Comparison of Different Procedures in a Combination of Ab Interno Microhook Trabeculotomy and Cataract Surgery
Abstract
1. Introduction
2. Materials and Methods
2.1. Methods
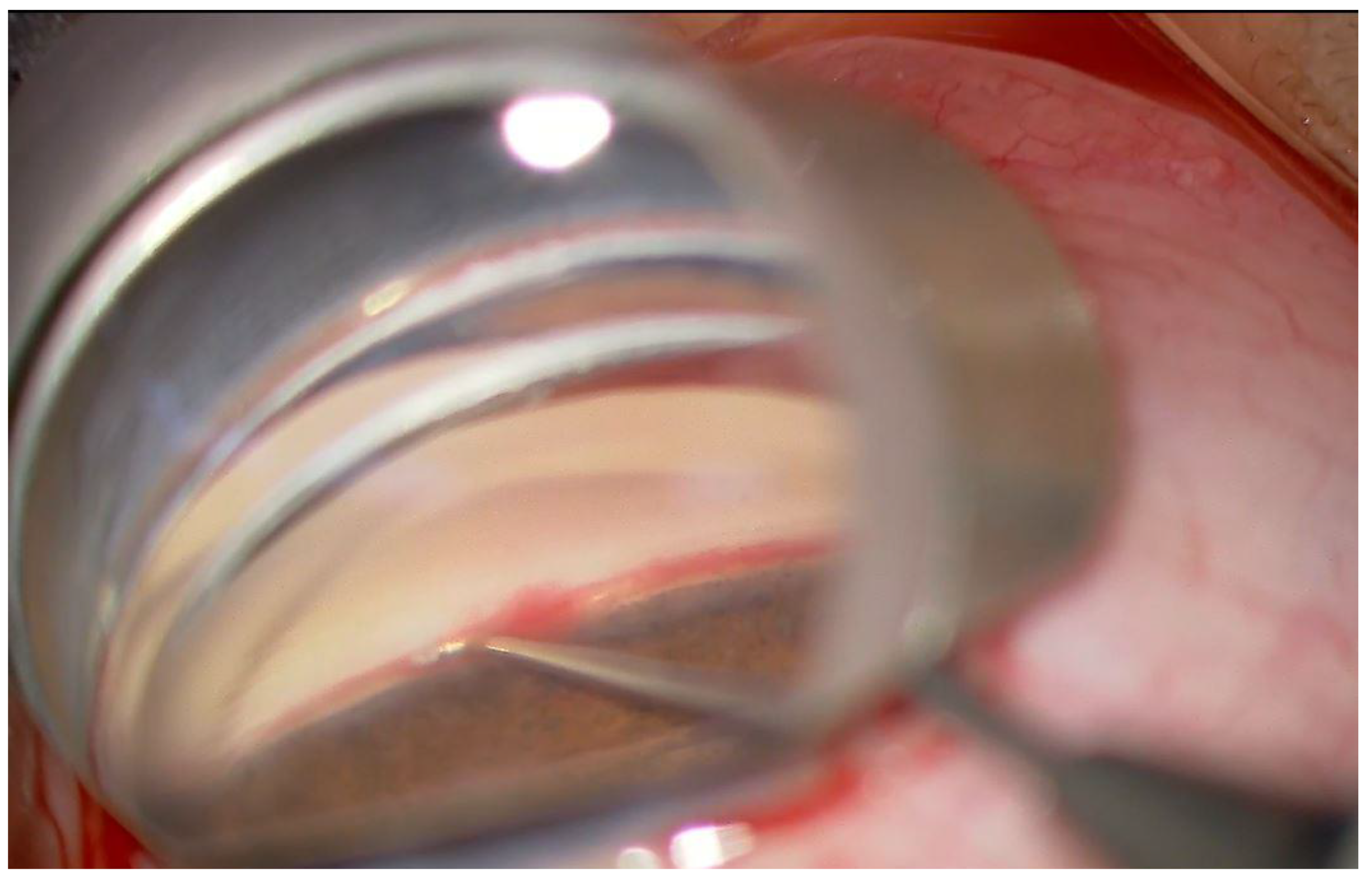
2.2. Surgical Procedure
2.3. Outcome Measures
2.4. Statistical Analysis
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chihara, E.; Nishida, A.; Kodo, M.; Yoshimura, N.; Matsumura, M.; Yamamoto, M.; Tsukada, T. Trabeculotomy ab externo: An alternative treatment in adult patients with primary open-angle glaucoma. Ophthalmic Surg. 1993, 24, 735–739. [Google Scholar] [CrossRef]
- Tanihara, H.; Negi, A.; Akimoto, M.; Terauchi, H.; Okudaira, A.; Kozaki, J.; Takeuchi, A.; Nagata, N. Surgical effects of trabeculotomy ab externo on adult eyes with primary open angle glaucoma and pseudoexfoliation syndrome. Arch. Ophthalmol. 1993, 111, 1653–1661. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Ohira, A.; Chihara, E. Surgical outcome of combined trabeculotomy and cataract surgery. J. Glaucoma 2001, 10, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Ohira, A.; Chihara, E. Factors leading to reduced intraocular pressure after combined trabeculotomy and cataract surgery. J. Glaucoma 2002, 11, 3–9. [Google Scholar] [CrossRef]
- Francis, B.A.; Singh, K.; Lin, S.C.; Hodapp, E.; Jampel, H.D.; Samples, J.R.; Smith, S.D. Novel glaucoma procedures: A report by the American Academy of Ophthalmology. Ophthalmology 2011, 118, 1466–1480. [Google Scholar] [CrossRef]
- Grover, D.S.; Godfrey, D.G.; Smith, O.; Feuer, W.J.; Montes de Oca, I.; Fellman, R.L. Gonioscopy-assisted transluminal trabeculotomy, ab interno trabeculotomy: Technique report and preliminary results. Ophthalmology 2014, 121, 855–861. [Google Scholar] [CrossRef]
- Sato, T.; Hirata, A.; Mizoguchi, T. Outcomes of 360 degrees suture trabeculotomy with deep sclerectomy combined with cataract surgery for primary open angle glaucoma and coexisting cataract. Clin. Ophthalmol. 2014, 8, 1301–1310. [Google Scholar] [CrossRef][Green Version]
- Sato, T.; Hirata, A.; Mizoguchi, T. Prospective, noncomparative, nonrandomized case study of short-term outcomes of 360 degrees suture trabeculotomy ab interno in patients with open-angle glaucoma. Clin. Ophthalmol. 2015, 9, 63–68. [Google Scholar] [CrossRef]
- Saheb, H.; Ahmed, I.I. Micro-invasive glaucoma surgery: Current perspectives and future directions. Curr. Opin. Ophthalmol. 2012, 23, 96–104. [Google Scholar] [CrossRef]
