Temporal Changes in the Oxyhemoglobin Dissociation Curve of Critically Ill COVID-19 Patients
Abstract
:1. Introduction
2. Materials and Methods
2.1. p50 Calculation with Variable Coefficient
2.2. The p50 Shift over Time
2.3. Outcomes
2.4. Statistical Analysis
3. Results
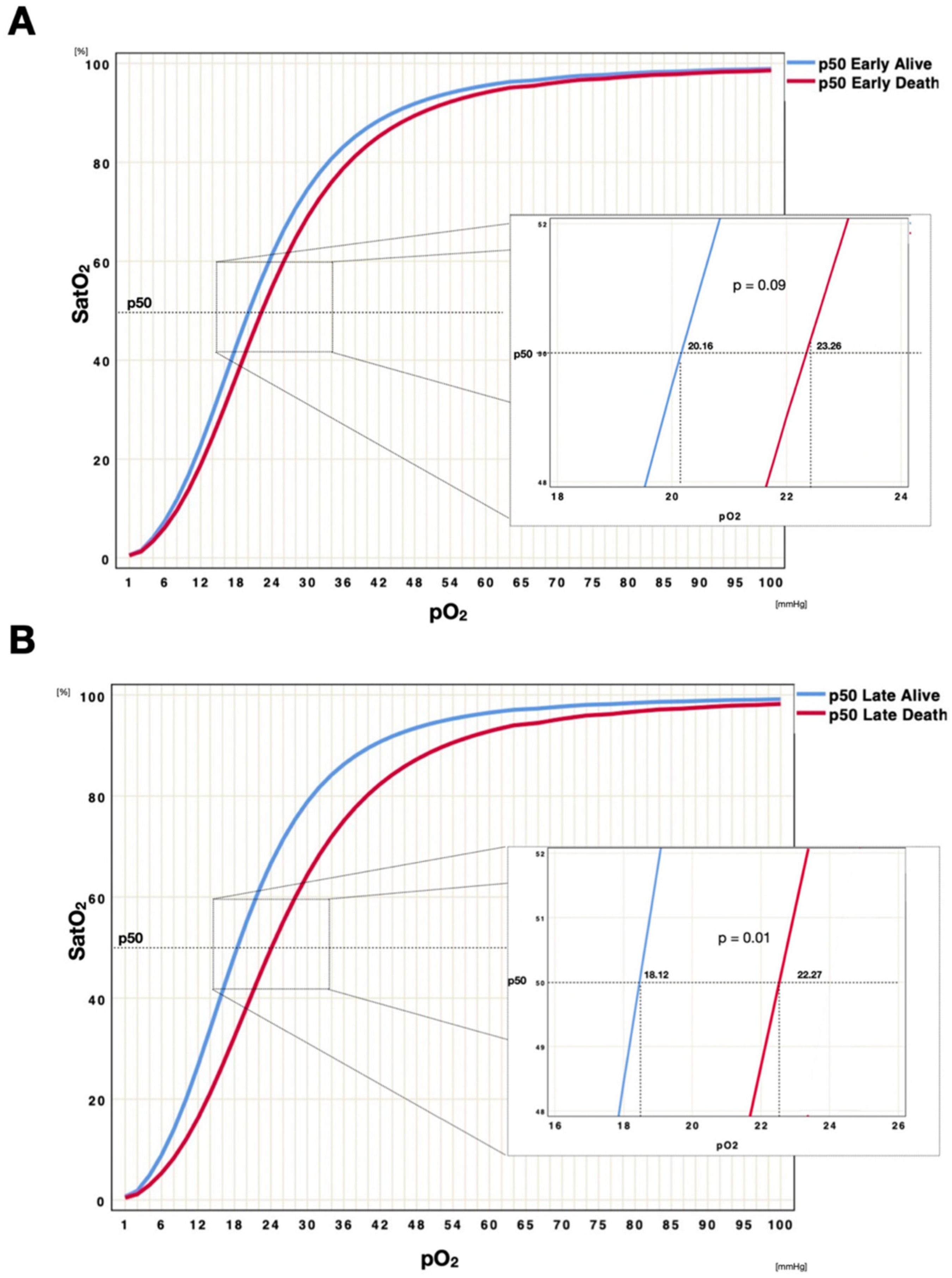
3.1. p50 Shift over Time
3.2. Subgroup Analysis
3.3. Variance Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Collins, J.A.; Rudenski, A.; Gibson, J.; Howard, L.; O’Driscoll, R. Relating oxygen partial pressure, saturation and content: The haemoglobin–oxygen dissociation curve. Breathe 2015, 11, 194–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kacmarek, R.M.; Stoller, J.K.; Heuer, A. (Eds.) Egan’s Fundamentals of Respiratory Care—E-Book; Elsevier Health Sciences: Frisco, CO, USA, 2019. [Google Scholar]
- Bohr, C.; Hasselbalch, K.; Krogh, A. Ueber einen in biologischer Ueziehung wichtigen Ein- fluss, den die Kohlensaurespannung des Blutes. Skand. Arch. Physiol. 1904, 16, 402–412. [Google Scholar] [CrossRef]
- West, J.B. Effect of slope and shape of dissociation curve on pulmonary gas exchange. Respir. Physiol. 1969, 8, 66–85. [Google Scholar] [CrossRef]
- Jensen, F.B. Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport. Acta Physiol. Scand. 2004, 182, 215–227. [Google Scholar] [CrossRef]
- Ivanov, L.A.; Chebotarev, N.D. Effect of Hyperoxia on the Oxyhemoglobin Dissociation Curve in Various Periods of Aging. Available online: https://pubmed.ncbi.nlm.nih.gov/6466848/ (accessed on 11 May 2021).
