Low miR 511-5p Expression as a Potential Predictor of a Poor Nutritional Status in Head and Neck Cancer Patients Subjected to Intensity-Modulated Radiation Therapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Characteristics of the Study Group
2.2. Treatment and Patient Assessment
2.3. miRNA Expression Analysis
2.4. Bioelectrical Impedance Analysis
2.5. Statistical Analysis
3. Results
3.1. Charasteristic of Study Group
3.2. Nutritional Assasement
3.3. Factors Affecting the Risk of Malnutrition According to the SGA Scale
3.4. Factors Affecting the Risk of Higher Nutritional Risk According to the NRS Scale
3.5. Factors Affecting the Risk of CWL
3.6. miR-511-3p Expression in Predicting the Occurrence of Nutritional Deficiencies
3.7. Comparison of miR-511-3p Relative Expression Depending on Demographic, Clinical and Nutritional Variables
3.8. Correlation between miR-511-3p Expression and Nutritional Status Indicators
3.9. Overall Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vigneswaran, N.; Williams, M.D. Epidemiologic trends in head and neck cancer and aids in diagnosis. Oral Maxillofac. Surg. Clin. N. Am. 2014, 26, 123–141. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and neck cancer: Changing epidemiology, diagnosis, and treatment. Mayo Clin. Proc. 2008, 83, 489–501. [Google Scholar] [CrossRef]
- Marur, S.; Forastiere, A.A. Head and Neck Squamous Cell Carcinoma: Update on Epidemiology, Diagnosis, and Treatment. Mayo Clin. Proc. 2016, 91, 386–396. [Google Scholar] [CrossRef]
- Kouloulias, V.; Thalassinou, S.; Platoni, K.; Zygogianni, A.; Kouvaris, J.; Antypas, C.; Efstathopoulos, E.; Nikolaos, K. The treatment outcome and radiation-induced toxicity for patients with head and neck carcinoma in the IMRT era: A systematic review with dosimetric and clinical parameters. BioMed Res. Int. 2013, 2013, 401261. [Google Scholar] [CrossRef]
- Ghosh, G.; Gupta, G.; Malviya, A.; Saroj, D. Comparison three-dimensional conformal radiotherapy versus intensity modulated radiation therapy in local control of head and neck cancer. J. Cancer Res. Ther. 2018, 14, 1412–1417. [Google Scholar] [CrossRef]
- Wang, X.; Eisbruch, A. IMRT for head and neck cancer: Reducing xerostomia and dysphagia. J. Radiat. Res. 2016, 57, i69–i75. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamgani, A.; Van Rooij, P.; Verduijn, G.M.; Mehilal, R.; Kerrebijn, J.D.; Levendag, P.C. The impact of treatment modality and radiation technique on outcomes and toxicity of patients with locally advanced oropharyngeal cancer. Laryngoscope 2013, 123, 386–393. [Google Scholar] [CrossRef]
- Meza-Valderrama, D.; Marco, E.; Dávalos-Yerovi, V.; Muns, M.D.; Tejero-Sánchez, M.; Duarte, E.; Sánchez-Rodríguez, D. Sarcopenia, malnutrition, and cachexia: Adapting definitions and terminology of nutritional disorders in older people with cancer. Nutrients 2021, 13, 761. [Google Scholar] [CrossRef] [PubMed]
