The Effect of Fibrates on Kidney Function and Chronic Kidney Disease Progression: A Systematic Review and Meta-Analysis of Randomised Studies
Abstract
:1. Introduction
2. Methods
2.1. Protocol and Registration
2.2. Eligibility Criteria
2.3. Information Sources and Search
2.4. Study Selection and Data Collection Process
2.5. Risk of Bias
2.6. Summary Measures and Synthesis of Results
- For continuous outcomes (i.e., eGFR, creatinine), we performed a generalised inverse variance analysis of standardised mean difference between patients in intervention and control group, pre and post administration of intervention/treatment/placebo using a random effects model.
- For categorical outcomes, relative risk was calculated using number of affected patients per outcome of interest from the included studies and a pooled estimate is presented using forest plots. Pooled estimates were calculated with a random-effects model (Der Simonian–Laird method) to account for both within and between study variability. Heterogeneity between synthesised studies were calculated using the I2 statistic and the presence of publication bias was investigated graphically by precision funnel plots. All statistical analyses were performed using STATA (Version 14, StataCorp, College Station, TX, USA).
3. Results
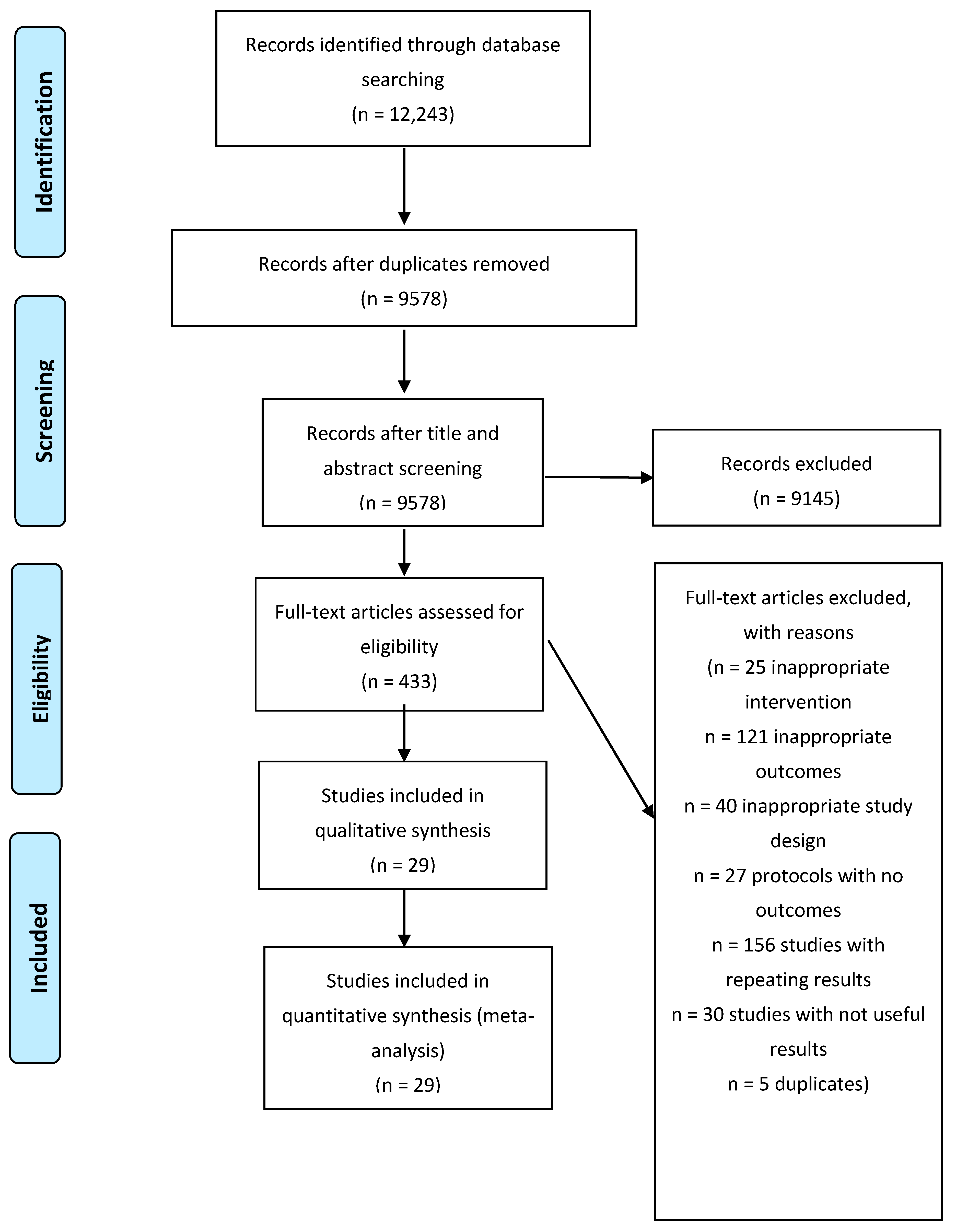
3.1. Study Selection
3.2. Study Characteristics
3.3. Risk of Bias within Studies
3.4. Synthesis of Results
3.5. Creatinine Change
3.6. eGFR
3.7. Albuminuria
3.8. Chronic Kidney Disease and End Stage Kidney Disease
3.9. Publication Bias across Studies
4. Discussion
Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drawz, P.; Rahman, M. Chronic kidney disease. Ann. Intern. Med. 2015, 162, ITC1–ITC14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, N.R.; Fatoba, S.T.; Oke, J.L.; Hirst, J.A.; O’Callaghan, C.A.; Lasserson, D.S.; Hobbs, F.D.R. Global prevalence of chronic kidney disease—A systematic review and meta-analysis. PLoS ONE 2016, 11, e0158765. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, R.N.; Parfrey, P.S.; Sarnak, M.J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 1998, 32 (Suppl. 3), S112–S119. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Liu, B.; Tao, W.; Hao, Z.; Liu, M. Fibrates for secondary prevention of cardiovascular disease and stroke. Cochrane Database Syst. Rev. 2015, 2015, CD009580. [Google Scholar] [CrossRef]
- Ferro, C.J.; Mark, P.B.; Kanbay, M.; Sarafidis, P.; Heine, G.H.; Rossignol, P.; Massy, Z.A.; Mallamaci, F.; Valdivielso, J.M.; Malyszko, J.; et al. Lipid management in patients with chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 727–749. [Google Scholar] [CrossRef] [Green Version]
- Keech, A.C.; Simes, R.J.; Barter, P.J.; Best, J.; Scott, R.A.P.; Taskinen, M.-R.; Forder, P.M.; Pillai, A.; Davis, T.M.; Glasziou, P.; et al. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): Randomised controlled trial. Lancet 2005, 366, 1849–1861. [Google Scholar] [CrossRef]
- The ACCORD Study Group; Ginsberg, H.N.; Elam, M.B.; Lovato, L.C.; Crouse, J.R.; Leiter, L.A.; Linz, P.; Friede-Wald, W.T.; Buse, J.B.; Gerstein, H.C.; et al. Effects of Combination Lipid Therapy in Type 2 Diabetes Mellitus. N. Engl. J. Med. 2010, 362, 1563–1574. [Google Scholar] [CrossRef]