- Ditzel, J. The Problem of Tissue Oxygenation in Diabetes Mellitus: The “Three-in-One Concept” for the Development of Diabetic Microangiopathy and a Rational Approach to Its Prophylaxis. Available online: https://pubmed.ncbi.nlm.nih.gov/1057374/ (accessed on 11 May 2021).
- Lanza, V.; Mercadante, S.; Pignataro, A. Effects of Halothane, Enflurane, and Nitrous Oxide on Oxyhemoglobin Affinity. Anesthesiology 1988, 68, 591–594. [Google Scholar] [CrossRef]
- Clerbaux, T.; Detry, B.; Reynaert, M.; Frans, A. Right shift of the oxyhemoglobin dissociation curve in acute respiratory distress syndrome. Pathol. Biol. 1997, 45, 269–273. [Google Scholar] [PubMed]
- Hill, A. The possible effects of the aggregation of the molecules of haemoglobin on its dissociation curves. J. Physiol. 1910, 40, 4–7. [Google Scholar]
- Severinghaus, J.W. Simple, Accurate Equations for Human Blood O2 Dissociation Computations. Available online: https://pubmed.ncbi.nlm.nih.gov/35496/ (accessed on 5 May 2021).
- Dash, R.K.; Korman, B.; Bassingthwaighte, J.B. Simple accurate mathematical models of blood HbO2 and HbCO2 dissociation curves at varied physiological conditions: Evaluation and comparison with other models. Eur. J. Appl. Physiol. 2016, 116, 97–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sohrabi, C.; Alsafi, Z.; O’Neill, N.; Khan, M.; Kerwan, A.; Al-Jabir, A.; Iosifidis, C.; Agha, R. World Health Organization declares global emergency: A review of the 2019 novel coronavirus (COVID-19). Int. J. Surg. 2020, 76, 71–76. [Google Scholar] [CrossRef]
- Singh, S.P.; Pritam, M.; Pandey, B.; Yadav, T.P. Microstructure, pathophysiology, and potential therapeutics of COVID-19: A comprehensive review. J. Med. Virol. 2021, 93, 275–299. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Tobin, M.J.; Laghi, F.; Jubran, A. Why COVID-19 silent hypoxemia is baffling to physicians. Am. J. Respir. Crit. Care Med. 2020, 202, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Wenzhong, L.; Hualan, L. COVID-19: Attacks the 1-beta Chain of Hemoglobin to Disrupt Respiratory Function and Escape Immunity. ChemRxiv 2022. preprint. [Google Scholar] [CrossRef]
- Lansiaux, E.; Pébaÿ, P.P.; Picard, J.L.; Son-Forget, J. COVID-19: Beta-thalassemia subjects immunised? Med. Hypotheses 2020, 142, 109827. [Google Scholar] [CrossRef]
- Vogel, D.J.; Formenti, F.; Retter, A.J.; Vasques, F.; Camporota, L. A left shift in the oxyhaemoglobin dissociation curve in patients with severe coronavirus disease 2019 (COVID-19). Br. J. Haematol. 2020, 191, 390–393. [Google Scholar] [CrossRef] [PubMed]
- Daniel, Y.; Hunt, B.J.; Retter, A.; Henderson, K.; Wilson, S.; Sharpe, C.C.; Shattock, M.J. Haemoglobin oxygen affinity in patients with severe COVID-19 infection. Br. J. Haematol. 2020, 190, e126–e127. [Google Scholar] [CrossRef] [PubMed]
- Pascual-Guàrdia, S.; Ferrer, A.; Díaz, Ó.; Caguana, A.O.; Tejedor, E.; Bellido-Calduch, S.; Rodríguez-Chiaradia, D.A.; Gea, J. Absence of Relevant Clinical Effects of SARS-COV-2 on the Affinity of Hemoglobin for O2 in Patients with COVID-19. Arch. Bronconeumol. 2021, 57, 757–763. [Google Scholar] [CrossRef]