- Gorenc, M.; Kozjek, N.R.; Strojan, P. Malnutrition and cachexia in patients with head and neck cancer treated with (chemo)radiotherapy. Rep. Pract. Oncol. Radiother. 2015, 20, 249–258. [Google Scholar] [CrossRef]
- Kwon, M.; Kim, R.B.; Roh, J.-L.; Lee, S.-W.; Kim, S.-B.; Choi, S.-H.; Nam, S.Y.; Kim, S.Y. Prevalence and clinical significance of cancer cachexia based on time from treatment in advanced-stage head and neck squamous cell carcinoma. Head Neck 2017, 39, 716–723. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Zhang, L. Cancer cachexia: Definition, staging, and emerging treatments. Cancer Manag. Res. 2020, 12, 5597–5605. [Google Scholar] [CrossRef]
- Cederholm, T.; Barazzoni, R.; Austin, P.; Ballmer, P.; Biolo, G.; Bischoff, S.C.; Compher, C.; Correia, I.; Higashiguchi, T.; Holst, M.; et al. ESPEN guidelines on definitions and terminology of clinical nutrition. Clin. Nutr. 2017, 36, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Santarpia, L.; Contaldo, F.; Pasanisi, F. Nutritional screening and early treatment of malnutrition in cancer patients. J. Cachexia Sarcopenia Muscle 2011, 2, 27–35. [Google Scholar] [CrossRef]
- Webster, J.M.; Kempen, L.J.A.P.; Hardy, R.S.; Langen, R.C.J. Inflammation and Skeletal Muscle Wasting During Cachexia. Front. Physiol. 2020, 11, 597675. [Google Scholar] [CrossRef]
- Jager-Wittenaar, H.; Dijkstra, P.U.; Vissink, A.; Van Der Laan, B.F.A.M.; Van Oort, R.P.; Roodenburg, J.L.N. Critical weight loss in head and neck cancer—Prevalence and risk factors at diagnosis: An explorative study. Support. Care Cancer 2007, 15, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Langius, J.A.E.; Bakker, S.; Rietveld, D.H.F.; Kruizenga, H.M.; Langendijk, J.A.; Weijs, P.J.M.; Leemans, C.R. Critical weight loss is a major prognostic indicator for disease-specific survival in patients with head and neck cancer receiving radiotherapy. Br. J. Cancer 2013, 109, 1093–1099. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Shen, L.J.; Guo, X.; Guo, X.M.; Qian, C.N.; Wu, P.H. Critical weight loss predicts poor prognosis in nasopharyngeal carcinoma. BMC Cancer 2016, 16, 169. [Google Scholar] [CrossRef] [PubMed]
- Lønbro, S.; Petersen, G.B.; Andersen, J.R.; Johansen, J. Prediction of critical weight loss during radiation treatment in head and neck cancer patients is dependent on BMI. Support. Care Cancer 2016, 24, 2101–2109. [Google Scholar] [CrossRef]
- Loumaye, A.; Thissen, J.P. Biomarkers of cancer cachexia. Clin. Biochem. 2017, 50, 1281–1288. [Google Scholar] [CrossRef] [PubMed]
- Cao, Z.; Zhao, K.; Jose, I.; Hoogenraad, N.J.; Osellame, L.D. Biomarkers for Cancer Cachexia: A Mini Review. Int. J. Mol. Sci. 2021, 22, 4501. [Google Scholar] [CrossRef]