- Ansquer, J.C.; Foucher, C.; Rattier, S.; Taskinen, M.R.; Steiner, G. Fenofibrate reduces progression to microalbuminuria over 3 years in a placebo-controlled study in type 2 diabetes: Results from the Diabetes Atherosclerosis Intervention Study (DAIS). Am. J. Kidney Dis. 2005, 45, 485–493. [Google Scholar] [CrossRef]
- Jun, M.; Zhu, B.; Tonelli, M.; Jardine, M.; Patel, A.; Neal, B.; Liyanage, T.; Keech, A.; Cass, A.; Perkovic, V. Effects of fibrates in kidney disease: A systematic review and meta-analysis. J. Am. Coll. Cardiol. 2012, 60, 2061–2071. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.; Meng, F.; Ma, N.; Li, C.; Ding, Z.; Wang, H.; Hou, R.; Qin, Y. Meta-analysis of safety of the coadministration of statin with fenofibrate in patients with combined hyperlipidemia. Am. J. Cardiol. 2012, 110, 1296–1301. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA Statement for Reporting Systematic Reviews and Meta-Analyses of Studies That Evaluate Health Care Interventions: Explanation and Elaboration. PLoS Med. 2009, 6, e1000100. [Google Scholar] [CrossRef]
- Kousios, A.; Hadjivasilis, A.; Kouis, P.; Panayiotou, A. The Effect of Fibrates on Kidney Function and Chronic Kidney Disease Progression: Protocol for a Systematic Review; Research Square: Durham, NC, USA, 2020. [Google Scholar] [CrossRef]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esenboga, K.; Çiçek, Ö.F.; Oktay, A.A.; Ayral, P.A.; Gürlek, A. Effect of fenofibrate on serum nitric oxide levels in patients with hypertriglyceridemia. Adv. Clin. Exp. Med. 2019, 28, 931–936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- La Fountaine, M.F.; Cirnigliaro, C.M.; Hobson, J.C.; Lombard, A.T.; Specht, A.F.; Dyson-Hudson, T.A.; Kirshblum, S.C.; Bauman, W.A. A Four Month Randomized Controlled Trial on the Efficacy of Once-daily Fenofibrate Monotherapy in Persons with Spinal Cord Injury. Sci. Rep. 2019, 9, 17166–17169. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Shirai, K.; Nagayama, D.; Nakamura, S.; Oka, R.; Tanaka, S.; Watanabe, Y.; Imamura, H.; Sato, Y.; Kawana, H.; et al. Bezafibrate ameliorates arterial stiffness assessed by cardio-ankle vascular index in hypertriglyceridemic patients with type 2 diabetes mellitus. J. Atheroscler. Thromb. 2019, 26, 659–669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arai, H.; Yamashita, S.; Yokote, K.; Araki, E.; Suganami, H.; Ishibashi, S. Efficacy and safety of pemafibrate versus fenofibrate in patients with high triglyceride and low HDL cholesterol levels: A multicenter, placebo-controlled, double-blind, randomized trial. J. Atheroscler. Thromb. 2018, 25, 521–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pinchbeck, J.L.; Moxon, J.V.; Rowbotham, S.E.; Bourke, M.; Lazzaroni, S.; Morton, S.K.; Matthews, E.O.; Hendy, K.; Jones, R.E.; Bourke, B.; et al. Randomized Placebo-Controlled Trial Assessing the Effect of 24-Week Fenofibrate Therapy on Circulating Markers of Abdominal Aortic Aneurysm: Outcomes From the FAME-2 Trial. J. Am. Heart Assoc. 2018, 7, e009866. [Google Scholar] [CrossRef] [Green Version]