- Lim, A.Y.H.; Goh, J.L.; Chua, M.C.W.; Heng, B.H.; Abisheganaden, J.A.; George, P.P. Temporal changes of haematological and radiological findings of the COVID-19 infection—A review of literature. BMC Pulm. Med. 2021, 21, 37. [Google Scholar] [CrossRef]
- To, K.K.-W.; Tsang, O.T.-Y.; Leung, W.-S.; Tam, A.R.; Wu, T.-C.; Lung, D.C.; Yip, C.C.-Y.; Cai, J.-P.; Chan, J.M.-C.; Chik, T.S.-H.; et al. Temporal profiles of viral load in posterior oropharyngeal saliva samples and serum antibody responses during infection by SARS-CoV-2: An observational cohort study. Lancet Infect. Dis. 2020, 20, 565–574. [Google Scholar] [CrossRef] [Green Version]
- Weiss, J.N. The Hill equation revisited: Uses and misuses. FASEB J. 1997, 11, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Tobin, M.J. Basing respiratory management of COVID-19 on physiological principles. Am. J. Respir. Crit. Care Med. 2020, 201, 1319–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gothe, B. Egan’S Fundamentals of Respiratory Care. Chest 1990, 98, 16. [Google Scholar] [CrossRef]
- Louw, A.; Cracco, C.; Cerf, C.; Harf, A.; Duvaldestin, P.; Lemaire, F.; Brochard, L. Accuracy of pulse oximetry in the intensive care unit. Intensive Care Med. 2001, 27, 1606–1613. [Google Scholar] [CrossRef] [PubMed]
- Maes, M.; Higginson, E.; Pereira-Dias, J.; Curran, M.D.; Parmar, S.; Khokhar, F.; Cuchet-Lourenço, D.; Lux, J.; Sharma-Hajela, S.; Ravenhill, B.; et al. Ventilator-associated pneumonia in critically ill patients with COVID-19. Crit. Care 2021, 25, 25. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Fan, J.; Yu, F.; Feng, B.; Lou, B.; Zou, Q.; Xie, G.; Lin, S.; Wang, R.; Yang, X.; et al. Viral load dynamics and disease severity in patients infected with SARS-CoV-2 in Zhejiang province, China, January–March 2020: Retrospective cohort study. BMJ 2020, 369, m1443. [Google Scholar] [CrossRef] [Green Version]
- Harutyunyan, G.; Harutyunyan, G.; Mkhoyan, G.; Harutyunyan, V.; Soghomonyan, S. Haemoglobin oxygen affinity in patients with severe COVID-19 infection: Still unclear. Br. J. Haematol. 2020, 190, 725–726. [Google Scholar] [CrossRef]
- Shattock, M.J.; Daniel, Y.; Hunt, B.J.; Retter, A.; Henderson, K.; Wilson, S.; Sharpe, C.C. COVID-19 and haemoglobin oxygen affinity: Some clarity? Br. J. Haematol. 2020, 190, 723–724. [Google Scholar] [CrossRef]
- Voet, D.; Voet, J.G. Biochemistry, 4th ed.; Wiley: New York, NY, USA, 2010; p. 1520. ISBN 978-0-470-57095-1. [Google Scholar]
- Gattinoni, L.; Coppola, S.; Cressoni, M.; Busana, M.; Rossi, S.; Chiumello, D. COVID-19 does not lead to a “typical” acute respiratory distress syndrome. Am. J. Respir. Crit. Care Med. 2020, 201, 1299–1300. [Google Scholar] [CrossRef] [Green Version]
- Bendjelid, K.; Raphaël, G. Treating Hypoxemic Patients with SARS-COV-2 Pneumonia: Back to Applied Physiology. Available online: https://www.sciencedirect.com/science/article/abs/pii/S2352556820300692?via%3Dihub (accessed on 5 October 2020).
