Effects of the Dental Caries Preventive Procedure on the White Spot Lesions during Orthodontic Treatment—An Open Label Randomized Controlled Trial
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trial Design
2.2. Setting
2.3. Inclusion Criteria
2.4. Sample Size Calculation
2.5. Randomization
2.6. Clinical Procedure
2.7. Outcome Variables
2.7.1. Assessment of WSLs
2.7.2. Assessment of the Cariogenic Bacteria
2.8. Next-Generation Sequence
2.8.1. Microbial DNA Extraction
2.8.2. Microbial Community Analysis
2.8.3. Bioinformatics Analysis
2.9. Statistical Analysis
2.9.1. QLF Data Analysis
2.9.2. Mixed Effect Modeling
2.9.3. Microbiome Analysis
2.10. Ethics
3. Results
3.1. Baseline Characteristics of the Patients Paticiapted in This Study
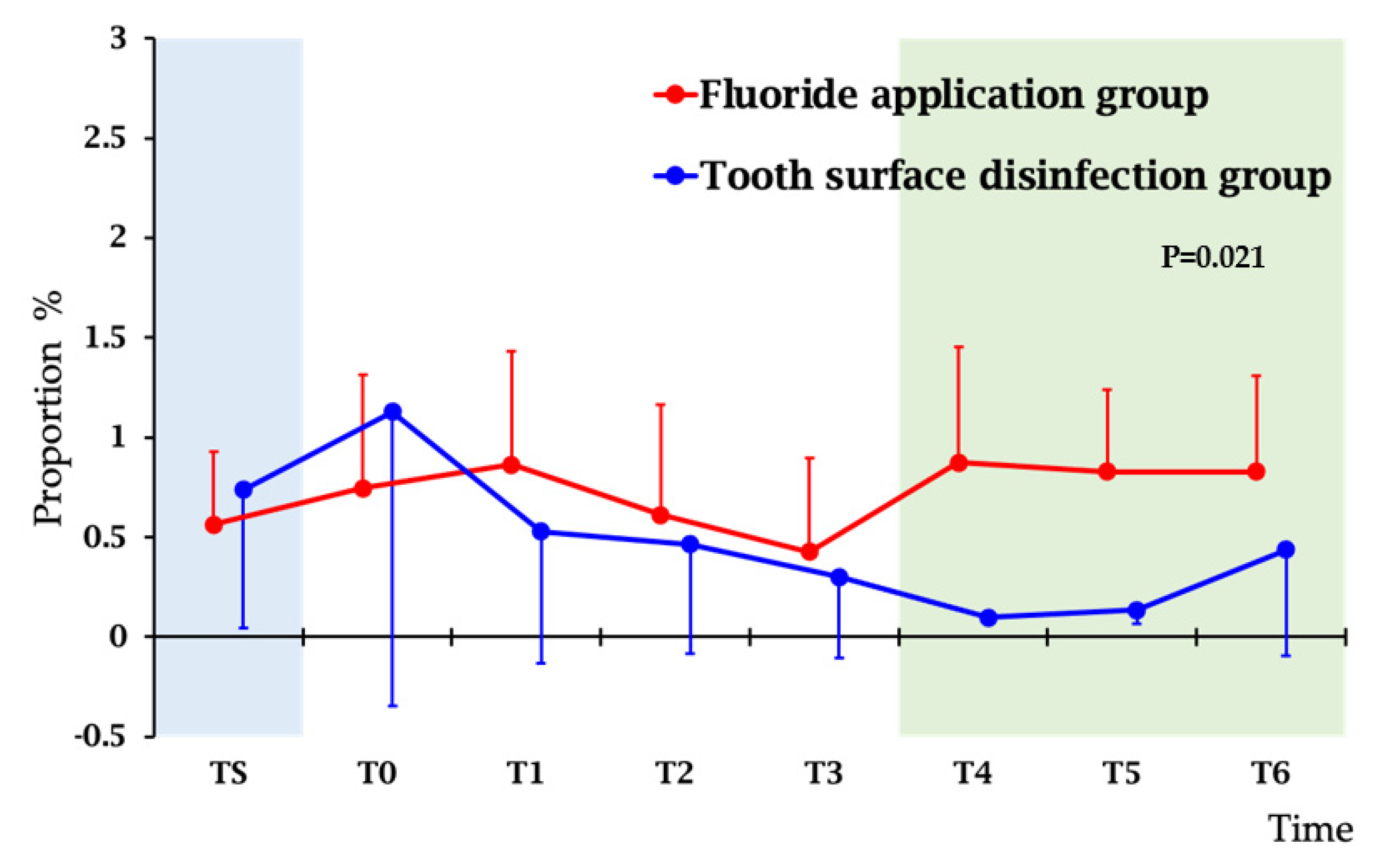
3.2. Monitoring the Effect of Treatment by Salvaru Levels of Mutans Streptococci
3.3. Site Level Changes of the White Spot Regions Evalate by QLF
3.4. Microbiome Analysis before and after Treatment
3.4.1. Sequence Data
3.4.2. Oral-Microbiome Structure
3.4.3. Effect of Tooth Surface Disinfection Treatment on Oral Microbiome
3.4.4. Correlation of White Spot and Oral Microbiome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Spyridon, N.P.; Damian, H.; Theodore, E. Outcomes of comprehensive fixed appliance orthodontic treatment: A systematic review with meta-analysis and methodological overview. Korean J. Orthod. 2017, 47, 401–413. [Google Scholar] [CrossRef] [Green Version]
- Chang, H.S.; Walsh, L.J.; Freer, T.J. Enamel demineralization during orthodontic treatment. Aetiology and prevention. Aust. Dent. J. 1997, 42, 322–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Julien, K.C.; BusChang, P.H.; CamPBell, P.M. Prevalence of white spot lesion formation during orthodontic treatment. Angle Orthod. 2013, 83, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Paolantonio, M.; D’ercole, S.; Perinetti, G.; Tripodi, D.; Catamo, G.; Serra, E.; Bruè, C.; Piccolomini, R. Clinical and microbiological effects of different restorative materials on the periodontal tissues adjacent to subgingival class V restorations. J. Clin. Periodontol. 2004, 31, 200–207. [Google Scholar] [CrossRef]
- Freitas, A.O.; Marquezan, M.; Mda, C.N.; Alviano, D.S.; Maia, L.C. The influence of orthodontic fixed appliances on the oral microbiota: A systematic review. Dental. Press J. Orthod. 2014, 19, 46–55. [Google Scholar] [CrossRef] [Green Version]
- Topaloglu-Ak, A.; Ertugrul, F.; Eden, E.; Ates, M.; Bulut, H. Effect of orthodontic appliances on oral microbiota—6 month followup. J. Clin. Pediatr. Dent. 2011, 35, 433–436. [Google Scholar] [CrossRef]
- Ogaard, B.; Rolla, G.; Arends, J.; Cate, J.M. Orthodontic appliances and enamel demineralization. Part 2. Prevention and treatment of lesions. Am. J. Orthod. Dentofac. Orthop. 1988, 94, 123–128. [Google Scholar] [CrossRef]
- Mattousch, T.J.; van der Veen, M.H.; Zentner, A. Caries lesions after orthodontic treatment followed by quantitative light-induced fluorescence: A 2-year follow-up. Eur. J. Orthod. 2007, 29, 294–298. [Google Scholar] [CrossRef] [Green Version]
