Intraindividual Changes in Rheumatoid Factor and Anti-Cyclic Citrullinated Peptide Antibody Tests in Korean Patients Visiting Local Clinics and Hospitals
Abstract
:1. Introduction
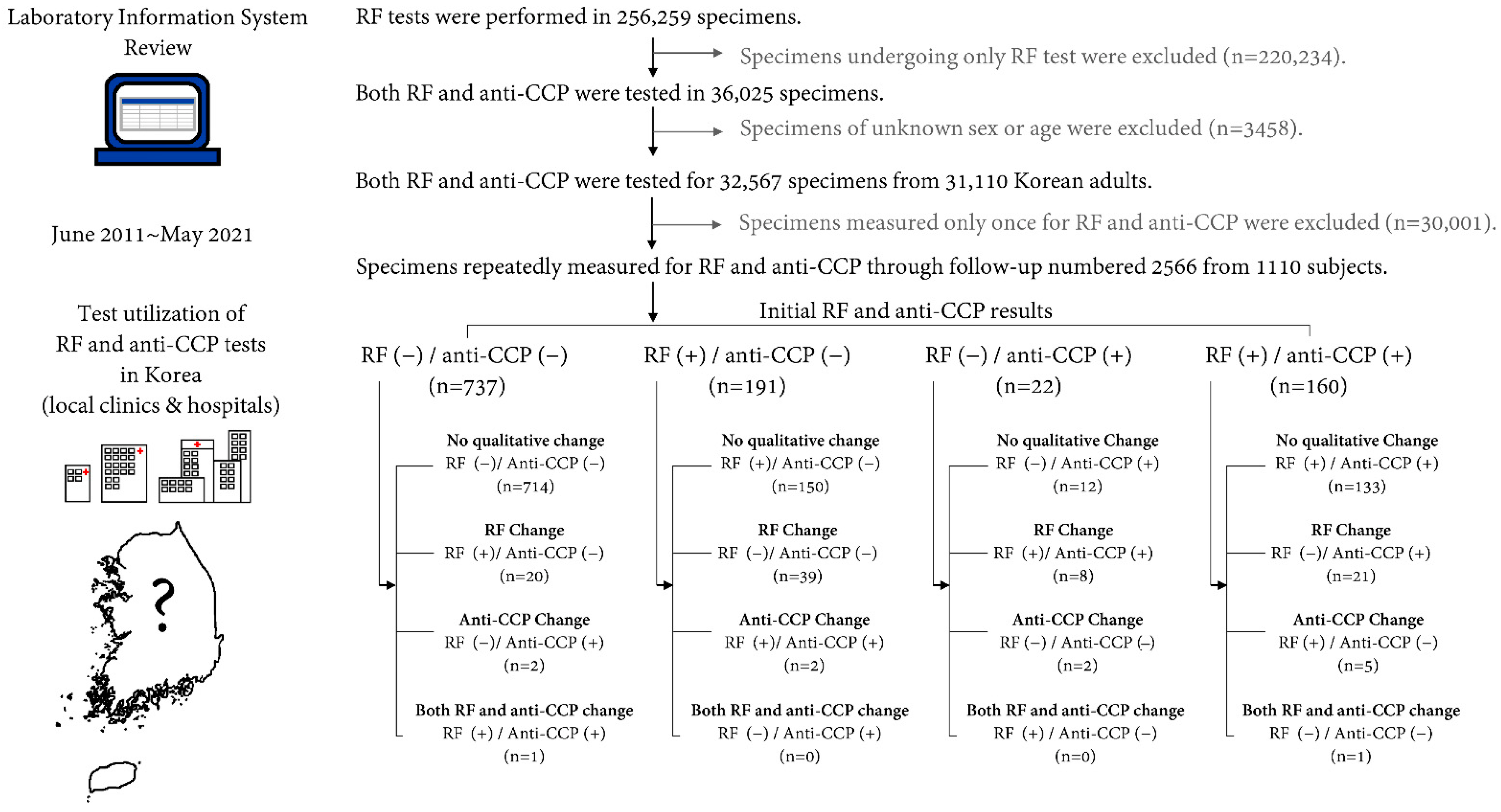
2. Materials and Methods
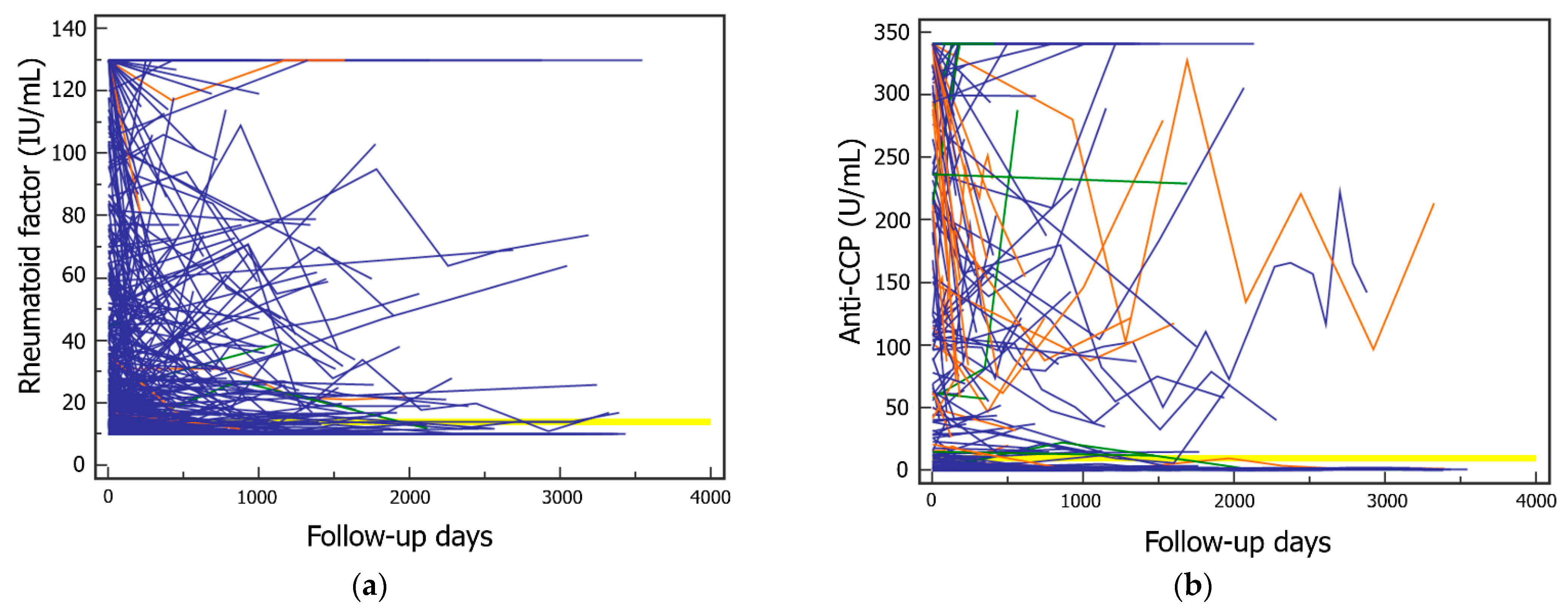
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Smolen, J.S.; Aletaha, D.; McInnes, I.B. Rheumatoid arthritis. Lancet 2016, 388, 2023–2038. [Google Scholar] [CrossRef]
- Aletaha, D.; Smolen, J.S. Diagnosis and Management of Rheumatoid Arthritis: A Review. JAMA 2018, 320, 1360–1372. [Google Scholar] [CrossRef] [PubMed]
- Khan, T.; Ricardo, J.; Renzoni, E.A.; Mouyis, M. A Closer Look at the Role of Anti-CCP Antibodies in the Pathogenesis of Rheumatoid Arthritis-Associated Interstitial Lung Disease and Bronchiectasis. Rheumatol. Ther. 2021, 8, 1463–1475. [Google Scholar] [CrossRef] [PubMed]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Rönnelid, J.; Turesson, C.; Kastbom, A. Autoantibodies in Rheumatoid Arthritis—Laboratory and Clinical Perspectives. Front. Immunol. 2021, 12, 685312. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 rheumatoid arthritis classification criteria: An American College of Rheumatology/European League against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef]
- Scofield, R.H. Autoantibodies as predictors of disease. Lancet 2004, 363, 1544–1546. [Google Scholar] [CrossRef]
- Barra, L.; Pope, J.; Bessette, L.; Haraoui, B.; Bykerk, V. Lack of seroconversion of rheumatoid factor and anti-cyclic citrullinated peptide in patients with early inflammatory arthritis: A systematic literature review. Rheumatology 2011, 50, 311–316. [Google Scholar] [CrossRef] [Green Version]
- Böhler, C.; Radner, H.; Smolen, J.S.; Aletaha, D. Serological changes in the course of traditional and biological disease modifying therapy of rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 241–244. [Google Scholar] [CrossRef] [Green Version]
- Ahn, S.S.; Ha, J.W.; Park, Y.B.; Lee, S.W. Rheumatoid factor positivity in antineutrophil cytoplasmic antibody-associated vasculitis: A distinct clinical entity or innocent bystander? Rheumatology 2021. [Google Scholar] [CrossRef]