- Cederholm, T.; Jensen, G.L.; Correia, M.I.T.D.; Gonzalez, M.C.; Fukushima, R.; Higashiguchi, T.; Baptista, G.; Barazzoni, R.; Blaauw, R.; Coats, A.; et al. GLIM criteria for the diagnosis of malnutrition—A consensus report from the global clinical nutrition community. Clin. Nutr. 2019, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Acunzo, M.; Croce, C.M. MicroRNA in Cancer and Cachexia—A Mini-Review. J. Infect. Dis. 2015, 212, S74–S77. [Google Scholar] [CrossRef]
- Reddy, K.B. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015, 15, 38. [Google Scholar] [CrossRef]
- Nowicka, Z.; Stawiski, K.; Tomasik, B.; Fendler, W. Extracellular miRNAs as biomarkers of head and neck cancer progression and metastasis. Int. J. Mol. Sci. 2019, 20, 4799. [Google Scholar] [CrossRef]
- Faraoni, I.; Antonetti, F.R.; Cardone, J.; Bonmassar, E. miR-155 gene: A typical multifunctional microRNA. Biochim. Biophys. Acta—Mol. Basis Dis. 2009, 1792, 497–505. [Google Scholar] [CrossRef]
- Kabzinski, J.; Maczynska, M.; Majsterek, I. Microrna as a novel biomarker in the diagnosis of head and neck cancer. Biomolecules 2021, 11, 844. [Google Scholar] [CrossRef]
- Chugh, P.; Dittmer, D.P. Potential pitfalls in microRNA profiling. Wiley Interdiscip. Rev. RNA 2012, 3, 601–616. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Springer, J. MicroRNAs in muscle wasting. J. Cachexia Sarcopenia Muscle 2018, 9, 1209–1212. [Google Scholar] [CrossRef]
- Yanai, K.; Kaneko, S.; Ishii, H.; Aomatsu, A.; Ito, K.; Hirai, K.; Ookawara, S.; Ishibashi, K.; Morishita, Y. Micrornas in sarcopenia: A systematic review. Front. Med. 2020, 7, 180. [Google Scholar] [CrossRef]
- Powrózek, T.; Mlak, R.; Brzozowska, A.; Mazurek, M.; Gołębiowski, P.; Małecka-Massalska, T. MiRNA-130a significantly improves accuracy of SGA nutritional assessment tool in prediction of malnutrition and cachexia in radiotherapy-treated head and neck cancer patients. Cancers 2018, 10, 294. [Google Scholar] [CrossRef] [PubMed]
- Kottorou, A.; Dimitrakopoulos, F.I.; Tsezou, A. Non-coding RNAs in cancer-associated cachexia: Clinical implications and future perspectives. Transl. Oncol. 2021, 14, 101101. [Google Scholar] [CrossRef] [PubMed]
- Rahman, S.; Vandewalle, J.; van Hamersveld, P.H.P.; Verseijden, C.; Welting, O.; Jongejan, A.; Casanova, P.; Meijer, S.L.; Libert, C.; Hakvoort, T.B.M.; et al. miR-511 Deficiency Protects Mice from Experimental Colitis by Reducing TLR3 and TLR4 Responses via WD Repeat and FYVE-Domain-Containing Protein 1. Cells 2022, 11, 58. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, M.L.; Pucci, F.; Magri, L.; Moi, D.; Gilfillan, G.D.; Ranghetti, A.; Casazza, A.; Mazzone, M.; Lyle, R.; Naldini, L.; et al. MiR-511-3p Modulates Genetic Programs of Tumor-Associated Macrophages. Cell Rep. 2012, 1, 141–154. [Google Scholar] [CrossRef]
