. Immunol. 2013, 174, 45–52. [Google Scholar] [CrossRef]
- Schwarz, J.; Scheckenbach, V.; Kugel, H.; Spring, B.; Pagel, J.; Härtel, C.; Pauluschke-Fröhlich, J.; Peter, A.; Poets, C.F.; Gille, C.; et al. Granulocytic myeloid-derived suppressor cells (GR-MDSC) accumulate in cord blood of preterm infants and remain elevated during the neonatal period. Clin. Exp. Immunol. 2017, 191, 328–337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leiber, A.; Schwarz, J.; Köstlin, N.; Spring, B.; Fehrenbacher, B.; Katava, N.; Poets, C.F.; Gille, C. Neonatal myeloid derived suppressor cells show reduced apoptosis and immunosuppressive activity upon infection with Escherichia coli. Eur. J. Immunol. 2017, 47, 1009–1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gervassi, A.; Lejarcegui, N.; Dross, S.; Jacobson, A.; Itaya, G.; Kidzeru, E.; Gantt, S.; Jaspan, H.; Horton, H. Myeloid Derived Suppressor Cells Are Present at High Frequency in Neonates and Suppress In Vitro T Cell Responses. PLoS ONE 2014, 9, e107816. [Google Scholar] [CrossRef] [Green Version]
- He, Y.-M.; Li, X.; Perego, M.; Nefedova, Y.; Kossenkov, A.V.; A Jensen, E.; Kagan, V.E.; Liu, Y.-F.; Fu, S.-Y.; Ye, Q.-J.; et al. Transitory presence of myeloid-derived suppressor cells in neonates is critical for control of inflammation. Nat. Med. 2018, 24, 224–231. [Google Scholar] [CrossRef] [PubMed]
- Köstlin, N.; Vogelmann, M.; Spring, B.; Schwarz, J.; Feucht, J.; Härtel, C.; Orlikowsky, T.W.; Poets, C.F.; Gille, C. Granulocytic myeloid-derived suppressor cells from human cord blood modulate T-helper cell response towards an anti-inflammatory phenotype. Immunology 2017, 152, 89–101. [Google Scholar] [CrossRef]
- Muniraman, H.; Sardesai, T.; Sardesai, S. Disorders of the Umbilical Cord. Pediatr. Rev. 2018, 39, 332–341. [Google Scholar] [CrossRef]
- Taghizadeh, R.; Cetrulo, K.; Cetrulo, C. Wharton’s Jelly stem cells: Future clinical applications. Placenta 2011, 32, S311–S315. [Google Scholar] [CrossRef]
- Rogers, I.; Casper, R.F. Umbilical cord blood stem cells. Best Pract. Res. Clin. Obstet. Gynaecol. 2004, 18, 893–908. [Google Scholar] [CrossRef]
- Gluckman, E.; Broxmeyer, H.E.; Auerbach, A.D.; Friedman, H.S.; Douglas, G.W.; Devergie, A.; Esperou, H.; Thierry, D.; Socie, G.; Lehn, P.; et al. Hematopoietic Reconstitution in a Patient with Fanconi’s Anemia by Means of Umbilical-Cord Blood from an HLA-Identical Sibling. N. Engl. J. Med. 1989, 321, 1174–1178. [Google Scholar] [CrossRef]
- Gluckman, E. Umbilical cord blood biology and transplantation. Curr. Opin. Hematol. 1995, 2, 413–416. [Google Scholar] [CrossRef]
- Mayani, H. Umbilical Cord Blood: Lessons Learned and Lingering Challenges after More Than 20 Years of Basic and Clinical Research. Arch. Med Res. 2011, 42, 645–651. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.-J.; Yan, D.-C.; Lee, Y.-C.; Hsiao, H.-S.; Lee, P.-T.; Liang, Y.-W.; Kuo, M.L. Umbilical Cord Blood Immunology—Relevance to Stem Cell Transplantation. Clin. Rev. Allerg. Immunol. 2012, 42, 45–57. [Google Scholar] [CrossRef] [PubMed]
- Dietz, S.; Schwarz, J.; Vogelmann, M.; Spring, B.; Molnár, K.; Orlikowsky, T.W.; Wiese, F.; Holzer, U.; Poets, C.F.; Gille, C.; et al. Cord blood granulocytic myeloid-derived suppressor cells impair monocyte T cell stimulatory capacity and response to bacterial stimulation. Pediatr. Res. 2019, 86, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Talmadge, J.E.; Gabrilovich, D.I. History of myeloid-derived suppressor cells. Nat. Cancer 2013, 13, 739–752. [Google Scholar] [CrossRef] [PubMed]