- Morgan, T.J.; Koch, D.; Morris, D.; Clague, A.; Purdie, D.M. Reduced red cell 2,3-diphosphoglycerate concentrations in critical illness without decreased in vivo P50. Anaesth. Intensive Care 2001, 29, 479–483. [Google Scholar] [CrossRef] [Green Version]





| Label | Unit | Mean (SD)/N (%) |
|---|---|---|
| Clinical characteristics | ||
| Arterial Hypertension | 14 (42.4) | |
| Diabetes | 12 (36.3) | |
| OSAS | 5 (15.1) | |
| COPD | 3 (9) | |
| Pulmonary Embolism | 5 (15.1) | |
| CVVHDF | 5 (15.1) | |
| SAPS II | 43 ± 6 | |
| SOFA | 6.5 (4.0–8.75) | |
| NEMS | 36 (19.25–39.0) | |
| LOS | days | 14 (12–28) |
| ICU overall survival | 25 (67.5) | |
| Laboratory | ||
| CRP | mg/L | 245 ± 111 |
| LDH | U/L | 595 (482–770) |
| Total bilirubin | μmol/L | 9.7 (7.6–16.4) |
| AST | U/L | 52.5 (42.7–83.0) |
| ALT | U/L | 42.0 (28.0–56.0) |
| Creatinine Kinase | U/L | 337 (82–562) |
| White cells | G/L | 7.7 (5.6–11.1) |
| Lymphocytes | G/L | 0.8 (0.5–1.0) |
| Lactate | mmol/L | 0.9 (0.8–1.5) |
| Ferritin | ng/mL | 2101 (1046–3857) |
| Creatinine | μmol/L | 88 (72–122) |
| Thrombocytes | G/L | 242 ± 89 |
| Arterial Blood gas analysis | ||
| Number | 3′514 | |
| pH | 7.39 ± 0.08 | |
| PaCO2 | mmHg | 48 ± 12 |
| PaO2 | mmHg | 82 ± 25 |
| HCO3− | mmol/L | 28 ± 5 |
| BE | mmol/L | 3 ± 4 |
| SatO2 | % | 95 ± 15 |
| Hemoglobin | g/dL | 11.5 ± 2.0 |
| Hematocrit | % | 35 ± 7 |
| COHb | % | 1.3 ± 0.5 |
| MetHb | % | 0.9 ± 0.4 |
| Na+ | mmol/L | 146 ± 6 |
| K+ | mmol/L | 4 ± 0.8 |
| Ca2+ | mmol/L | 1.14 ± 0.08 |
| Cl− | mmol/L | 110 ± 7 |
| Glucose | mmol/L | 8.9 ± 3.1 |
| Temperature | °C | 37.1 ± 0.4 |
| Lactate | mmol/L | 1.3 ± 0.5 |
| Pt 1 | Pt 2 | Pt 3 | Pt 4 | Pt 5 | Pt 6 | Pt 7 | Pt 8 | ||
| n | 168 | 21 | 79 | 236 | 88 | 66 | 75 | 46 | |
| pH | 7.37 ± 0.04 | 7.44 ± 0.03 | 7.40 ± 0.05 | 7.41 ± 0.07 | 7.44 ± 0.05 | 7.44 ± 0.03 | 7.43 ± 0.04 | 7.44 ± 0.04 | |
| PaO2 | [mmHg] | 86.9 ± 20.1 | 87.3 ± 33.8 | 108.2 ± 43.9 | 85.3 ± 28.6 | 102.2 ± 29.5 | 92.9 ± 34.4 | 100.9 ± 32.5 | 112.2 ± 33.8 |
| PaCO2 | [mmHg] | 43 ± 5.6 | 37.8 ± 4.9 | 42 ± 5.7 | 47.7 ± 8.9 | 44.7 ± 6.7 | 42 ± 4.4 | 36.7 ± 4.2 | 40.4 ± 6.4 |
| HCO3 | [mmol/L] | 24.9 ± 2.0 | 25.2 ± 2.1 | 25.9 ± 3.4 | 29.9 ± 4.4 | 30 ± 3.3 | 28.7 ± 3.1 | 24.6 ± 3.7 | 27.2 ± 2.9 |