- Curtale, G.; Renzi, T.A.; Drufuca, L.; Rubino, M.; Locati, M. Glucocorticoids downregulate TLR4 signaling activity via its direct targeting by miR-511-5p. Eur. J. Immunol. 2017, 47, 2080–2089. [Google Scholar] [CrossRef]
- Lee, D.E.; Brown, J.L.; Rosa-Caldwell, M.E.; Blackwell, T.A.; Perry, R.A.; Brown, L.A.; Khatri, B.; Seo, D.; Bottje, W.G.; Washington, T.A.; et al. Cancer cachexia-induced muscle atrophy: Evidence for alterations in microRNAs important for muscle size. Physiol. Genom. 2017, 49, 253–260. [Google Scholar] [CrossRef]
- Awuah, D.; Ruisinger, A.; Alobaid, M.; Mbadugha, C.; Ghaemmaghami, A.M. MicroRNA-511-3p mediated modulation of the peroxisome proliferator-activated receptor gamma (PPARγ) controls LPS-induced inflammatory responses in human monocyte derived DCs. bioRxiv 2020, 2, 104–117. [Google Scholar] [CrossRef]
- Prasad, N.; Sinha, A.; Gupta, A.; Bhadauria, D.; Manjunath, R.; Kaul, A.; Sharma, R.K. Validity of nutrition risk index as a malnutrition screening tool compared with subjective global assessment in end-stage renal disease patients on peritoneal dialysis. Indian J. Nephrol. 2016, 26, 27–32. [Google Scholar] [CrossRef]
- Rutten, L.J.F.; Augustson, E.M.; Moser, R.P.; Beckjord, E.B.; Hesse, B.W. Smoking knowledge and behavior in the United States: Sociodemographic, smoking status, and geographic patterns. Nicotine Tob. Res. 2008, 10, 1559–1570. [Google Scholar] [CrossRef]
- Koelmeyer, L.A.; Ward, L.C.; Dean, C.; Boyages, J. Body Positional Effects on Bioimpedance Spectroscopy Measurements for Lymphedema Assessment of the Arm. Lymphat. Res. Biol. 2020, 18, 464–473. [Google Scholar] [CrossRef]
- Ward, L.C.; Dylke, E.; Czerniec, S.; Isenring, E.; Kilbreath, S.L. Reference Ranges for Assessment of Unilateral Lymphedema in Legs by Bioelectrical Impedance Spectroscopy. Lymphat. Res. Biol. 2011, 9, 43–46. [Google Scholar] [CrossRef]
- Ward, L.C.; Dylke, E.; Czerniec, S.; Isenring, E.; Kilbreath, S.L. Confirmation of the Reference Impedance Ratios Used for Assessment of Breast Cancer-Related Lymphedema by Bioelectrical Impedance Spectroscopy. Lymphat. Res. Biol. 2011, 9, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Saroul, N.; Pastourel, R.; Mulliez, A.; Farigon, N.; Dupuch, V.; Mom, T.; Boirie, Y.; Gilain, L. Which Assessment Method of Malnutrition in Head and Neck Cancer? Otolaryngol.—Head Neck Surg. 2018, 158, 1065–1071. [Google Scholar] [CrossRef]
- Labayen, I.; Moreno, L.A.; Marti, A.; González-Lamuño, D.; Wárnberg, J.; Ortega, F.B.; Bueno, G.; Nova, E.; Ruiz, J.R.; Garagorri, J.M.; et al. Effect of the Ala12 allele in the PPARγ-2 gene on the relationship between birth weight and body composition in adolescents: The AVENA study. Pediatr. Res. 2007, 62, 615–619. [Google Scholar] [CrossRef] [PubMed]