- Koopal, C.; Marais, A.D.; Westerink, J.; Van Der Graaf, Y.; Visseren, F.L.J. Effect of adding bezafibrate to standard lipid-lowering therapy on post-fat load lipid levels in patients with familial dysbetalipoproteinemia. A randomized placebo-controlled crossover trial. J. Lipid Res. 2017, 58, 2180–2187. [Google Scholar] [CrossRef] [Green Version]
- Foucher, C.; Aubonnet, P.; Reichert, P.; Berli, M.; Schaeffer, A.; Vargas, C.G.C.; Lochocka, A.; Belenky, D.; Koch, H.-F.; Cholib study Investigators. New Fixed-Dose Combinations of Fenofibrate/Simvastatin Therapy Significantly Improve the Lipid Profile of High-Risk Patients with Mixed Dyslipidemia Versus Monotherapies. Cardiovasc. Ther. 2015, 33, 329–337. [Google Scholar] [CrossRef]
- Makariou, E.S.; Elisaf, M.; Kei, A.; Challa, A.; DiNicolantonio, J.J.; Liberopoulos, E. No effect of switching to high-dose rosuvastatin, add-on nicotinic acid, or addon fenofibrate on serum vitamin D levels in patients with mixed dyslipidemia. Hippokratia 2015, 19, 136–140. [Google Scholar]
- Chen, Y.-P.; Chang, K.-C.; Tseng, W.-K.; Yin, W.-H.; Chen, J.-W.; Lee, Y.-T.; Wu, C.-C. Increased Rosuvastatin Dose versus Concomitant Fenofibrate and Rosuvastatin Therapy to Achieve Lipid Goal in Patients with Diabetes or Atherosclerosis with Metabolic Syndrome. Acta Cardiol. Sin. 2013, 29, 421–428. Available online: http://www.ncbi.nlm.nih.gov/pubmed/27122739 (accessed on 11 December 2020). [PubMed]
- Li, X.P.; Gong, H.R.; Huang, X.S.; Huang, W.Y.; Zhao, S.P. The influence of statin-fibrate combination therapy on lipids profile and apolipoprotein A5 in patients with acute coronary syndrome. Lipids Health Dis. 2013, 12, 133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weinstein, D.L.; Williams, L.A.; Carlson, D.M.; Kelly, M.T.; Burns, K.M.; Setze, C.M.; Lele, A.; Stolzenbach, J.C. A randomized, double-blind study of fenofibric acid plus rosuvastatin compared with rosuvastatin alone in stage 3 chronic kidney disease. Clin. Ther. 2013, 35, 1186–1198. [Google Scholar] [CrossRef]
- Lee, S.H.; Cho, K.I.; Kim, J.Y.; Ahn, Y.K.; Rha, S.-W.; Kim, Y.-J.; Choi, Y.-S.; Choi, S.W.; Jeon, D.W.; Min, P.-K.; et al. Non-lipid effects of rosuvastatin-fenofibrate combination therapy in high-risk Asian patients with mixed hyperlipidemia. Atherosclerosis 2012, 221, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.M.E.; Ting, R.; Best, J.D.; Donoghoe, M.W.; Drury, P.L.; Sullivan, D.R.; Jenkins, A.J.; O’Connell, R.L.; Whiting, M.J.; Glasziou, P.P.; et al. Effects of fenofibrate on renal function in patients with type 2 diabetes mellitus: The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) Study. Diabetologia 2011, 54, 280–290. [Google Scholar] [CrossRef] [Green Version]
- Chan, D.C.; Hamilton, S.J.; Rye, K.A.; Chew, G.T.; Jenkins, A.J.; Lambert, G.; Watts, G.F. Fenofibrate concomitantly decreases serum proprotein convertase subtilisin/kexin type 9 and very-low-density lipoprotein particle concentrations in statin-treated type 2 diabetic patients. Diabetes Obes. Metab. 2010, 12, 752–756. [Google Scholar] [CrossRef] [PubMed]