- Moriyama, C.M.; Rodrigues, J.A.; Lussi, A.; Diniz, M.B. Effectiveness of Fluorescence-Based Methods to Detect in situ Demineralization and Remineralization on Smooth Surfaces. Caries Res. 2014, 48, 507–514. [Google Scholar] [CrossRef]
- Demito, C.F.; Rodrigues, G.V.; Ramos, A.L.; Bowman, S.J. Efficacy of a fluoride varnish in preventing white-spot lesions as measured with laser fluorescence. J. Clin. Orthod. 2011, 45, 25–29. [Google Scholar]
- Rosenbloom, R.G.; Tinanoff, N. Salivary Streptococcus mutans levels in patients before, during, and after orthodontic treatment. Am. J. Orthod. Dentofac. Orthop. 1991, 100, 35–37. [Google Scholar] [CrossRef]
- Mummolo, S.; Nota, A.; Albani, F.; Marchetti, E.; Gatto, R.; Marzo, G.; Quinzi, V.; Tecco, S. Salivary levels of Streptococcus mutans and Lactobacilli and other salivary indices in patients wearing clear aligners versus fixed orthodontic appliances: An observational study. PLoS ONE 2020, 15, e0228798. [Google Scholar] [CrossRef]
- Al Mulla, A.H.; Al Kharsa, S.; Birkhed, D. Modified fluoride toothpaste technique reduces caries in orthodontic patients: A longitudinal, randomized clinical trial. Am. J. Orthod. Dentofac. Orthop. 2010, 138, 285–291. [Google Scholar] [CrossRef]
- Farhadian, N.; Miresmaeili, A.; Eslami, B.; Mehrabi, S. Effect of fluoride varnish on enamel demineralization around brackets: An in-vivo study. Am. J. Orthod. Dentofac. Orthop. 2008, 133, S95–S98. [Google Scholar] [CrossRef]
- Rijkom, H.; Truin, G.; Van’t Hof, M. A meta-analysis of clinical studies on the caries-inhibiting effect of chlorhexidine treatment. J. Dent. Res. 1996, 75, 790–795. [Google Scholar] [CrossRef]
- Maruo, I.T.; Rosa, E.A.; Maruo, H.; Tanaka, O.; Guariza, F.O.; Ignácio, S.A.; Camargo, E.S. Effect of chlorhexidine mouth rinse on Streptococci counts of tooth tissue borne palatal expander biofilm. Orthod. Craniofac. Res. 2008, 11, 136–142. [Google Scholar] [CrossRef]
- Reynolds, E.C.; Cai, F.; Cochrane, N.J.; Shen, P.; Walker, G.D.; Morgan, M.V.; Reynolds, C. Fluoride and casein phosphopeptide-amorphous calcium phosphate. J. Den. Res. 2008, 87, 344–348. [Google Scholar] [CrossRef]
- Alves, P.V.M.; Alviano, W.S.; Bolognese, A.M.; Nojima, L.I. Treatment protocol to control Streptococcus mutans level in an orthodontic patient with high caries risk. Am. J. Orthod. Dentofac. Orthop. 2008, 133, 91–94. [Google Scholar] [CrossRef]
- Al-Bazi, S.M.; Abbassy, M.A.; Bakry, A.S.; Merdad, L.A.; Hassan, A.H. Effects of chlorhexidine (gel) application on bacterial levels and orthodontic brackets during orthodontic treatment. J. Oral Sci. 2016, 58, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Restrepo, M.; Bussaneli, D.G.; Jeremias, F.; Cordeiro, R.C.L.; Magalhães, A.C.; Spolidorio, D.M.P.; Santos, P.L. Control of White Spot Lesion Adjacent to Orthodontic Bracket with Use of Fluoride Varnish or Chlorhexidine Gel. Sci. World J. 2015, 2015, 218452. [Google Scholar] [CrossRef]