| BE | [mmol/L] | −0.4 ± 1.8 | 1.2 ± 1.6 | 0.9 ± 3.3 | 4.5 ± 4.3 | 5.1 ± 3.1 | 4 ± 3.0 | 0.5 ± 3.7 | 2.7 ± 2.3 |
| SaO2 | [%] | 95 ± 6 | 97 ± 2 | 97 ± 5 | 95 ± 7 | 97 ± 4 | 96 ± 2 | 97 ± 4 | 98 ± 2 |
| Hb | [g/L] | 9.7 ± 1.3 | 14.6 ± 1.6 | 13.2 ± 1.1 | 11.1 ± 2.8 | 12 ± 0.9 | 13.5 ± 2.7 | 11.4 ± 2.8 | 12.5 ± 3.1 |
| Hct | [%] | 30 ± 4 | 45 ± 5 | 40 ± 4 | 34 ± 9 | 37 ± 3 | 41 ± 8 | 35 ± 8 | 38 ± 9 |
| O2Hb | [%] | 93 ± 5 | 95 ± 2 | 95 ± 5 | 93 ± 4 | 95 ± 4 | 94 ± 3 | 95 ± 4 | 95 ± 2 |
| COHb | [%] | 1.6 ± 0.3 | 1.2 ± 0.3 | 1.0 ± 0.4 | 1.7 ± 0.4 | 1.3 ± 0.2 | 0.8 ± 0.3 | 0.7 ± 0.3 | 1.0 ± 0.4 |
| MetHb | [%] | 0.8 ± 0.4 | 0.4 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.2 | 0.8 ± 0.3 | 1.1 ± 0.3 | 1.2 ± 0.3 | 1.1 ± 0.3 |
| Na+ | [mmol/L] | 143 ± 5 | 141 ± 2 | 143 ± 3 | 152 ± 6 | 150 ± 4 | 148 ± 2 | 141 ± 4 | 147 ± 4 |
| K+ | [mmol/L] | 4.4 ± 0.5 | 3.8 ± 0.2 | 3.9 ± 0.3 | 3.9 ± 0.4 | 4.2 ± 2.3 | 3.7 ± 0.3 | 4.0 ± 0.4 | 3.8 ± 0.4 |
| Ca2+ | [mmol/L] | 1.09 ± 0.08 | 1.08 ± 0.04 | 1.17 ± 0.05 | 1.11 ± 0.07 | 1.16 ± 0.05 | 1.13 ± 0.05 | 1.11 ± 0.04 | 1.17 ± 0.06 |
| Cl− | [mmol/L] | 107 ± 5 | 109 ± 2 | 111 ± 4 | 114 ± 6 | 111 ± 5 | 111 ± 3 | 110 ± 4 | 113 ± 5 |
| Glucose | [mmol/L] | 9.7 ± 2.2 | 5.7 ± 1.3 | 7.3 ± 1.1 | 10.2 ± 4.1 | 9.5 ± 1.8 | 9.8 ± 1.9 | 6.2 ± 0.9 | 9.7 ± 1.8 |
| Lactate | [mmol/L] | 1.3 ± 0.3 | 0.8 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.7 | 1.1 ± 0.3 | 1.0 ± 0.3 | 1.3 ± 0.4 | 1.1 ± 0.3 |
| Pt 9 | Pt 10 | Pt 11 | Pt 12 | Pt 13 | Pt 14 | Pt 15 | Pt 16 | ||
| n | 155 | 75 | 164 | 103 | 77 | 56 | 125 | 204 | |
| pH | 7.44 ± 0.08 | 7.42 ± 0.04 | 7.34 ± 0.07 | 7.38 ± 0.12 | 7.40 ± 0.06 | 7.43 ± 0.04 | 7.45 ± 0.04 | 7.40 ± 0.06 | |
| PaO2 | [mmHg] | 97.8 ± 37.7 | 89.8 ± 26.3 | 78.3 ± 24.8 | 83 ± 21.9 | 90.5 ± 20.2 | 85.4 ± 21.9 | 78.3 ± 14.8 | 72.8 ± 13.4 |
| PaCO2 | [mmHg] | 46.4 ± 13.9 | 46.1 ± 6.9 | 53.3 ± 10.3 | 49 ± 17.1 | 42.4 ± 5.8 | 44.6 ± 4.1 | 41.5 ± 8.9 | 51.1 ± 10.3 |
| HCO3 | [mmol/L] | 30.6 ± 4.1 | 29.8 ± 4.1 | 28.4 ± 3.5 | 27.3 ± 3.5 | 26.2 ± 3.2 | 29.3 ± 2.7 | 28.7 ± 5.2 | 30.8 ± 3.3 |
| BE | [mmol/L] | 5.6 ± 2.7 | 4.6 ± 3.6 | 1.9 ± 3.6 | 1.3 ± 3.8 | 1.1 ± 3.4 | 4.3 ± 2.8 | 4.2 ± 4.2 | 5.1 ± 2.8 |