- Derosa, G.; Maffioli, P.; Salvadeo, S.A.T.; Ferrari, I.; Gravina, A.; Mereu, R.; Palumbo, I.; D’Angelo, A.; Cicero, A. Fenofibrate, simvastatin and their combination in the management of dyslipidaemia in type 2 diabetic patients. Curr. Med. Res. Opin. 2009, 25, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Mohiuddin, S.M.; Pepine, C.J.; Kelly, M.T.; Buttler, S.M.; Setze, C.M.; Sleep, D.J.; Stolzenbach, J.C. Efficacy and safety of ABT-335 (fenofibric acid) in combination with simvastatin in patients with mixed dyslipidemia: A phase 3, randomized, controlled study. Am. Heart J. 2009, 157, 195–203. [Google Scholar] [CrossRef]
- Jones, P.H.; Davidson, M.H.; Kashyap, M.L.; Kelly, M.T.; Buttler, S.M.; Setze, C.M.; Sleep, D.J.; Stolzenbach, J.C. Efficacy and safety of ABT-335 (fenofibric acid) in combination with rosuvastatin in patients with mixed dyslipidemia: A phase 3 study. Atherosclerosis 2009, 204, 208–215. [Google Scholar] [CrossRef]
- Ansquer, J.C.; Dalton, R.N.; Caussé, E.; Crimet, D.; Le Malicot, K.; Foucher, C. Effect of Fenofibrate on Kidney Function: A 6-Week Randomized Crossover Trial in Healthy People. Am. J. Kidney Dis. 2008, 51, 904–913. [Google Scholar] [CrossRef]
- Saito, Y.; Yamada, N.; Shirai, K.; Sasaki, J.; Ebihara, Y.; Yanase, T.; Fox, J.C. Effect of rosuvastatin 5-20 mg on triglycerides and other lipid parameters in Japanese patients with hypertriglyceridemia. Atherosclerosis 2007, 194, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Athyros, V.G.; Mikhailidis, D.P.; Papageorgiou, A.A.; Didangelos, T.P.; Peletidou, A.; Kleta, D.; Karagiannis, A.; Kakafika, A.I.; Tziomalos, K.; Elisaf, M. Targeting vascular risk in patients with metabolic syndrome but without diabetes. Metabolism 2005, 54, 1065–1074. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, J.; Yamamoto, K.; Ageta, M. Effects of fenofibrate on high-density lipoprotein particle size in patients with hyperlipidemia: A randomized, double-blind, placebo-controlled, multicenter, crossover study. Clin Ther. 2002, 24, 1614–1626. [Google Scholar] [CrossRef]
- Levin, A.; Duncan, L.; Djurdjev, O.; Shapiro, R.J.; Frohlich, J.; Belanger, A.; Dumas, R.; Ross, S. A randomized placebo-controlled double-blind trial of lipid lowering strategies in patients with renal insufficiency: Diet modification with or without fenofibrate. Clin. Nephrol. 2000, 53, 140–146. [Google Scholar]
- Samuelsson, O.; Attman, P.O.; Knight-Gibson, C.; Kron, B.; Larsson, R.; Mulec, H.; Weiss, L.; Alaupovic, P. Effect of gemfibrozil on lipoprotein abnormalities in chronic renal insufficiency: A controlled study in human chronic renal disease. Nephron 1997, 75, 286–294. [Google Scholar] [CrossRef]
- Bruce, R.; Daniels, A.; Candy, T. Renal function changes in diabetic nephropathy induced by bezafibrate. Nephron 1996, 73, 490. [Google Scholar] [CrossRef]
- Barbir, M.; Hunt, B.; Kushwaha, S.; Kehely, A.; Prescot, R.; Thompson, G.R.; Mitchell, A.; Yacoub, M. Maxepa versus bezafibrate in hyperlipidemic cardiac transplant recipients. Am. J. Cardiol. 1992, 70, 1596–1601. [Google Scholar] [CrossRef]
- Jones, I.R.; Swai, A.; Taylor, R.; Miller, M.; Laker, M.F.; Alberti, K.G.M.M. Lowering of plasma glucose concentrations with bezafibrate in patients with moderately controlled NIDDM. Diabetes Care 1990, 13, 855–863. [Google Scholar] [CrossRef]