- Benson, P.E.; Pender, N.; Higham, S.M. Quantifying enamel demineralization from teeth with orthodontic brackets—a comparison of two methods. Part 1: Repeatability and agreement. Eur. J. Orthod. 2003, 25, 149–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamaki, Y.; Nomura, Y.; Takeuchi, H.; Ida, H.; Arakawa, H.; Tsurumoto, A.; Kumagai, T.; Hanada, N. Study of the clinical usefulness of a dental drug system for selective reduction of mutans streptococci using a case series. J. Oral Sci. 2006, 48, 111–116. [Google Scholar] [CrossRef] [Green Version]
- Benic, G.Z.; Farella, M.; Morgan, X.C.; Viswam, J.; Nicolas, D.H.; Cannon, R.D.; Li, M. Oral probiotics reduce halitosis in patients wearing orthodontic braces: A randomized, triple-blind, placebo-controlled trial. J. Breath Res. 2019, 13, 036010. [Google Scholar] [CrossRef]
- Koopman, J.E.; van der Kaaij, N.C.; Buijs, M.J.; Elyassi, Y.; van der Veen, M.H.; Crielaard, W.; Ten Cate, J.M.; Zaura, E. The Effect of Fixed Orthodontic Appliances and Fluoride Mouthwash on the Oral Microbiome of Adolescents—A Randomized Controlled Clinical Trial. PLoS ONE 2015, 10, e0137318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, I.; Chung, J.; Vella, R.; Weinstock, G.M.; Zhou, Y.; Jheon, A.H. Alterations in subgingival microbiota during full-fixed appliance orthodontic treatment—A prospective study. Orthod. Craniofac. Res. 2021, 19. [Google Scholar] [CrossRef] [PubMed]
- Vidović, B.; Gušić, I.; Tamaš, I.; Mihajlović, D.; Mitić, V.; Obradović, R.; Radovanović, M.; Brkić, S. The effect of the octenidine-based oral antiseptic on the structure of microbial communities and periodontal status in patients with fixed orthodontic treatment. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8598–8605. [Google Scholar] [CrossRef] [PubMed]
- Kado, I.; Hisatsune, J.; Tsuruda, K.; Tanimoto, K.; Sugai, M. The impact of fixed orthodontic appliances on oral microbiome dynamics in Japanese patients. Sci. Rep. 2020, 10, 21989. [Google Scholar] [CrossRef]
- Rechmann, P.; Kinsel, R.; Featherstone, J.D.B. Integrating Caries Management by Risk Assessment (CAMBRA) and Prevention Strategies into the Contemporary Dental Practice. Compend. Contin. Educ. Dent. 2018, 39, 226–233. [Google Scholar]
- Nomura, Y.; Takeuchi, H.; Kaneko, N.; Matin, K.; Iguchi, R.; Toyoshima, Y.; Kono, Y.; Ikemi, T.; Imai, S.; Nishizawa, T.; et al. Feasibility of eradication of mutans streptococci from oral cavities. J. Oral Sci. 2004, 46, 179–183. [Google Scholar] [CrossRef] [Green Version]
- Nomura, Y.; Senpuku, H.; Tsuge, S.; Hayashi, M.; Sasaki, A.; Tamura, H.; Ida, H.; Yoshikawa, E.; Nishikawara, F.; Kawamura, S.; et al. Controlling opportunistic pathogens in the oral cavity of preschool children by the use of 3DS. Jpn. J. Infect. Dis. 2001, 54, 199–200. [Google Scholar]
- Takeuchi, H.; Fukushima, K.; Senpuku, H.; Nomura, Y.; Kaneko, N.; Yano, A.; Morita, E.; Imai, S.; Nisizawa, T.; Kono, Y.; et al. Clinical study of mutans streptococci using 3DS and monoclonal antibodies. Jpn. J. Infect. Dis. 2001, 54, 34–36. [Google Scholar]