- Camargo, R.G.; Quintas Teixeira Ribeiro, H.; Geraldo, M.V.; Matos-Neto, E.; Neves, R.X.; Carlos Carnevali, L.; Donatto, F.F.; Alcântara, P.S.M.; Ottoch, J.P.; Seelaender, M. Cancer Cachexia and MicroRNAs. Mediat. Inflamm. 2015, 2015, 367561. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, H.; Li, P.; Zhao, Y.; Liu, M.; Tang, H. NF-κ B-modulated miR-130a targets TNF-α in cervical cancer cells. 2014, 12, 155. J. Transl. Med. 2014, 12, 155. [Google Scholar] [CrossRef]
- Li, Z.C.; Han, N.; Li, X.; Li, G.; Liu, Y.Z.; Sun, G.X.; Wang, Y.; Chen, G.T.; Li, G.F. Decreased expression of microRNA-130a correlates with TNF-α in the development of osteoarthritis. Int. J. Clin. Exp. Pathol. 2015, 8, 2555–2564. [Google Scholar]
- Narasimhan, A.; Ghosh, S.; Stretch, C.; Greiner, R.; Bathe, O.F.; Baracos, V.; Damaraju, S. Small RNAome profiling from human skeletal muscle: Novel miRNAs and their targets associated with cancer cachexia. J. Cachexia Sarcopenia Muscle 2017, 8, 405–416. [Google Scholar] [CrossRef] [PubMed]
- Hitachi, K.; Nakatani, M.; Tsuchida, K. Myostatin signaling regulates Akt activity via the regulation of miR-486 expression. Int. J. Biochem. Cell Biol. 2014, 47, 93–103. [Google Scholar] [CrossRef]
- Kulyté, A.; Lorente-Cebrián, S.; Gao, H.; Mejhert, N.; Agustsson, T.; Arner, P.; Rydén, M.; Dahlman, I. MicroRNA profiling links miR-378 to enhanced adipocyte lipolysis in human cancer cachexia. Am. J. Physiol.—Endocrinol. Metab. 2014, 306, E267–E274. [Google Scholar] [CrossRef] [PubMed]
- Rebane, A.; Akdis, C.A. MicroRNAs: Essential players in the regulation of inflammation. J. Allergy Clin. Immunol. 2013, 132, 15–26. [Google Scholar] [CrossRef]
- Marinho, R.; Alcântara, P.S.M.; Ottoch, J.P.; Seelaender, M. Role of Exosomal MicroRNAs and myomiRs in the Development of Cancer Cachexia-Associated Muscle Wasting. Front. Nutr. 2018, 4, 69. [Google Scholar] [CrossRef] [PubMed]
- Powrózek, T.; Brzozowska, A.; Mazurek, M.; Mlak, R.; Sobieszek, G.; Małecka-Massalska, T. Combined analysis of miRNA-181a with phase angle derived from bioelectrical impedance predicts radiotherapy-induced changes in body composition and survival of male patients with head and neck cancer. Head Neck 2019, 41, 3247–3257. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Yao, L.; Toiyama, Y.; Yamamoto, A.; Shigemori, T.; Yin, C.; Omura, Y.; Ide, S.; Kitajima, T.; Shimura, T.; et al. Prognostic impact of sarcopenia and its correlation with circulating miR-21 in colorectal cancer patients. Oncol. Rep. 2018, 39, 1555–1564. [Google Scholar] [CrossRef] [PubMed]
- Okugawa, Y.; Toiyama, Y.; Hur, K.; Yamamoto, A.; Yin, C.; Ide, S.; Kitajima, T.; Fujikawa, H.; Yasuda, H.; Koike, Y.; et al. Circulating miR-203 derived from metastatic tissues promotes myopenia in colorectal cancer patients. J. Cachexia Sarcopenia Muscle 2019, 10, 536–548. [Google Scholar] [CrossRef] [PubMed]