| SaO2 | [%] | 97 ± 2 | 96 ± 4 | 95 ± 5 | 96 ± 3 | 97 ± 2 | 96 ± 5 | 95 ± 2 | 94 ± 3 |
| Hb | [g/L] | 10.9 ± 2.1 | 12.4 ± 1.4 | 11.8 ± 2.0 | 13.7 ± 1.9 | 12.1 ± 1.6 | 12.4 ± 0.7 | 14.8 ± 1.0 | 10.5 ± 2.1 |
| Hct | [%] | 34 ± 6 | 38 ± 4 | 36 ± 6 | 42 ± 6 | 37 ± 5 | 38 ± 2 | 45 ± 3 | 32 ± 6 |
| O2Hb | [%] | 95 ± 3 | 94 ± 4 | 93 ± 5 | 94 ± 3 | 95 ± 2 | 94 ± 5 | 93 ± 2 | 91 ± 3 |
| COHb | [%] | 1.1 ± 0.4 | 0.6 ± 0.4 | 1.5 ± 0.2 | 1.5 ± 0.2 | 1.0 ± 0.4 | 1.3 ± 0.2 | 1.3 ± 0.3 | 1.8 ± 0.3 |
| MetHb | [%] | 1.2 ± 0.3 | 1.1 ± 0.3 | 0.6 ± 0.2 | 0.6 ± 0.2 | 1.0 ± 0.5 | 0.6 ± 0.2 | 1.2 ± 0.2 | 1.0 ± 0.3 |
| Na+ | [mmol/L] | 152 ± 5 | 146 ± 5 | 147 ± 2 | 146 ± 4 | 148 ± 5 | 146 ± 3 | 153 ± 13 | 148 ± 3 |
| K+ | [mmol/L] | 3.8 ± 2.2 | 3.6 ± 0.3 | 4.3 ± 0.6 | 4.0 ± 0.6 | 3.9 ± 0.4 | 3.8 ± 0.3 | 3.8 ± 0.4 | 4.1 ± 0.4 |
| Ca2+ | [mmol/L] | 1.10 ± 0.04 | 1.13 ± 0.06 | 1.1 ± 0.1 | 1.12 ± 0.6 | 1.13 ± 0.1 | 1.16 ± 0.1 | 1.16 ± 0.1 | 1.14 ± 0.05 |
| Cl− | [mmol/L] | 113 ± 5 | 107 ± 5 | 111 ± 3 | 111 ± 5 | 103 ± 4 | 108 ± 3 | 117 ± 6 | 111 ± 3 |
| Glucose | [mmol/L] | 7.6 ± 1.4 | 6.9 ± 1.3 | 10.3 ± 2.6 | 9.0 ± 2.0 | 1.0 ± 0.3 | 11.1 ± 2.3 | 9.7 ± 2.5 | 8.9 ± 1.7 |
| Lactate | [mmol/L] | 1.2 ± 0.3 | 1.2 ± 0.4 | 2.1 ± 0.6 | 1.4 ± 0.5 | 1.0 ± 0.3 | 1.5 ± 0.5 | 1.5 ± 0.4 | 0.9 ± 0.3 |
| Pt 17 | Pt 18 | Pt 19 | Pt 20 | Pt 21 | Pt 22 | Pt 23 | Pt 24 | ||
| n | 251 | 41 | 265 | 25 | 159 | 68 | 92 | 212 | |
| pH | 7.40 ± 0.07 | 7.27 ± 0.07 | 7.38 ± 0.07 | 7.42 ± 0.03 | 7.41 ± 0.08 | 7.42 ± 0.06 | 7.41 ± 0.05 | 7.36 ± 0.07 | |
| PaO2 | [mmHg] | 75.4 ± 18.5 | 73.8 ± 19.9 | 79.1 ± 20.4 | 72.7 ± 18.8 | 83.7 ± 22.1 | 82.6 ± 24.0 | 74.7 ± 15.8 | 72.1 ± 16.0 |
| PaCO2 | [mmHg] | 40.7 ± 7.9 | 52.0 ± 11.0 | 59.4 ± 15.8 | 37.7 ± 2.7 | 46.6 ± 11.9 | 43.7 ± 8.6 | 54.2 ± 9.0 | 55.4 ± 9.0 |
| HCO3 | [mmol/L] | 24.7 ± 3.0 | 23.2 ± 2.8 | 34.9 ± 8.5 | 24.7 ± 2.4 | 29.0 ± 4.5 | 28.2 ± 3.9 | 34.1 ± 4.2 | 30.9 ± 4.4 |
| BE | [mmol/L] | −0.1 ± 3.0 | −4.2 ± 2.1 | 8.5 ± 7.8 | 0.4 ± 2.5 | 3.8 ± 3.3 | 3.2 ± 3.3 | 7.7 ± 3.5 | 4.5 ± 4.4 |
| SaO2 | [%] | 94 ± 4 | 92 ± 6 | 94 ± 4 | 94 ± 4 | 102 ± 70 | 96 ± 4 | 94 ± 5 | 94 ± 4 |
| Hb | [g/L] | 8.8 ± 1.2 | 14.5 ± 1.0 | 9.5 ± 1.8 | 14.1 ± 0.8 | 11.9 ± 1.1 | 13.9 ± 0.9 | 13.5 ± 0.9 | 9.7 ± 1.8 |