- Ramos-Gomez, F.J.; Crystal, Y.O.; Domejean, S.; Featherstone, J.D. Minimal intervention dentistry: Part 3. Paediatric dental care--prevention and management protocols using caries risk assessment for infants and young children. Br. Dent. J. 2012, 213, 501–508. [Google Scholar] [CrossRef] [Green Version]
- Enneking, F.K.; Radhakrishnan, N.S.; Berg, K.; Patel, S.; Wishin, J.M.; Vasilopoulos, T. Patient-centered anesthesia triage system predicts ASA physical status. Anesth. Analg. 2017, 124, 1957–1962. [Google Scholar] [CrossRef]
- Aykan, O.A.; Taner, O.; Mustafa, M.F.; Ahmet, Y. A comparative assessment of orthodontic treatment outcomes using the quantitative light-induced fluorescence (QLF) method between direct bonding and indirect bonding techniques in adolescents: A single-centre, single-blind randomized controlled trial. Eur. J. Orthod. 2020, 42, 441–453. [Google Scholar] [CrossRef]
- Kim, O.S.; Cho, Y.J.; Lee, K.; Yoon, S.H.; Kim, M.; Na, H.; Park, S.C.; Jeon, Y.S.; Lee, J.H.; Yi, H.; et al. Introducing EzTaxon-e: A prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int. J. Syst. Evol. Microbiol. 2012, 62, 716–721. [Google Scholar] [CrossRef]
- Yoon, S.H.; Ha, S.M.; Kwon, S.; Lim, J.; Kim, Y.; Seo, H.; Chun, J. Introducing EzBioCloud: A taxonomically united database of 16S rRNA and whole genome assemblies. Int. J. Syst. Evol. Microbiol. 2017, 67, 1613–1617. [Google Scholar] [CrossRef]
- Nomura, Y.; Morozumi, T.; Nakagawa, T.; Sugaya, T.; Kawanami, M.; Suzuki, F.; Takahashi, K.; Abe, Y.; Sato, S.; Makino-Oi, A.; et al. Site-level progression of periodontal disease during a follow-up period. PLoS ONE 2017, 12, e0188670. [Google Scholar] [CrossRef]
- Nomura, Y.; Otsuka, R.; Wint, W.Y.; Okada, A.; Hasegawa, R.; Hanada, N. Tooth-level analysis of dental caries in primary dentition in Myanmar children. Int. J. Environ. Res. Public Health 2020, 17, 7613. [Google Scholar] [CrossRef]
- Nomura, Y.; Otsuka, R.; Hasegawa, R.; Hanada, N. Oral Microbiome of Children Living in an Isolated Area in Myanmar. Int. J. Environ. Res. Public Health 2020, 17, 4033. [Google Scholar] [CrossRef]
- Nomura, Y.; Kakuta, E.; Okada, A.; Otsuka, R.; Shimada, M.; Tomizawa, Y.; Taguchi, C.; Arikawa, K.; Daikoku, H.; Sato, T.; et al. Oral microbiome in four female centenarians. Appl. Sci. 2020, 10, 5312. [Google Scholar] [CrossRef]
- Emel, S.; Ilhan, B. Microbiological Evaluation of 0.2% Chlorhexidine Gluconate Mouth Rinse in Orthodontic Patients. Angle Orthod. 2007, 77, 881–884. [Google Scholar] [CrossRef]
- Oltramari-Navarro, P.V.P.; Titarelli, J.M.; Marsicano, J.; Henriques, J.F.C.; Janson, G.; Lauris, J.; Buzalaf, M.A.R. Effectiveness of 0.50% and 0.75% chlorhexidine dentifrices in orthodontic patients: A double-blind and randomized controlled trial. Am. J. Orthod. Dentofac. Orthop. 2009, 136, 651–656. [Google Scholar] [CrossRef] [PubMed]