- Davidson, M.H.; Rooney, M.W.; Drucker, J.; Eugene Griffin, H.; Oosman, S.; Beckert, M. Efficacy and tolerability of atorvastatin/fenofibrate fixed-dose combination tablet compared with atorvastatin and fenofibrate monotherapies in patients with dyslipidemia: A 12-week, multicenter, double-blind, randomized, parallel-group study. Clin. Ther. 2009, 31, 2824–2838. [Google Scholar] [CrossRef]
- Tanaka, T.; Higashijima, Y.; Wada, T.; Nangaku, M. The potential for renoprotection with incretin-based drugs. Kidney Int. 2014, 86, 701–711. [Google Scholar] [CrossRef] [Green Version]
- Jakob, T.; Nordmann, A.J.; Schandelmaier, S.; Ferreira-González, I.; Briel, M. Fibrates for primary prevention of cardiovascular disease events. Cochrane Database Syst. Rev. 2016, 11, CD009753. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, K.; Pattharanitima, P.; Patel, N.; Duffy, A.; Saha, A.; Chaudhary, K.; Debnath, N.; Van Vleck, T.; Chan, L.; Nadkarni, G.N.; et al. Rate of correction of hypernatremia and health outcomes in critically ill patients. Clin. J. Am. Soc. Nephrol. 2019, 14, 656–663. [Google Scholar] [CrossRef]
- Lipscombe, J.; Lewis, G.F.; Cattran, D.; Bargman, J.M. Deterioration in renal function associated with fibrate therapy. Clin Nephrol. 2001, 55, 39–44. Available online: https://europepmc.org/article/med/11200866 (accessed on 4 December 2020). [PubMed]
- Nicolaou, O.; Kousios, A.; Sokratous, K.; Potamiti, L.; Koniali, L.; Neophytou, G.; Papacharalampous, R.; Zanti, M.; Ioannou, K.; Hadjisavvas, A.; et al. Alport syndrome: Proteomic analysis identifies early molecular pathway alterations in Col4a3 knock out mice. Nephrology 2020, 25, 937–949. [Google Scholar] [CrossRef] [PubMed]
- Harzandi, A.; Lee, S.; Bidkhori, G.; Saha, S.; Hendry, B.M.; Mardinoglu, A.; Shoaie, S.; Sharpe, C.C. Acute Kidney Injury Leading to CKD is Associated with a Persistence of Metabolic Dysfunction and Hypertriglyceridemia. iScience 2021, 24, 102046. [Google Scholar] [CrossRef] [PubMed]
- Park, C.W.; Zhang, Y.; Zhang, X.; Wu, J.; Chen, L.; Cha, D.; Su, D.; Hwang, M.-T.; Fan, X.; Davis, L.; et al. PPARα agonist fenofibrate improves diabetic nephropathy in db/db mic. Kidney Int. 2006, 69, 1511–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, H.W.; Lim, J.H.; Kim, M.Y.; Shin, S.J.; Chung, S.; Choi, B.S.; Kim, H.W.; Kim, Y.-S.; Park, C.W.; Chang, Y.S. High-fat diet-induced renal cell apoptosis and oxidative stress in spontaneously hypertensive rat are ameliorated by fenofibrate through the PPARα-FoxO3a-PGC-1α pathway. Nephrol. Dial. Transplant. 2012, 27, 2213–2225. [Google Scholar] [CrossRef] [Green Version]
- Ting, R.D.; Keech, A.C.; Drury, P.L.; Donoghoe, M.W.; Hedley, J.; Jenkins, A.J.; Davis, T.M.; Lehto, S.; Celermajer, D.; Simes, R.J.; et al. Benefits and safety of long-term fenofibrate therapy in people with type 2 diabetes and renal impairment: The FIELD study. Diabetes Care. 2012, 35, 218–225. [Google Scholar] [CrossRef] [Green Version]
- Tonelli, M.; Collins, D.; Robins, S.; Bloomfield, H.; Curhan, G.C. Gemfibrozil for secondary prevention of cardiovascular events in mild to moderate chronic renal insufficiency. Kidney Int. 2004, 66, 1123–1130. [Google Scholar] [CrossRef] [Green Version]