| Hct | [%] | 27 ± 4 | 44 ± 3 | 29 ± 5 | 43 ± 2 | 37 ± 3 | 43 ± 3 | 41 ± 3 | 30 ± 6 |
| O2Hb | [%] | 92 ± 4 | 90 ± 5 | 92 ± 4 | 92 ± 4 | 94 ± 2 | 94 ± 3 | 92 ± 5 | 92 ± 3 |
| COHb | [%] | 1.4 ± 0.6 | 1.1 ± 0.1 | 1.7 ± 0.6 | 1.1 ± 0.1 | 1.2 ± 0.2 | 1.3 ± 0.2 | 1.1 ± 0.2 | 1.5 ± 0.3 |
| MetHb | [%] | 0.9 ± 0.3 | 0.9 ± 0.3 | 1.0 ± 0.4 | 0.8 ± 0.3 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.2 | 1.0 ± 0.3 |
| Na+ | [mmol/L] | 141 ± 4 | 145 ± 3 | 149 ± 4 | 144 ± 2 | 149 ± 6 | 146 ± 5 | 147 ± 4 | 146 ± 5 |
| K+ | [mmol/L] | 4.4 ± 0.6 | 4.4 ± 0.6 | 3.8 ± 0.4 | 3.6 ± 0.3 | 4.0 ± 0.4 | 3.8 ± 0.4 | 4.1 ± 0.4 | 3.9 ± 0.4 |
| Ca2+ | [mmol/L] | 1.16 ± 0.1 | 1.11 ± 0.1 | 1.15 ± 0.08 | 1.13 ± 0.03 | 1.16 ± 0.08 | 1.11 ± 0.05 | 1.20 ± 0.06 | 1.21 ± 0.04 |
| Cl− | [mmol/L] | 107 ± 4 | 115 ± 4 | 108 ± 10 | 110 ± 2.8 | 113 ± 5 | 109 ± 13 | 104 ± 3 | 109 ± 6 |
| Glucose | [mmol/L] | 9.6 ± 3.7 | 9.1 ± 1.2 | 8.7 ± 1.7 | 5.7 ± 0.6 | 8.4 ± 2.5 | 6.0 ± 1.1 | 8.9 ± 2.0 | 7.7 ± 1.8 |
| Lactate | [mmol/L] | 1.2 ± 0.4 | 2.1 ± 0.6 | 1.4 ± 0.8 | 1.2 ± 0.3 | 1.3 ± 0.5 | 1.3 ± 0.4 | 1.7 ± 0.5 | 1.1 ± 0.3 |
| Pt 25 | Pt 26 | Pt 27 | Pt 28 | Pt 29 | Pt 30 | Pt 31 | Pt 32 | ||
| n | 41 | 130 | 14 | 224 | 54 | 38 | 36 | 126 | |
| pH | 7.40 ± 0.06 | 7.39 ± 0.09 | 7.40 ± 0.04 | 7.34 ± 0.06 | 7.44 ± 0.03 | 7.45 ± 0.04 | 7.46 ± 0.03 | 7.31 ± 0.11 | |
| PaO2 | [mmHg] | 96.7 ± 29.0 | 80.8 ± 19.6 | 65.4 ± 14.0 | 78.6 ± 18.7 | 80.2 ± 24.9 | 85.7 ± 19.6 | 61.0 ± 10.4 | 71.4 ± 14.0 |
| PaCO2 | [mmHg] | 40.6 ± 4.9 | 54.8 ± 13.3 | 37.2 ± 3.8 | 49.1 ± 7.4 | 41.3 ± 5.0 | 41.7 ± 3.7 | 37.9 ± 6.5 | 60.8 ± 19.1 |
| HCO3 | [mmol/L] | 25.2 ± 2.4 | 32.9 ± 5.8 | 23.2 ± 3.6 | 26.5 ± 2.4 | 27.7 ± 2.9 | 28.8 ± 2.5 | 27.7 ± 1.3 | 29.6 ± 3.4 |
| BE | [mmol/L] | 0.4 ± 3.0 | 6.5 ± 5.4 | −1.4 ± 3.7 | 0.3 ± 2.4 | 3.1 ± 2.5 | 4.3 ± 2.6 | 3.7 ± 1.3 | 2.5 ± 2.9 |
| SaO2 | [%] | 97 ± 3 | 95 ± 3 | 91 ± 9 | 95 ± 3 | 95 ± 2 | 94 ± 14 | 91 ± 4 | 93 ± 4 |
| Hb | [g/L] | 14.7 ± 1.0 | 13.5 ± 1.1 | 10.7 ± 1.3 | 10.6 ± 2.1 | 12.2 ± 0.5 | 13.3 ± 1.5 | 14.5 ± 1.0 | 11.4 ± 1.8 |
| Hct | [%] | 45 ± 3 | 41 ± 3 | 33 ± 4 | 33 ± 7 | 37 ± 2 | 41 ± 5 | 44 ± 3 | 35 ± 5 |
| O2Hb | [%] | 95 ± 3 | 93 ± 3 | 89 ± 8 | 93 ± 3 | 93 ± 3 | 94 ± 4 | 90 ± 4 | 90 ± 4 |