- Langius, J.A.E.; Twisk, J.; Kampman, M.; Doornaert, P.; Kramer, M.H.H.; Weijs, P.J.M.; Leemans, C.R. Prediction model to predict critical weight loss in patients with head and neck cancer during (chemo)radiotherapy. Oral Oncol. 2016, 52, 91–96. [Google Scholar] [CrossRef] [PubMed]


| Variable | Study Group (n = 60) | ||
|---|---|---|---|
| Gender | Male | 51 (85.0%) | |
| Female[M1] [m2] | 9 (15.0%) | ||
| Age [years] | Mean ± standard deviation, median (range) | 65 ± 9.26 63 (42–87) | |
| Age [years] | ≥ 63 | 23 (38.3%) | |
| < 63 | 37 (61.7%) | ||
| Histopathological diagnosis | Squamous cell carcinoma | 58 (96.7%) | |
| Other | 2 (3.3%) | ||
| Tumor location | Oropharyngeal | 23 (38.3%) | |
| Larynx | 33 (55.0%) | ||
| Others | 4 (6.7%) | ||
| T stage | T1 | 1 (1.7%) | |
| T2 | 8 (13.3%) | ||
| T3 | 21 (35.0%) | ||
| T4 | 30 (50.0%) | ||
| N stage | N0 | 18 (30.0%) | |
| N1 | 8 (13.3%) | ||
| N2 | 29 (48.3%) | ||
| N3 | 5 (8.3%) | ||
| M stage | M0 | 59 (98.3%) | |
| M1 | 1 (1.7%) | ||
| Disease stage (TNM) | III | 16 (26.7%) | |
| IVA | 35 (58.3%) | ||
| IVB | 3 (5.0%) | ||
| IVC | 6 (10.0%) | ||
| Performance status | ≤1 | 51 (85.0%) | |
| >1 | 9 (15.0%) | ||
| Type of treatment | Surgery + RT | 28 (46.7%) | |
| Surgery + C-RT | 18 (30.0%) | ||
| RT alone | 8 (13.3%) | ||
| C-RT | 6 (10.0%) | ||
| Alcohol consumption | Yes | 27 (45.0%) | |
| No | 33 (55.0%) | ||
| Smoking status | Smoker | 44 (73.3%) | |
| Non-smoker | 16 (26.7%) | ||
| Smoking status | Current smoker | 41 (93.2%) | |
| Former smoker | 3 (6.8%) | ||
| Smoking during treatment | Yes | 37 (90.2%) | |
| No | 4 (9.8%) | ||
| Parenteral nutrition | Yes | 7 (11.7%) | |
| No | 53 (88.3%) | ||
| Weight [kg] | Mean ± standard deviation, median (range) | 65 ± 11.40 66 (43–91) | |
| BMI [kg/m2] | Mean ± standard deviation, median (range) | 22.94 ± 4.28 22.84 (14.5–34.4) | |
| SGA | A | 9 (15.0%) | |
| B | 29 (48.3%) | ||
| C | 22 (36.7%) | ||
| NRS-2002 | 2 | 41 (68.3%) | |
| 3 | 16 (26.7%) | ||
| 4 | 2 (3.3%) | ||
| 5 | 1 (1.7%) | ||
| CWL | Yes | 20 (33.3%) | |
| No | 40 (66.7%) | ||
| Nutrition risk index (NRI) | Normal | 1(1.67%) | |
| Mild | 3 (5.0%) | ||
| Moderate | 53 (88.33%) | ||
| Severe | 3 (5.0%) | ||
| Interval of time between surgery and nutritional assessment [days] | Mean ± standard deviation, median (range) | 70.92 ±35.26 63(25–259) | |
| Extent of surgery | Tumor resection | 3 (6.52%) | |
| Tumor resection with lymphadenectomy | 43 (93.48%) | ||
| Nutritional support in the post-operational period | PEG | 2 (3.34%) | |
| Feeding tube | 20 (33.33%) | ||
| No | 38 (63.33%) | ||
| Nutritional support in the post-operational time [days] | Mean ± standard deviation, median (range) | 15.95 ± 4.89 15.50 (9–28) | |
| Oral nutritional support during RT | Yes | 3 (5.00%) | |
| No | 57 (95.00%) | ||
| Parenteral nutrition during RT | Yes | 7 (11.67%) | |