| First Author | Year | Study Name | Intervention | Control | No Intervention | No Control |
|---|---|---|---|---|---|---|
| Esenboga | 2019 | fenofibrate 250 mg/d | placebo | 30 | 26 | |
| La Fountaine | 2019 | fenofibrate 145 mg | control | 10 | 8 | |
| Yamaguchi | 2019 | bezafibrate 400 mg | eicosapentaenoic acid 1.8 g/day | 33 | 31 | |
| Arai | 2018 | pemafibrate (0.1 or 0.2 or 0.4 mg) or fenofibrate (100 mg or 200) | placebo | pemafibrate 0.1 mg: 45, pemafibrate 0.2 mg: 128, pemafibrate 0.4 mg: 84, fenofibrate 100 mg: 85, fenofibrate 200 mg: 140 | 43 | |
| Pinchbeck | 2018 | FAME | 145 mg fenofibrate | placebo | 70 | 70 |
| Koopal | 2017 | bezafibrate | placebo | 15 in total | crossover | |
| Foucher | 2015 | fenofibrate/simvastatin 145/20 mg or 145/40 mg | simvastatin 20 mg or 40 mg | fenofibrate/simvastatin 145/20 mg: 109, fenofibrate/simvastatin 145/40 mg: 110 | simvastatin 20 mg: 114, simvastatin 40 mg: 112 | |
| Makariou | 2014 | add-on-statin micronised fenofibrate (200 mg) | rosuvastatin 40 mg | 13 | 17 | |
| Chen | 2013 | fenofibrate 80 mg + rosuvastatin 5 mg | fosuvastatin 10 mg | 50 | 62 | |
| Li Xiang-ping | 2013 | atorvastatin 20 mg + bezafibrate 200 mg | atorvastatin 20 mg | 52 | 52 | |
| Weinstein | 2013 | fenofibric acid + rosuvastatin 5 then 10 mg | rosuvastatin 5 then 10 | 140 | 140 | |
| Lee | 2012 | rosuvastatin10 mg + fenofibrate160 mg | rosuvastatin10 mg | 90 | 90 | |
| Davis | 2011 | FIELD | fenofibrate | placebo | 4895 | 4900 |
| Ginsberg | 2010 | ACCORD | fenofibrate + simvastaatin | placebo + simvastatin | 2765 | 2753 |
| Chan | 2010 | fenofibrate (145 mg/day) | placebo | 15 in total | crossover | |
| Derosa | 2009 | fenofibrate 145 mg + simvastatin 40 mg/d | simvastatin 40 mg/d | 79 | 82 | |
| Davidson | 2009 | atorvastatin 40 mg and fenofibrate 100 mg | atorvastatin 40 mg, or fenofibrate 145 mg | 73 | 74 for statin | |
| Mohiuddin | 2009 | fenofibric acid 135 mg+ rosuvastatin 20 mg OR fenofibric acids 135 mg + rosuvastatin 40 mg | rosuvastatin 20 mg OR rosuvastatin 40 mg | fenofibric acid 135 mg+ rosuvastatin 20 mg: 113, fenofibric acids 135 mg + rosuvastatin 40 mg: 111 | rosuvastatin 20 mg: 116, rosuvastatin 40 mg: 112 | |
| Jones | 2009 | fenofibric acid 135 mg + rosuvastatin 10 mg OR fenofibric acids 135 mg + rosuvastatin 20 mg | rosuvastatin 10 mg OR rosuvastatin 20 mg | fenofibric acid 135 mg + rosuvastatin 10 mg: 261, fenofibric acids 135 mg + rosuvastatin 20 mg: 262 | rosuvastatin 10 mg: 265, rosuvastatin 20 mg: 266 | |
| Ansquer | 2008 | fenofibrate (160-mg/ | placebo | 21 in total | crossover | |
| Saito | 2007 | bezafibrate 200 mg | placebo | 27 | 35 | |
| Ansquer | 2005 | DAIS | 200 mg of micronised fenofibrate | placebo | 155 | 159 |
| Athyros | 2005 | fenofibrate 200 mg OR fenofibrate 200 mg + atorvastatin 20 mg | control (diet) OR atorvastatin, 20 mg/d | fenofibrate 200 mg: 100, fenofibrate 200 mg + atorvastatin 20 mg: 100 | control (diet): 100, atorvastatin 20 mg: 100 | |
| Sasaki | 2002 | fenofibrate 300 mg | placebo | 50 crossover | Data for creatinine from 35 patients | |
| Levin | 2000 | fenofibrate | placebo | 16 | 12 | |
| Samuelsson | 1997 | gemfibrozil | dietary | 28 | 29 | |