| COHb | [%] | 1.08 ± 0.1 | 1.4 ± 0.2 | 1.26 ± 0.1 | 1.7 ± 0.3 | 1.0 ± 0.1 | 1.0 ± 0.2 | 1.02 ± 0.1 | 1.43 ± 0.3 |
| MetHb | [%] | 0.9 ± 0.4 | 1.1 ± 0.3 | 0.7 ± 0.2 | 0.7 ± 0.3 | 1.2 ± 0.3 | 1.2 ± 0.3 | 0.9 ± 0.4 | 1.1 ± 0.3 |
| Na+ | [mmol/L] | 146 ± 3 | 145 ± 4 | 138 ± 5 | 148 ± 5 | 144 ± 15 | 142 ± 3 | 141 ± 1 | 145 ± 3 |
| K+ | [mmol/L] | 3.8 ± 0.2 | 4.0 ± 0.5 | 3.8 ± 0.2 | 4.4 ± 0.5 | 3.8 ± 0.4 | 3.8 ± 0.3 | 3.6 ± 0.3 | 4.5 ± 1.0 |
| Ca2+ | [mmol/L] | 1.12 ± 0.04 | 1.13 ± 0.04 | 0.98 ± 0.37 | 1.19 ± 0.06 | 1.11 ± 0.07 | 1.10 ± 0.04 | 1.11 ± 0.04 | 1.18 ± 0.04 |
| Cl− | [mmol/L] | 112 ± 4 | 107 ± 5 | 104 ± 4 | 111 ± 8 | 108 ± 5 | 106 ± 4 | 106 ± 2 | 109 ± 10 |
| Glucose | [mmol/L] | 6.2 ± 0.8 | 8.8 ± 1.5 | 6.3 ± 2.8 | 10.1 ± 7.3 | 10.3 ± 3.6 | 7.5 ± 1.1 | 6.1 ± 1.2 | 8.0 ± 1.5 |
| Lactate | [mmol/L] | 1.6 ± 0.4 | 1.2 ± 0.3 | 0.6 ± 0.2 | 1.3 ± 0.4 | 1.7 ± 0.8 | 1.1 ± 0.2 | 1.6 ± 0.3 | 1.2 ± 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ceruti, S.; Minotti, B.; Glotta, A.; Biggiogero, M.; Bona, G.; Marzano, M.; Greco, P.; Spagnoletti, M.; Garzoni, C.; Bendjelid, K. Temporal Changes in the Oxyhemoglobin Dissociation Curve of Critically Ill COVID-19 Patients. J. Clin. Med. 2022, 11, 788. https://doi.org/10.3390/jcm11030788
Ceruti S, Minotti B, Glotta A, Biggiogero M, Bona G, Marzano M, Greco P, Spagnoletti M, Garzoni C, Bendjelid K. Temporal Changes in the Oxyhemoglobin Dissociation Curve of Critically Ill COVID-19 Patients. Journal of Clinical Medicine. 2022; 11(3):788. https://doi.org/10.3390/jcm11030788
Chicago/Turabian StyleCeruti, Samuele, Bruno Minotti, Andrea Glotta, Maira Biggiogero, Giovanni Bona, Martino Marzano, Pietro Greco, Marco Spagnoletti, Christian Garzoni, and Karim Bendjelid. 2022. "Temporal Changes in the Oxyhemoglobin Dissociation Curve of Critically Ill COVID-19 Patients" Journal of Clinical Medicine 11, no. 3: 788. https://doi.org/10.3390/jcm11030788
APA StyleCeruti, S., Minotti, B., Glotta, A., Biggiogero, M., Bona, G., Marzano, M., Greco, P., Spagnoletti, M., Garzoni, C., & Bendjelid, K. (2022). Temporal Changes in the Oxyhemoglobin Dissociation Curve of Critically Ill COVID-19 Patients. Journal of Clinical Medicine, 11(3), 788. https://doi.org/10.3390/jcm11030788