- Chopra, A.; Lin, H.Y.; Navarra, S.V.; Saeed, M.A.; Sockalingam, S.; Thongpooswan, S.; Jois, R.; Salim, B.; Gavin Lee, K.W.; Lau, T.C.; et al. Rheumatoid arthritis management in the APLAR region: Perspectives from an expert panel of rheumatologists, patients and community oriented program for control of rheumatic diseases. Int. J. Rheum. Dis. 2021, 24, 1106–1111. [Google Scholar] [CrossRef]
- Falkenburg, W.J.J.; von Richthofen, H.J.; Koers, J.; Weykamp, C.; Schreurs, M.W.J.; Bakker-Jonges, L.E.; Haagen, I.A.; Lems, W.F.; Hamann, D.; van Schaardenburg, D.; et al. Clinically relevant discrepancies between different rheumatoid factor assays. Clin. Chem. Lab. Med. 2018, 56, 1749–1758. [Google Scholar] [CrossRef] [Green Version]
- CLSI. User Protocol for Evaluation of Qualitative Test Performance; Approved Guideline—Second Edition; CLSI Document EP12A2; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008. [Google Scholar]
- Jonsson, M.K.; Hensvold, A.H.; Hansson, M.; Aga, A.B.; Sexton, J.; Mathsson-Alm, L.; Cornillet, M.; Serre, G.; Lillegraven, S.; Fevang, B.S.; et al. The role of anti-citrullinated protein antibody reactivities in an inception cohort of patients with rheumatoid arthritis receiving treat-to-target therapy. Arthritis Res. Ther. 2018, 20, 146. [Google Scholar] [CrossRef] [Green Version]
- Murata, K.; Ito, H.; Hashimoto, M.; Murakami, K.; Watanabe, R.; Tanaka, M.; Yamamoto, W.; Matsuda, S. Fluctuation in anti-cyclic citrullinated protein antibody level predicts relapse from remission in rheumatoid arthritis: KURAMA cohort. Arthritis Res. Ther. 2020, 22, 268. [Google Scholar] [CrossRef]
- Li, F. Serum anti-citrullinated protein antibodies and rheumatoid factor increase the risk of rheumatoid arthritis-related interstitial lung disease: A meta-analysis. Clin. Rheumatol. 2021, 40, 4533–4543. [Google Scholar] [CrossRef]
- Natalini, J.G.; Baker, J.F.; Sing, N.; Mahajan, T.D.; Roul, P.; Thiele, G.M.; Sauer, B.C.; Johnson, C.R.; Kawut, S.M.; Mikuls, T.R.; et al. Autoantibody Seropositivity and Risk for Interstitial Lung Disease in a Prospective Male-Predominant Rheumatoid Arthritis Cohort of U.S. Veterans. Ann. Am. Thorac. Soc. 2021, 18, 598–605. [Google Scholar] [CrossRef]