| Bruce | 1996 | bezafibrate 400 mg | placebo | 12 | 12 | |
| Barbir | 1992 | bezafibrate | placebo (maxepa (fish oil)) | 43 | 44 | |
| Jones | 1990 | bezafibrate (200 mg 3 times/day | placebo | 20 | 17 |
| Author | Year | Study Name | Random Sequence Generation (Selection Bias) | Allocation Concealment (Selection Bias) | Blinding of Participants and Personnel (Performance Bias) | Blinding of Outcome Assessment (Detection Bias) | Incomplete Outcome Data (Attrition Bias) | Selective Reporting (Reporting Bias) | Other Bias |
|---|---|---|---|---|---|---|---|---|---|
| Esenboga | 2019 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| La Fountaine | 2019 | LOW | LOW | HIGH | HIGH | NOT CLEAR | LOW | NOT CLEAR | |
| Yamaguchi | 2019 | LOW | LOW | HIGH | HIGH | LOW | LOW | LOW | |
| Arai | 2018 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Pinchbeck | 2018 | FAME | LOW | LOW | LOW | LOW | LOW | LOW | NOT CLEAR |
| Koopal | 2017 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Foucher | 2015 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Makariou | 2014 | LOW | LOW | HIGH | HIGH | NOT CLEAR | LOW | NOT CLEAR | |
| Chen | 2013 | LOW | LOW | HIGH | LOW | LOW | LOW | LOW | |
| Li, Xiang ping | 2013 | LOW | LOW | HIGH | HIGH | LOW | LOW | LOW | |
| Weinstein | 2013 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Lee | 2012 | LOW | LOW | HIGH | HIGH | LOW | LOW | NOT CLEAR | |
| Davis | 2011 | FIELD | LOW | LOW | LOW | LOW | LOW | LOW | LOW |
| Ginsberg | 2010 | ACCORD | LOW | LOW | LOW | LOW | LOW | LOW | LOW |
| Chan | 2010 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Derosa | 2009 | LOW | NOT CLEAR | LOW | LOW | LOW | LOW | HIGH | |
| Davidson | 2009 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Mohiuddin | 2009 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Jones | 2009 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Ansquer | 2008 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Saito | 2007 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Ansquer | 2005 | DAIS | LOW | LOW | LOW | LOW | LOW | LOW | LOW |
| Athyros | 2005 | LOW | LOW | HIGH | HIGH | LOW | LOW | LOW | |
| Sasaki | 2002 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Levin | 2000 | LOW | LOW | LOW | LOW | LOW | LOW | LOW | |
| Samuelsson | 1997 | LOW | LOW | HIGH | HIGH | LOW | LOW | LOW | |
| Bruce | 1996 | LOW | LOW | LOW | LOW | LOW | LOW | NOT CLEAR | |
| Barbir | 1992 | LOW | LOW | HIGH | HIGH | LOW | LOW | LOW | |
| Jones | 1990 | LOW | LOW | LOW | LOW | LOW | LOW | LOW |
| Low Risk | Not Clear | High Risk | |
|---|---|---|---|
| Random sequence generation (selection bias) | 100% | 0% | 0% |
| Allocation concealment (selection bias) | 96.55% | 3.45% | 0% |
| Blinding of participants and personnel (performance bias) | 68.97% | 0% | 31.03% |
| Blinding of outcome assessment (detection bias) | 72.41% | 0% | 27.59% |
| Incomplete outcome data (attrition bias) | 79.31% | 20.69% | 0% |
| Selective reporting (reporting bias) | 100% | 0% | 0% |
| Other bias | 79.31% | 17.24% | 3.45% |
| Outcome | Method | Effect Estimate | 95% CI Lower Limit | 95% CI Upper Limit | Heterogeneity I2 % |
|---|---|---|---|---|---|