- Tanito, M. Microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery. Clin. Ophthalmol. 2018, 12, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Sugihara, K.; Tsutsui, A.; Hara, K.; Manabe, K.; Matsuoka, Y. Midterm results of microhook ab interno trabeculotomy in initial 560 eyes with glaucoma. J. Clin. Med. 2021, 10, 814. [Google Scholar] [CrossRef]
- Tanito, M.; Ikeda, Y.; Fujihara, E. Effectiveness and safety of combined cataract surgery and microhook ab interno trabeculotomy in Japanese eyes with glaucoma: Report of an initial case series. Jpn. J. Ophthalmol. 2017, 61, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Hirabayashi, M.T.; King, J.T.; Lee, D.; An, J.A. Outcome of phacoemulsification combined with excisional goniotomy using the Kahook Dual Blade in severe glaucoma patients at 6 months. Clin. Ophthalmol. 2019, 13, 715–721. [Google Scholar] [CrossRef] [PubMed]
- Kashiwagi, K.; Kogure, S.; Mabuchi, F.; Chiba, T.; Yamamoto, T.; Kuwayama, Y.; Araie, M. Change in visual acuity and associated risk factors after trabeculectomy with adjunctive mitomycin C. Acta Ophthalmol. 2016, 94, e561–e570. [Google Scholar] [CrossRef] [PubMed]
- Tanito, M.; Sano, I.; Ikeda, Y.; Fujihara, E. Short-term results of microhook ab interno trabeculotomy, a novel minimally invasive glaucoma surgery in Japanese eyes: Initial case series. Acta Ophthalmol. 2017, 95, e354–e360. [Google Scholar] [CrossRef] [PubMed]
- Richter, G.M.; Coleman, A.L. Minimally invasive glaucoma surgery: Current status and future prospects. Clin. Ophthalmol. 2016, 10, 189–206. [Google Scholar]
- Fontana, L.; De Maria, M. Letter to the Editor: Surgical Management of Pseudoexfoliative Glaucoma: A Review of Current Clinical Considerations and Surgical Outcomes. J. Glaucoma 2021, 30, e378. [Google Scholar] [CrossRef] [PubMed]
- Fontana, L.; De Maria, M.; Iannetta, D.; Moramarco, A. Gonioscopy-assisted transluminal trabeculotomy for chronic angle-closure glaucoma: Preliminary results. Graefes Arch. Clin. Exp. Ophthalmol. 2021, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Grover, D.S.; Smith, O.; Fellman, R.L.; Godfrey, D.G.; Gupta, A.; de Oca, I.M.; Feuer, W.J. J. Gonioscopy assisted transluminal trabeculotomy: An ab interno circumferential trabeculotomy: 24 months follow-up. J. Glaucoma 2018, 27, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Boese, E.A.; Shah, M. Gonioscopy-assisted Transluminal Trabeculotomy (GATT) is An Effective Procedure for Steroid-induced Glaucoma. J. Glaucoma 2019, 28, 803–807. [Google Scholar] [CrossRef] [PubMed]

| Group 1 | Group 2 | p | |