| No | 53 (88.33%) | ||
| Nutritional Status | Median (Interquartile Range) | ROC Analysis | Risk Analysis | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Median (Interquartile Range) | p | Cut-Off Value | Sensitivity | Specificity | AUC [95% CI] | p | Low [<Cut Off] (%) | High [≥Cut Off] (%) | OR [95% CI] | p | ||
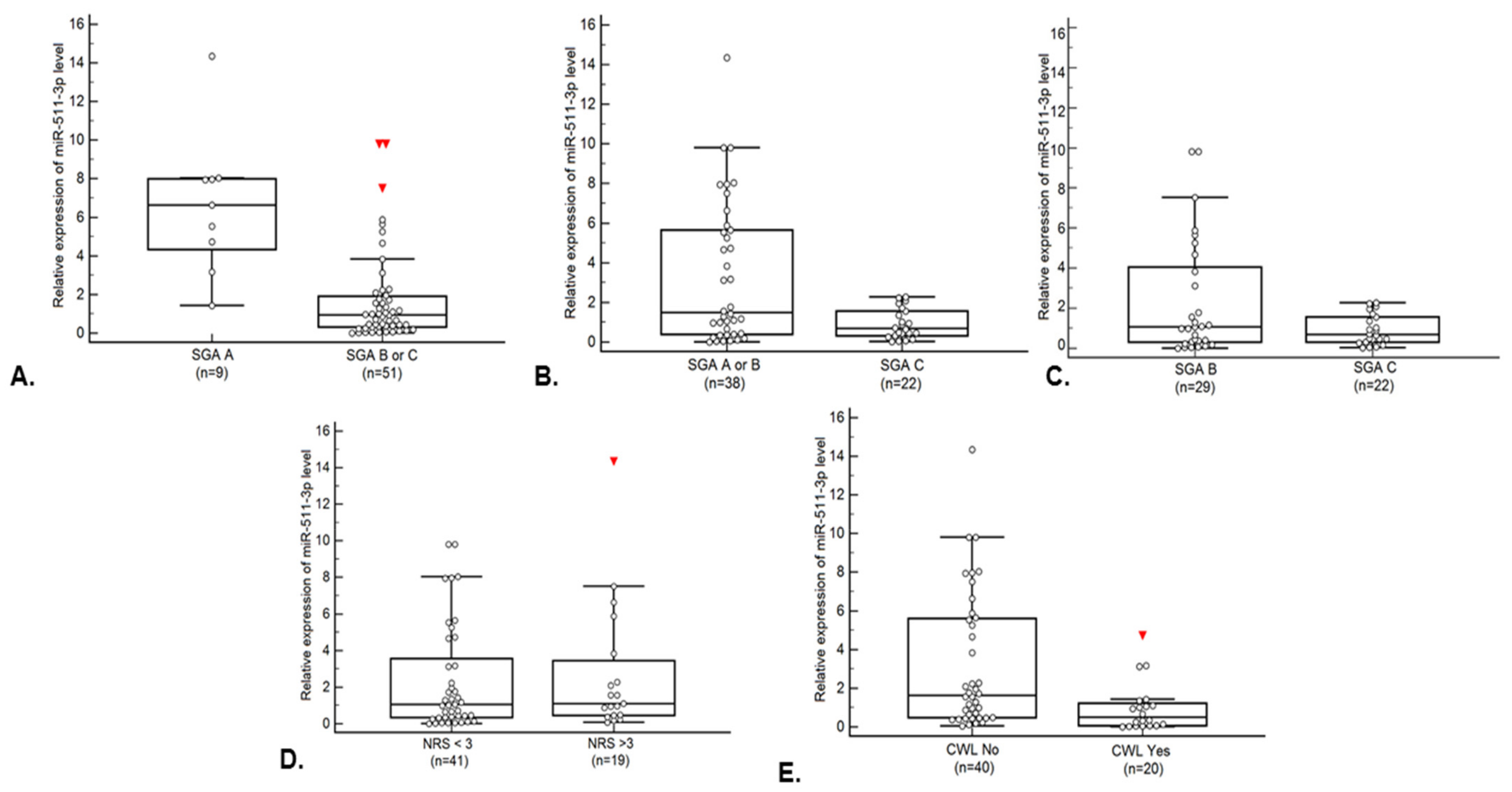
| SGA | A | 6.27 (4.35–7.99) | 0.0001 * | ≤3.12 | 84.3 | 88.90 | 0.90 [0.80–0.96] | <0.0001 * | 1 (2.32%) | 8 (47.05%) | 37.33 [4.14–336.94] | 0.0013 * |
| B or C | 0.93 (0.32–1.91) | 42 (97.67%) | 9 (52.95%) | |||||||||
| SGA | A or B | 1.49 (0.40–5.65) | 0.0251 * | ≤2.28 | 100.00 | 44.70 | 0.675 [0.54–0.79] | 0.0110 * | 21 (50.00%) | 17 (94.44%) | 17.00 [2.07–139.61] | 0.0084 * |
| C | 0.68 (0.32–1.55) | 21 (50.00%) | 1 (5.56%) | |||||||||
| SGA | B | 1.06 (0.31–4.04) | 0.3135 | ≤2.28 | 100.00 | 31.03 | 0.583 [0.44–0.72] | 0.3049 | 20 (48.78%) | 9 (90.00%) | 9.45 [1.09–81.52] | 0.0411 * |
| C | 0.68 (0.44–1.37) | 21 (51.22%) | 1 (10.00%) | |||||||||
| NRS-2002 | <3 | 1.06 (0.33–3.55) | 0.5945 | >0.14 | 94.70 | 19.50 | 0.543 [0.41–0.67] | 0.5844 | 7 (87.50%) | 34 (65.38%) | 3.71 [0.42–32.52] | 0.2371 |
| >3 | 1.09 (0.47–3.45) | 1 (12.50%) | 18 (34.62%) | |||||||||
| CWL | No | 1.64 (0.48–5.59) | 0.0025 * | ≤0.34 | 50.00 | 90.00 | 0.741 [0.61–0.85] | 0.0003 * | 4 (30.77%) | 36 (76.60%) | 7.36 [1.89–28.62] | 0.0039 * |
| Yes | 0.51 (0.06–1.22) | 9 (69.23%) | 11 (23.40%) | |||||||||
| Variable | Relative Expression of miR-511-3p | |
|---|---|---|
| rho | p | |
| Age [years] | −0.073 | 0.5768 |
| Weight [kg] | 0.114 | 0.3845 |
| BMI [kg/m2] | 0.317 | 0.0136 * |
| Total protein [g/L] | −0.045 | 0.7295 |
| Albumin [g/L] | 0.322 | 0.0094 * |
| Prealbumin[g/dL] | 0.281 | 0.8314 |
| CRP [mg/L] | −0.340 | 0.0048 * |
| Transferrin [g/L] | 0.160 | 0.2206 |
| FM [kg] | 0.001 | 0.9946 |
| FM% | 0.001 | 0.9926 |
| FFM [kg] | −0.311 | 0.0156 * |
| FFM% | −0.013 | 0.9208 |
| FFMI [kg/m2] | −0.256 | 0.0486 * |
| (nFFMI) [kg/m2] | −0.232 | 0.0747 |
| SGA | −0.440 | 0.0004 * |
| NRS-2002 | 0.062 | 0.6386 |
| T stage | −0.296 | 0.0218 * |
| N stage | −0.298 | 0.0205 * |
| M stage | −0.064 | 0.6276 |
| Disease stage (TNM) | −0.443 | 0.0004 * |
| NRI | 0.317 | 0.0130 * |
| Interval of time between surgery and nutritional assessment [days] | −0.001 | 0.8826 |
| Nutritional support in the post-operational time [days] | −0.250 | 0.2467 |
| Variable | Log-Rank Test | |||
|---|---|---|---|---|
| Univariable Analysis | Multivariable Analysis # | |||
| HR [95% CI] | p | HR [95% CI] | p | |
| Gender (male) | 1.61 [0.67–3.85] | 0.2931 | 1.95 [0.59–6.46] | 0.2763 |
| Age (≥ 63 years) | 1.70 [0.83–3.48] | 0.0861 | 1.62 [0.82–3.22] | 0.1695 |
| Smoking history (yes) | 0.68 [0.32–1.44] | 0.2414 | 0.74 [0.36–1.51] | 0.4061 |