- James, P.; Worthington, H.V.; Parnell, C.; Harding, M.; Lamont, T.; Cheung, A.; Whelton, H.; Riley, P. Chlorhexidine mouthrinse as an adjunctive treatment for gingival health. Cochrane Database Syst. Rev. 2017, 3, CD008676. [Google Scholar] [CrossRef] [PubMed]
- Pitts, N.; Ekstrand, K.; The ICDAS Foundation. International Caries Detection and Assessment System (ICDAS) and its International Caries Classification and Management System (ICCMS)—Methods for staging of the caries process and enabling dentists to manage caries. Community Dent. Oral Epidemiol. 2013, 41, e41–e52. [Google Scholar] [CrossRef]
- Inada, E.; Saitoh, I.; Yu, Y.; Tomiyama, D.; Murakami, D.; Takemoto, Y.; Morizono, K.; Iwasaki, T.; Iwase, Y.; Yamasaki, Y. Quantitative evaluation of toothbrush and arm-joint motion during tooth brushing. Clin. Oral Investig. 2015, 19, 1451–1462. [Google Scholar] [CrossRef] [Green Version]
- Spiguel, M.H.; Tovo, M.F.; Kramer, P.F.; Franco, K.S.; Alves, K.M.; Delbem, A.C. Evaluation of laser fluorescence in the monitoring of the initial stage of the de-/remineralization process: An in vitro and in situ study. Caries Res. 2009, 43, 302–307. [Google Scholar] [CrossRef]
- Cochrane, N.J.; Walker, G.D.; Manton, D.J.; Reynolds, E.C. Comparison of quantitative light-induced fluorescence, digital photography and transverse microradiography for quantification of enamel remineralization. Aust. Dent. J. 2012, 57, 271–276. [Google Scholar] [CrossRef]
- Hall, A.F.; DeSchepper, E.; Ando, M.; Stookey, G.K. In vitro studies of laser fluorescence for detection and quantification of mineral loss from dental caries. Adv. Dent. Res. 1997, 11, 507–514. [Google Scholar] [CrossRef]
- Aljehani, A.; Tranæus, S.; Forsberg, C.-M.; Angmar-Mansson, B.; Shi, X.-O. In vitro quantification of white spot enamel lesions adjacent to fixed orthodontic appliances using quantitative light-induced fluorescence and DIAGNOdent. Acta Odontol. Scand. 2004, 62, 313–318. [Google Scholar] [CrossRef]
- Boersma, J.; Van der Veen, M.; Lagerweij, M.; Bokhout, B.; PrahlAndersen, B. Caries prevalence measured with QLF after treatment with fixed orthodontic appliances: Influencing factors. Caries Res. 2005, 39, 41–47. [Google Scholar] [CrossRef]
- Van Der Veen, M.H.; Attin, R.; Schwestka-Polly, R.; Wiechmann, D. Caries outcomes after orthodontic treatment with fixed appliances: Do lingual brackets make a difference? J. Eur. J. Oral Sci. 2010, 118, 298–303. [Google Scholar] [CrossRef]
- Tranaeus, S.; Al-Khateeb, S.; Björkman, S.; Twetman, S.; Angmar-Mansson, B. Application of quantitative lightinduced fluorescence to monitor incipient lesions in cariesactive children. A comparative study of remineralisation by fluoride varnish and professional cleaning. Eur. J. Oral Sci. 2001, 109, 71–75. [Google Scholar] [CrossRef]
- Silva, V.M.; Massaro, C.; Buzalaf, M.A.R.; Janson, G.; Garib, D. Prevention of non-cavitated lesions with fluoride and xylitol varnishes during orthodontic treatment: A randomized clinical trial. Clin. Oral Investig. 2021, 25, 3421–3430. [Google Scholar] [CrossRef]