|---|---|---|---|
| Number of Eyes | 20 | 20 | |
| Mean age (range), years | 72.7 ± 9.4 (51–85) | 74.2 ± 6.5 (60–82) | 0.73 |
| Sex | |||
| Male | 9 (45.0%) | 5 (25.0%) | |
| Female | 11 (55.0%) | 15 (75.0%) | |
| Eye | |||
| Right | 7 (35.0%) | 8 (40.0%) | |
| Left | 13 (65.0%) | 12 (60.0%) | |
| Glaucoma Type | |||
| POAG | 9 (45.0%) | 9 (45.0%) | |
| EXG | 3 (15.0%) | 6 (30.0%) | |
| SIG | 3 (15.0%) | 2 (10.0%) | |
| UG | 3 (15.0%) | 2 (10.0%) | |
| Other | 2 (10.0%) | 1 (5.0%) | |
| Visual field mean deviation (dB) | −7.6 ± 6.4 | −7.2 ± 7.3 | 0.57 |
| Follow-up (months) | 6.0 ± 0.73 | 5.6 ± 0.75 | 0.13 |
| Group 1 | Group 2 | p | |
|---|---|---|---|
| IOP (mmHg) | |||
| Preoperative | 26.1± 12.3 | 20.6 ± 8.8 | 0.13 |
| Postoperative | 14.1 ± 3.3 | 12.9 ± 3.2 | 0.33 |
| Medication | |||
| Preoperative | 4.0 (3–5) | 3.5 (2–4.25) | 0.11 |
| Postoperative | 2.5 (2–3) | 1.0 (0–3) | 0.06 |
| BCVA (logMAR) | |||
| Preoperative | 0.28 ± 0.28 | 0.21 ± 0.45 | 0.13 |
| Postoperative | −0.040 ± 0.18 | 0.058 ± 0.38 | 0.55 |
| Surgical time (min) | 17.3 ± 5.7 | 20.9 ± 6.9 | 0.13 |
| Group 1 | Group 2 | p | |
|---|---|---|---|
| Intraoperative complications | |||
| Blood reflux | 20 (100%) | 20 (100%) | |
| Postoperative complications | |||
| Hyphema with niveau formation | 1 (5.0%) | 6 (30.0%) | 0.046 |
| Transient IOP elevation (>30 mmHg) | 3 (15.0%) | 4 (20.0%) | 0.50 |
| Blood accumulation in the lens bag | 0 | 1 (5.0%) | 0.50 |
| Postoperative intervention | |||
| Hyphema washout | 0 | 2 (10.0%) | 0.24 |
| Nd:YAG laser capsulotomy | 0 | 1 (5.0%) | 0.50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miura, Y.; Fukuda, K. Comparison of Different Procedures in a Combination of Ab Interno Microhook Trabeculotomy and Cataract Surgery. J. Clin. Med. 2022, 11, 738. https://doi.org/10.3390/jcm11030738
Miura Y, Fukuda K. Comparison of Different Procedures in a Combination of Ab Interno Microhook Trabeculotomy and Cataract Surgery. Journal of Clinical Medicine. 2022; 11(3):738. https://doi.org/10.3390/jcm11030738
Chicago/Turabian StyleMiura, Yusaku, and Ken Fukuda. 2022. "Comparison of Different Procedures in a Combination of Ab Interno Microhook Trabeculotomy and Cataract Surgery" Journal of Clinical Medicine 11, no. 3: 738. https://doi.org/10.3390/jcm11030738
APA StyleMiura, Y., & Fukuda, K. (2022). Comparison of Different Procedures in a Combination of Ab Interno Microhook Trabeculotomy and Cataract Surgery. Journal of Clinical Medicine, 11(3), 738. https://doi.org/10.3390/jcm11030738