- Hiwa, R.; Ohmura, K.; Nakabo, S.; Terao, C.; Murakami, K.; Nakashima, R.; Imura, Y.; Yukawa, N.; Yoshifuji, H.; Hashimoto, M.; et al. Only rheumatoid factor-positive subset of anti-citrullinated peptide/protein antibody-negative rheumatoid arthritis may seroconvert to anti-citrullinated peptide/protein antibody-positive. Int. J. Rheum. Dis. 2017, 20, 731–736. [Google Scholar] [CrossRef]
- Smolen, J.S.; Landewé, R.B.M.; Bijlsma, J.W.J.; Burmester, G.R.; Dougados, M.; Kerschbaumer, A.; McInnes, I.B.; Sepriano, A.; van Vollenhoven, R.F.; de Wit, M.; et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann. Rheum. Dis. 2020, 79, 685–699. [Google Scholar] [CrossRef] [Green Version]
- Park, E.-J.; Kim, H.; Jung, S.M.; Sung, Y.-K.; Baek, H.J.; Lee, J. The Use of Biological Disease-modifying Antirheumatic Drugs for Inflammatory Arthritis in Korea: Results of a Korean Expert Consensus. Korean J. Intern. Med. 2020, 35, 41–59. [Google Scholar] [CrossRef]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; Clair, E.W.S.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021, 73, 1108–1123. [Google Scholar] [CrossRef]
- Belhassen, M.; Tubach, F.; Hudry, C.; Woronoff-Lemsi, M.; Levy-Bachelot, L.; Van Ganse, E.; Fautrel, B. Impact of persistence with tumour necrosis factor inhibitors on healthcare resource utilization and costs in chronic inflammatory joint diseases. Br. J. Clin. Pharmacol. 2021, 87, 163–177. [Google Scholar] [CrossRef] [PubMed]
- Vos, I.; Mol, C.V.; Trouw, L.A.; Mahler, M.; Bakker, J.A.; Offel, J.V.; Clerck, L.D.; Huizinga, T.W. Anti-citrullinated protein antibodies in the diagnosis of rheumatoid arthritis (RA): Diagnostic performance of automated anti-CCP-2 and anti-CCP-3 antibodies assays. Clin. Rheumatol. 2017, 36, 1487–1492. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Total (n = 1110) | Men (n = 392) | Women (n = 718) | |||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | Median | IQR | |