| Smoking during treatment (yes) | 0.80 [0.41–1.56] | 0.4681 | 0.80 [0.40–1.58] | 0.5219 |
| Alcohol consumption (yes) | 0.81 [0.42–1.54] | 0.4963 | 0.73 [0.37–1.43] | 0.3587 |
| Performance status (>0) | 2.20 [0.79–6.12] | 0.0343 * | 2.03 [0.87–4.75] | 0.1019 |
| Tumor location (oropharyngeal) | 1.05 [0.54–2.05] | 0.8773 | 1.07 [0.54–2.11] | 0.8431 |
| Tumor location (larynx) | 0.91 [0.47–1.73] | 0.7497 | 0.97 [0.49–1.93] | 0.9307 |
| T stage (T4) | 1.27 [0.66–2.44] | 0.4288 | 1.17 [0.60–2.29] | 0.6483 |
| N stage (N1–3) | 1.34 [0.68–2.62] | 0.3815 | 1.26 [0.61–2.61] | 0.5337 |
| M stage (M1) | 15.06 [0.01–27,725.05] | 0.0003 * | 10.81 [1.04–112.32] | 0.0473 * |
| TNM stage (IV) | 1.99 [0.78–5.05] | 0.0555 | 2.08 [0.95–4.55] | 0.0666 |
| Parenteral nutrition (yes) | 1.88 [0.56–6.28] | 0.1654 | 1.46 [0.51–4.18] | 0.4855 |
| Treatment (concurrent C-RT) | 1.21 [0.63–2.33] | 0.5314 | 1.26 [0.64–2.45] | 0.5013 |
| SGA (C) | 1.43 [0.62–3.29] | 0.4339 | 1.45 [0.56–3.74] | 0.4473 |
| SGA (BC) | 1.00 [0.52–1.93] | 0.9961 | 0.84 [0.41–1.70] | 0.6293 |
| NRS-2002 (≥3) | 1.23 [0.57–2.64] | 0.5451 | 1.49 [0.68–3.27] | 0.3224 |
| CWL (yes) | 0.85 [0.42–1.72] | 0.6507 | 0.90 [0.43–1.88] | 0.7856 |
| Relative expression of miR-511–3p (high)(≥2.84) | 0.93 [0.46–1.86] | 0.8225 | 0.94 [0.45–1.93] | 0.8587 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazurek, M.; Mlak, R.; Homa-Mlak, I.; Powrózek, T.; Brzozowska, A.; Kwaśniewski, W.; Opielak, G.; Małecka-Massalska, T. Low miR 511-5p Expression as a Potential Predictor of a Poor Nutritional Status in Head and Neck Cancer Patients Subjected to Intensity-Modulated Radiation Therapy. J. Clin. Med. 2022, 11, 805. https://doi.org/10.3390/jcm11030805
Mazurek M, Mlak R, Homa-Mlak I, Powrózek T, Brzozowska A, Kwaśniewski W, Opielak G, Małecka-Massalska T. Low miR 511-5p Expression as a Potential Predictor of a Poor Nutritional Status in Head and Neck Cancer Patients Subjected to Intensity-Modulated Radiation Therapy. Journal of Clinical Medicine. 2022; 11(3):805. https://doi.org/10.3390/jcm11030805
Chicago/Turabian StyleMazurek, Marcin, Radosław Mlak, Iwona Homa-Mlak, Tomasz Powrózek, Anna Brzozowska, Wojciech Kwaśniewski, Grzegorz Opielak, and Teresa Małecka-Massalska. 2022. "Low miR 511-5p Expression as a Potential Predictor of a Poor Nutritional Status in Head and Neck Cancer Patients Subjected to Intensity-Modulated Radiation Therapy" Journal of Clinical Medicine 11, no. 3: 805. https://doi.org/10.3390/jcm11030805
APA StyleMazurek, M., Mlak, R., Homa-Mlak, I., Powrózek, T., Brzozowska, A., Kwaśniewski, W., Opielak, G., & Małecka-Massalska, T. (2022). Low miR 511-5p Expression as a Potential Predictor of a Poor Nutritional Status in Head and Neck Cancer Patients Subjected to Intensity-Modulated Radiation Therapy. Journal of Clinical Medicine, 11(3), 805. https://doi.org/10.3390/jcm11030805