- Kronenberg, O.; Lussi, A.; Ruf, S. Preventive Effect of Ozone on the Development of White Spot Lesions during Multibracket Appliance Therapy. Angle Orthod. 2009, 79, 64–69. [Google Scholar] [CrossRef] [Green Version]
- Sonesson, M.; Brechter, A.; Lindman, R.; Abdulraheem, S.; Twetman, S. Fluoride varnish for white spot lesion prevention during orthodontic treatment: Results of a randomized controlled trial 1 year after debonding. Eur. J. Orthod. 2021, 43, 473–477. [Google Scholar] [CrossRef]
- Beerens, M.W.; Cate, J.M.; Buijs, M.J.; van der Veen, M.H. Long-term remineralizing effect of MI Paste Plus on regression of early caries after orthodontic fixed appliance treatment: A 12-month follow-up randomized controlled trial. Eur. J. Orthod. 2018, 40, 457–464. [Google Scholar] [CrossRef] [Green Version]
- Jablonski-Momeni, A.; Nothelfer, R.; Morawietz, M.; Kiesow, A.; Korbmacher-Steiner, H. Impact of self-assembling peptides in remineralization of artificial early enamel lesions adjacent to orthodontic brackets. Sci. Rep. 2020, 10, 15132. [Google Scholar] [CrossRef]
- Nomura, Y.; Fujita, Y.; Ishihara, Y.; Kakuta, E.; Okada, A.; Maki, K.; Hanada, N. Effects of cariogenic bacteria and sealant evaluated by International Caries Detection Assessment System. Open Dent. J. 2019, 13, 512–519. [Google Scholar] [CrossRef] [Green Version]
- Kilian, M.; Chapple, I.L.; Hannig, M.; Marsh, P.D.; Meuric, V.; Pedersen, A.M.L.; Tonetti, M.S.; Wade, W.G.; Zaura, E. The oral microbiome—An update for oral healthcare professionals. Br. Dent. J. 2016, 221, 657–666. [Google Scholar] [CrossRef]



| White Spot Area (mm2) | ΔF (%) | ΔFmax (%) | ΔQ(% × mm2) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | Coefficient (95% CI) | p-Value | |||
| Fixed effect | ||||||||||
| Intercept | 0.375 (0.314–0.435) | <0.001 | −2.195 (−2.234–−2.156) | <0.001 | −2.662 (−2.606–−2.718) | <0.001 | −1.063 (−1.357–−0.769) | <0.001 | ||
| Treatment | Tooth surface disinfection | −0.142 (−0.211–−0.074) | <0.001 | 0.076 (0.027–0.125) | 0.003 | 0.223 (0.155–0.292) | <0.001 | 0.636 (0.260–1.012) | 0.001 | |
| Fluoride application | Reference | Reference | Reference | Reference | ||||||
| Time | After 6 months | −0.043 (−0.061–−0.025) | <0.001 | 0.038 (0.027–0.048) | <0.001 | 0.106 (0.084–0.128) | <0.001 | 0.345 (0.295–0.396) | <0.001 | |
| Baseline | Reference | Reference | Reference | Reference | ||||||
| Tooth type | Maxillary | 1 | 0.054 (−0.002–0.11) | 0.059 | 0.094 (0.069–0.120) | <0.001 | 0.116 (0.079–0.153) | <0.001 | 0.005 (0.193–0.202) | 0.963 |
| 2 | −0.024 (−0.082–0.034) | 0.413 | 0.056 (0.030–0.082) | <0.001 | 0.090 (0.052–0.128) | <0.001 | 0.394 (0.190–0.598) | <0.001 | ||
| 3 | 0.082 (0.031–0.134) | 0.002 | 0.014 (0.010–0.037) | 0.249 | −0.002 (0.036–0.032) | 0.898 | −0.123 (0.305–0.059) | 0.186 | ||
| Mandibular | 1 | −0.097 (−0.149–−0.045) | <0.001 | 0.061 (0.038–0.085) | <0.001 | 0.103 (0.069–0.138) | <0.001 | 0.566 (0.381–0.750) | <0.001 | |
| 2 | −0.092 (−0.147–−0.037) | 0.001 | 0.055 (0.030–0.080) | <0.001 | 0.082 (0.045–0.118) | <0.001 | 0.476 (0.283–0.670) | <0.001 | ||