- Bizymi, N.; Bjelica, S.; Kittang, A.O.; Mojsilovic, S.; Velegraki, M.; Pontikoglou, C.; Roussel, M.; Ersvær, E.; Santibañez, J.F.; Lipoldová, M.; et al. Myeloid-Derived Suppressor Cells in Hematologic Diseases: Promising Biomarkers and Treatment Targets. HemaSphere 2019, 3, e168. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Du, W.; Yan, F.; Wang, Y.; Li, H.; Cao, S.; Yu, W.; Shen, C.; Liu, J.; Ren, X. Myeloid-Derived Suppressor Cells Suppress Antitumor Immune Responses through IDO Expression and Correlate with Lymph Node Metastasis in Patients with Breast Cancer. J. Immunol. 2013, 190, 3783–3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.-C.; Sun, H.-W.; Chen, H.-T.; Liang, J.; Yu, X.-J.; Wu, C.; Wang, Z.; Zheng, L. Circulating hematopoietic stem and progenitor cells are myeloid-biased in cancer patients. Proc. Natl. Acad. Sci. USA 2014, 111, 4221–4226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, M.-Y.; Lim, B.-G.; Kim, S.; Sohn, H.-J.; Kim, S.; Kim, T.-G. GM-CSF Promotes the Expansion and Differentiation of Cord Blood Myeloid-Derived Suppressor Cells, Which Attenuate Xenogeneic Graft-vs.-Host Disease. Front. Immunol. 2019, 10, 183. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.-Y.; Ryu, D.-B.; Park, M.-Y.; Lee, S.-E.; Park, G.; Kim, T.-G.; Min, C.-K. Ex Vivo Generated Human Cord Blood Myeloid-Derived Suppressor Cells Attenuate Murine Chronic Graft-versus-Host Diseases. Biol. Blood Marrow Transplant. 2018, 24, 2381–2396. [Google Scholar] [CrossRef] [Green Version]
- Zoso, A.; Mazza, E.M.C.; Bicciato, S.; Mandruzzato, S.; Bronte, V.; Serafini, P.; Inverardi, L. Human fibrocytic myeloid-derived suppressor cells express IDO and promote tolerance via Treg-cell expansion. Eur. J. Immunol. 2014, 44, 3307–3319. [Google Scholar] [CrossRef]
- Mazza, E.M.C.; Zoso, A.; Mandruzzato, S.; Bronte, V.; Serafini, P.; Inverardi, L.; Bicciato, S. Gene expression profiling of human fibrocytic myeloid-derived suppressor cells (f-MDSCs). Genom. Data 2014, 2, 389–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macholdová, K.; Macháčková, E.; Prošková, V.; Hromadníková, I.; Klubal, R. Latest findings on the placenta from the point of view of immunology, tolerance and mesenchymal stem cells. Ceska Gynekol. 2019, 84, 154–160. [Google Scholar] [PubMed]
- Zheng, Z.; Yang, H.; Lai, Z.; Wang, C.; Yang, S.; Li, M.; Shao, J. Myeloid-derived suppressor cells in obstetrical and gynecological diseases. Am. J. Reprod. Immunol. 2020, 84, e13266. [Google Scholar] [CrossRef] [PubMed]
- Köstlin, N.; Hofstädter, K.; Ostermeir, A.-L.; Spring, B.; Leiber, A.; Haen, S.; Abele, H.; Bauer, P.; Pollheimer, J.; Hartl, D.; et al. Granulocytic Myeloid-Derived Suppressor Cells Accumulate in Human Placenta and Polarize toward a Th2 Phenotype. J. Immunol. 2016, 196, 1132–1145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Liu, Y.; Shu, C.; Wan, J.; Shan, Y.; Zhi, X.; Sun, L.; Yi, H.; Yang, Y.-G.; He, J. Inhibition of pregnancy-associated granulocytic myeloid-derived suppressor cell expansion and arginase-1 production in preeclampsia. J. Reprod. Immunol. 2018, 127, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Heinemann, A.S.; Pirr, S.; Fehlhaber, B.; Mellinger, L.; Burgmann, J.; Busse, M.; Ginzel, M.; Friesenhagen, J.; von Köckritz-Blickwede, M.; Ulas, T.; et al. In neonates S100A8/S100A9 alarmins prevent the expansion of a specific inflammatory monocyte population promoting septic shock. FASEB J. 2017, 31, 1153–1164. [Google Scholar] [CrossRef] [Green Version]
- Demosthenous, C.; Sakellari, I.; Douka, V.; Papayanni, P.; Anagnostopoulos, A.; Gavriilaki, E. The Role of Myeloid-Derived Suppressor Cells (MDSCs) in Graft-versus-Host Disease (GVHD). J. Clin. Med. 2021, 10, 2050. [Google Scholar] [CrossRef]
- McDonald, C.A.; Penny, T.R.; Paton, M.C.B.; Sutherland, A.E.; Nekkanti, L.; Yawno, T.; Castillo-Melendez, M.; Fahey, M.C.; Jones, N.M.; Jenkin, G.; et al. Effects of umbilical cord blood cells, and subtypes, to reduce neuroinflammation following perinatal hypoxic-ischemic brain injury. J. Neuroinflamm. 2018, 15, 47. [Google Scholar] [CrossRef]
- Yang, S.; Wei, Y.; Sun, R.; Lu, W.; Lv, H.; Xiao, X.; Cao, Y.; Jin, X.; Zhao, M. Umbilical cord blood-derived mesenchymal stromal cells promote myeloid-derived suppressor cell proliferation by secreting HLA-G to reduce acute graft-versus-host disease after hematopoietic stem cell transplantation. Cytotherapy 2020, 22, 718–733. [Google Scholar] [CrossRef]