| Creatinine all studies | SMD | 1.05 | 0.63 | 1.46 | 99.1 |
| Creatinine studies using fenofibrate | SMD | 1.34 | 0.82 | 1.86 | 99.4 |
| Creatinine fenofibrate vs. placebo | SMD | 1.22 | 0.74 | 1.89 | 94 |
| Creatinine fenofibrate + statin vs. statin | SMD | 1.07 | 0.34 | 1.79 | 99.3 |
| Creatinine studies using bezafibrates | SMD | 0.68 | 0.01 | 1.34 | 88.8 |
| Creatinine bezafibrate vs. placebo | SMD | 0.79 | −0.01 | 1.59 | 88.9 |
| Short term creatinine all studies | SMD | 0.97 | 0.67 | 1.26 | 93.6 |
| Short term creatinine studies using fenofibrate | SMD | 1.23 | 0.88 | 1.58 | 94.1 |
| Short term creatinine fenofibrate vs. placebo | SMD | 2.73 | 1.53 | 3.94 | 96 |
| Short term creatinine fenofibrate plus statin vs. statin | SMD | 1.02 | 0.70 | 1.34 | 92.8 |
| Short term creatinine studies using bezafibrate | SMD | 0.65 | −0.11 | 1.42 | 91 |
| Short term creatinine bezafibrate vs. placebo | SMD | 0.79 | −0.17 | 1.75 | 91.7 |
| Creatinine in patients with diabetes all studies | SMD | 1.49 | 0.29 | 2.71 | 99.8 |
| Creatinine in patients with diabetes, fenofibrate vs. placebo | SMD | 0.86 | 0.35 | 1.37 | 91.8 |
| eGFR all studies | SMD | −1.99 | −3.42 | −0.48 | 99.5 |
| eGFR all studies with fenofibrates | SMD | −2.69 | −4.47 | −0.91 | 99.4 |
| eGFR fenofibrate vs. placebo | SMD | −2.53 | −4.46 | −0.60 | 99.3 |
| eGFR fenofibrate plus statin vs. statin | SMD | −2.98 | −8.00 | 2.05 | 99.5 |
| Short term eGFR all studies | SMD | −1.88 | −3.02 | −0.73 | 98.4 |
| Short term eGFR studies using fenofibrate | SMD | −2.64 | −4.55 | −0.72 | 98.9 |
| Short term eGFR fenofibrate vs. placebo | SMD | −2.38 | −4.20 | −0.57 | 97.8 |
| Short term eGFR fenofibrate plus statin vs. statin | SMD | −2.98 | −8.00 | 2.05 | 99.5 |
| Progression of albuminuria | RR | 0.86 | 0.76 | 0.98 | 63.5 |
| Regression of albuminuria | RR | 1.19 | 1.08 | 1.31 | 0 |
| Urinary protein excretion change | SMD | −0.14 | −0.56 | 0.29 | 0 |
| End stage kidney disease development | RR | 0.85 | 0.49 | 1.49 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hadjivasilis, A.; Kouis, P.; Kousios, A.; Panayiotou, A. The Effect of Fibrates on Kidney Function and Chronic Kidney Disease Progression: A Systematic Review and Meta-Analysis of Randomised Studies. J. Clin. Med. 2022, 11, 768. https://doi.org/10.3390/jcm11030768
Hadjivasilis A, Kouis P, Kousios A, Panayiotou A. The Effect of Fibrates on Kidney Function and Chronic Kidney Disease Progression: A Systematic Review and Meta-Analysis of Randomised Studies. Journal of Clinical Medicine. 2022; 11(3):768. https://doi.org/10.3390/jcm11030768
Chicago/Turabian StyleHadjivasilis, Alexandros, Panayiotis Kouis, Andreas Kousios, and Andrie Panayiotou. 2022. "The Effect of Fibrates on Kidney Function and Chronic Kidney Disease Progression: A Systematic Review and Meta-Analysis of Randomised Studies" Journal of Clinical Medicine 11, no. 3: 768. https://doi.org/10.3390/jcm11030768
APA StyleHadjivasilis, A., Kouis, P., Kousios, A., & Panayiotou, A. (2022). The Effect of Fibrates on Kidney Function and Chronic Kidney Disease Progression: A Systematic Review and Meta-Analysis of Randomised Studies. Journal of Clinical Medicine, 11(3), 768. https://doi.org/10.3390/jcm11030768