| 3 | Reference | Reference | Reference | Reference | ||||||
| Random effect | ||||||||||
| Level1 (Subject) | 0.005 (0.002–0.012) | 0.012 | −0.003 (−0.006–−0.002) | 0.004 | −0.006 (−0.012–−0.003) | 0.004 | −0.184 (−0.363–−0.094) | 0.004 | ||
| Level2 (Tooth) | 0.006 (0.004–0.010) | <0.001 | −0.001 (−0.002–−0.001) | 0.001 | <0.001 | - | −0.093 (−0.138–−0.063) | <0.001 | ||
| Level3 (Ordination) | 0.041 (0.036–0.048) | <0.001 | −0.009 (−0.011–−0.008) | <0.001 | −0.021 (−0.028–−0.017) | <0.001 | −0.535 (−0.598–−0.478) | <0.001 | ||
| Fitness index | ||||||||||
| AICC | 2959.631 | −2033.758 | 2904.679 | 11,802.447 | ||||||
| BIC | 2991.11 | −2002.287 | 2936.152 | 11,833.918 | ||||||
| Distribution | Normal, Link: Identity | Γ, Link: log | Γ, Link: log | Γ, Link: log | ||||||
| (A) | |||||||
| Species (0.1% or More Reduction) | Baseline (%) | After 6 Months (%) | Difference (%) | Species (0.1% or More Increase) | Baseline (%) | After 6 Months (%) | Difference (%) |
| Haemophilus parainfluenzae group | 11.18 | 7.16 | −4.02 | Streptococcus sinensis group | 1.91 | 4.59 | 2.68 |
| Neisseria_uc | 2.07 | 0.07 | −1.99 | Streptococcus parasanguinis group | 0.93 | 2.83 | 1.90 |
| Porphyromonas pasteri | 4.24 | 2.61 | −1.62 | Streptococcus salivarius group | 1.07 | 2.66 | 1.60 |
| Streptococcus pneumoniae group | 8.18 | 7.13 | −1.04 | Neisseria subflava | 11.56 | 13.01 | 1.45 |
| Gemella haemolysans group | 1.75 | 0.74 | −1.02 | Veillonella rogosae | 1.96 | 3.16 | 1.20 |
| Veillonella parvula group | 1.40 | 0.86 | −0.55 | Prevotella nanceiensis group | 0.43 | 1.35 | 0.91 |
| Fusobacterium nucleatum group | 1.28 | 0.77 | −0.51 | Veillonella atypica | 0.72 | 1.39 | 0.67 |
| Granulicatella adiacens group | 3.04 | 2.57 | −0.47 | Prevotella histicola | 0.31 | 0.80 | 0.49 |
| Prevotella denticola | 0.51 | 0.09 | −0.42 | Prevotella melaninogenica | 4.56 | 5.05 | 0.49 |
| Streptococcus gordonii group | 0.68 | 0.36 | −0.32 | Haemophilus pittmaniae | 0.05 | 0.48 | 0.43 |
| Haemophilus sputorum | 0.76 | 0.49 | −0.27 | Actinomyces graevenitzii | 0.11 | 0.50 | 0.39 |
| Neisseria elongata group | 0.68 | 0.42 | −0.26 | PAC001346_s | 0.26 | 0.63 | 0.38 |
| Haemophilus haemolyticus | 0.30 | 0.04 | −0.26 | Neisseria oralis | 0.17 | 0.53 | 0.36 |
| Capnocytophaga leadbetteri | 0.58 | 0.34 | −0.24 | Neisseria sicca group | 3.14 | 3.46 | 0.32 |
| Abiotrophia defectiva | 0.49 | 0.28 | −0.20 | FJ976422_s | 0.12 | 0.44 | 0.31 |
| Capnocytophaga sputigena | 0.51 | 0.33 | −0.18 | Prevotella pallens | 0.53 | 0.80 | 0.27 |
| KV831974_s group | 2.48 | 2.29 | −0.18 | Streptococcus_uc | 0.25 | 0.50 | 0.25 |
| Rothia dentocariosa | 0.42 | 0.24 | −0.18 | Lautropia mirabilis | 0.27 | 0.52 | 0.25 |
| KI272869_s | 0.32 | 0.17 | −0.14 | Prevotella jejuni | 0.14 | 0.37 | 0.23 |
| AM420132_s | 0.17 | 0.03 | −0.13 | Prevotella aurantiaca | 0.00 | 0.20 | 0.20 |
| JQ463704_s | 0.32 | 0.20 | −0.12 | Actinomyces odontolyticus | 0.09 | 0.29 | 0.20 |