| Age (years) | 53.1 | 42.2 to 61.8 | 49.4 | 35.7 to 58.8 | 54.5 | 46.2 to 62.7 |
| Rheumatoid factor (IU/mL) | 10.0 | 10.0 to 19.0 | 10.0 | 10.0 to 12.5 | 10.0 | 10.0 to 23.0 |
| Anti-CCP (U/mL) | 1.2 | 0.8 to 2.0 | 1.1 | 0.7 to 1.6 | 1.3 | 0.8 to 2.3 |
| Qualitative test results of initial measure, number of positive (%) | ||||||
| Rheumatoid factor (≥14 IU/mL) | 351 | (31.6%) | 90 | (20.3%) | 261 | (36.4%) |
| Anti-CCP (>10 U/mL) | 182 | (16.4%) | 45 | (11.5%) | 137 | (19.1%) |
| Anti-CCP | At Initial Measurement | Overall Measurement | ||||
|---|---|---|---|---|---|---|
| Rheumatoid Factor | Rheumatoid Factor | |||||
| Negative | Positive | Total | Negative | Positive | Total | |
| Not positive 1 | 737 (66.4%) | 191 (17.2%) | 928 (83.6%) | 1660 (64.7%) | 433 (16.9%) | 2093 (81.6%) |
| Positive | 22 (2.0%) | 160 (14.4%) | 182 (16.4%) | 65 (2.5%) | 408 (15.9%) | 473 (18.4%) |
| Total | 759 (68.4%) | 351 (31.6%) | 1110 | 1725 (67.2%) | 841 (32.8%) | 2566 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, R.; Lee, S.G.; Lee, E.H. Intraindividual Changes in Rheumatoid Factor and Anti-Cyclic Citrullinated Peptide Antibody Tests in Korean Patients Visiting Local Clinics and Hospitals. J. Clin. Med. 2022, 11, 832. https://doi.org/10.3390/jcm11030832
Choi R, Lee SG, Lee EH. Intraindividual Changes in Rheumatoid Factor and Anti-Cyclic Citrullinated Peptide Antibody Tests in Korean Patients Visiting Local Clinics and Hospitals. Journal of Clinical Medicine. 2022; 11(3):832. https://doi.org/10.3390/jcm11030832
Chicago/Turabian StyleChoi, Rihwa, Sang Gon Lee, and Eun Hee Lee. 2022. "Intraindividual Changes in Rheumatoid Factor and Anti-Cyclic Citrullinated Peptide Antibody Tests in Korean Patients Visiting Local Clinics and Hospitals" Journal of Clinical Medicine 11, no. 3: 832. https://doi.org/10.3390/jcm11030832
APA StyleChoi, R., Lee, S. G., & Lee, E. H. (2022). Intraindividual Changes in Rheumatoid Factor and Anti-Cyclic Citrullinated Peptide Antibody Tests in Korean Patients Visiting Local Clinics and Hospitals. Journal of Clinical Medicine, 11(3), 832. https://doi.org/10.3390/jcm11030832