- Qi, J.; Tang, X.; Li, W.; Chen, W.; Yao, G.; Sun, L. Mesenchymal stem cells inhibited the differentiation of MDSCs via COX2/PGE2 in experimental sialadenitis. Stem Cell Res. Ther. 2020, 11, 325. [Google Scholar] [CrossRef]
- Morton, J.J.; Keysar, S.B.; Perrenoud, L.; Chimed, T.; Reisinger, J.; Jackson, B.; Le, P.N.; Nieto, C.; Gomez, K.; Miller, B.; et al. Dual use of hematopoietic and mesenchymal stem cells enhances engraftment and immune cell trafficking in an allogeneic humanized mouse model of head and neck cancer. Mol. Carcinog. 2018, 57, 1651–1663. [Google Scholar] [CrossRef] [PubMed]
- Rocha, V.; Gluckman, E.; on behalf of the Eurocord-Netcord registry and European Blood and Marrow Transplant group. Improving outcomes of cord blood transplantation: HLA matching, cell dose and other graft- and transplantation-related factors. Br. J. Haematol. 2009, 147, 262–274. [Google Scholar] [CrossRef] [PubMed]
- Lecchi, L.; Ratti, I.; Lazzari, L.; Rebulla, P.; Sirchia, G. Reasons for discard of umbilical cord blood units before cryopreservation. Transfusion 2000, 40, 122–124. [Google Scholar] [CrossRef] [PubMed]
- Roura, S.; Pujal, J.-M.; Gálvez-Montón, C.; Bayes-Genis, A. The role and potential of umbilical cord blood in an era of new therapies: A review. Stem Cell Res. Ther. 2015, 6, 123. [Google Scholar] [CrossRef] [Green Version]

| Study | Progenitor Cells-Origin | Technique | Generated Cells | Importance |
|---|---|---|---|---|
| Yu et al. [66] | UCB-CD33+ cells-human | Co-culture with MDA-MB-231 human breast cancer cells | CD45+CD33+CD13+CD14−CD15− cells | Identification of (a) the contact-dependent manner of the immunosuppression of MDSCs and (b) targets, i.e., MDSCs, molecules, and pathways for possible novel therapies |
| Wu et al. [67] | UCB-CD34+ cells-human | Culture with GM-CSF with G-CSF and/or IL-6 | CD11b+CD14+HLA-DR-/low cells | Cytokines produced from solid tumors lead to the transition of the hematopoietic stem cells to immature myeloid cells, and, subsequently, to MDSCs with suppressive character |
| Park et al. [68] | UCB-CD34+ cells-human | Culture with rh-GM-CSF/SCF | HLA-DRlowCD11b+CD33+CD14+CD15- cells | Evidence that generated MDSCs can be used in the treatment of aGVHD and human inflammatory diseases |
| Lim et al. [69] | UCB-CD34+ cells-human | Culture with rh-GM-CSF and rh-SCF | CD14+HLA-DRlowCD11b+CD33+ cells | MDSCs generated ex vivo from human UCB can be used in treatment regimens not only against aGVHD, but also against cGVHD |
| Zoso et al. [70], Mazza et al. [71] | UCB cells-human | Culture with rh-GM-CSF and rh-G-CSF | f-MDSCs (co-express markers of MDSC, tDCs, and fibrocytes, i.e., CD33, IL-4Rα, CD11b, CD11c, CD13, CD14, CD15, HLA-DR, CD86, CD40, collagen V, and a-SMA) | f-MDSCs can serve as a tool for treatment of allograft rejection and in vitro generation of T regulatory cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizymi, N.; Georgopoulou, A.; Mastrogamvraki, N.; Matheakakis, A.; Gontika, I.; Fragiadaki, I.; Mavroudi, I.; Papadaki, H.A. Myeloid-Derived Suppressor Cells (MDSC) in the Umbilical Cord Blood: Biological Significance and Possible Therapeutic Applications. J. Clin. Med. 2022, 11, 727. https://doi.org/10.3390/jcm11030727
Bizymi N, Georgopoulou A, Mastrogamvraki N, Matheakakis A, Gontika I, Fragiadaki I, Mavroudi I, Papadaki HA. Myeloid-Derived Suppressor Cells (MDSC) in the Umbilical Cord Blood: Biological Significance and Possible Therapeutic Applications. Journal of Clinical Medicine. 2022; 11(3):727. https://doi.org/10.3390/jcm11030727
Chicago/Turabian StyleBizymi, Nikoleta, Anthie Georgopoulou, Natalia Mastrogamvraki, Angelos Matheakakis, Ioanna Gontika, Irene Fragiadaki, Irene Mavroudi, and Helen A. Papadaki. 2022. "Myeloid-Derived Suppressor Cells (MDSC) in the Umbilical Cord Blood: Biological Significance and Possible Therapeutic Applications" Journal of Clinical Medicine 11, no. 3: 727. https://doi.org/10.3390/jcm11030727