| Prevotella loescheii | 0.17 | 0.06 | −0.12 | PAC001345_s | 0.24 | 0.44 | 0.20 |
| Haemophilus_uc | 0.17 | 0.05 | −0.11 | Streptococcus peroris group | 0.37 | 0.54 | 0.17 |
| Dialister invisus | 0.25 | 0.13 | −0.11 | LT608321_s | 0.04 | 0.19 | 0.16 |
| Haemophilus influenzae group | 0.18 | 0.07 | −0.11 | Peptostreptococcus massiliae group | 0.06 | 0.20 | 0.14 |
| Prevotella oris | 0.19 | 0.08 | −0.10 | Atopobium parvulum | 0.27 | 0.39 | 0.12 |
| EU681966_s | 0.10 | 0.00 | −0.10 | JN713562_g_uc | 0.00 | 0.12 | 0.12 |
| FJ976402_s | 0.04 | 0.16 | 0.12 | ||||
| CAGY_s | 0.02 | 0.13 | 0.11 | ||||
| Megasphaera micronuciformis | 0.41 | 0.51 | 0.10 | ||||
| (B) | |||||||
| Species (0.1% or More Reduction) | Baseline (%) | After 6 Months (%) | Difference (%) | Species (0.1% or More Increase) | Baseline (%) | After 6 Months (%) | Difference (%) |
| Neisseria perflava | 14.06 | 5.21 | −8.85 | Prevotella veroralis | 0.02 | 0.14 | 0.11 |
| Neisseria subflava | 13.80 | 9.67 | −4.13 | JQ463704_s | 0.19 | 0.31 | 0.12 |
| Porphyromonas pasteri | 4.46 | 2.65 | −1.80 | Streptococcus gordonii group | 0.23 | 0.35 | 0.12 |
| Fusobacterium periodonticum group | 3.66 | 1.86 | −1.80 | Streptococcus salivarius group | 1.75 | 1.88 | 0.13 |
| FJ976422_s | 1.02 | 0.03 | −1.00 | Campylobacter gracilis | 0.08 | 0.21 | 0.13 |
| Prevotella nanceiensis group | 1.39 | 0.51 | −0.88 | Corynebacterium matruchotii | 0.06 | 0.20 | 0.14 |
| LT608321_s | 0.60 | 0.10 | −0.50 | JVLH_s | 0.04 | 0.19 | 0.15 |
| Neisseria elongata group | 1.07 | 0.58 | −0.50 | Dialister invisus | 0.08 | 0.24 | 0.16 |
| Peptostreptococcus massiliae group | 0.46 | 0.06 | −0.41 | Streptococcus peroris group | 0.57 | 0.73 | 0.17 |
| Prevotella aurantiaca | 0.32 | 0.08 | −0.24 | Streptococcus_uc | 0.33 | 0.51 | 0.18 |
| Prevotella pallens | 0.56 | 0.34 | −0.23 | Streptococcus sanguinis group | 0.77 | 0.95 | 0.18 |
| Haemophilus influenzae group | 0.37 | 0.18 | −0.20 | KE952139_s | 0.17 | 0.36 | 0.19 |
| Haemophilus sputorum | 0.40 | 0.23 | −0.17 | Prevotella jejuni | 0.34 | 0.54 | 0.20 |
| KI272869_s | 0.29 | 0.12 | −0.16 | Rothia aeria | 0.39 | 0.60 | 0.21 |
| Aggregatibacter aphrophilus | 0.48 | 0.37 | −0.11 | PAC001350_s | 0.13 | 0.35 | 0.22 |
| Capnocytophaga gingivalis | 0.15 | 0.04 | −0.11 | Veillonella rogosae | 2.79 | 3.01 | 0.22 |
| JF239777_s | 0.13 | 0.03 | −0.10 | Lautropia mirabilis | 0.41 | 0.66 | 0.25 |
| Haemophilus pittmaniae | 0.45 | 0.35 | −0.10 | Veillonella atypica | 0.68 | 0.95 | 0.28 |
| JX294356_s | 0.11 | 0.00 | −0.10 | Prevotella salivae | 0.16 | 0.47 | 0.30 |
| Atopobium parvulum | 0.22 | 0.53 | 0.32 | ||||
| Prevotella_uc | 0.08 | 0.41 | 0.32 | ||||
| Abiotrophia defectiva | 0.15 | 0.49 | 0.33 | ||||
| Rothia dentocariosa | 0.15 | 0.59 | 0.44 | ||||
| KV831974_s group | 1.41 | 1.88 | 0.47 | ||||
| Rothia mucilaginosa group | 0.44 | 0.96 | 0.52 | ||||
| Streptococcus parasanguinis group | 1.73 | 2.27 | 0.54 | ||||
| Prevotella melaninogenica | 3.85 | 4.43 | 0.59 | ||||
| Prevotella histicola | 0.17 | 0.76 | 0.59 | ||||
| Streptococcus pneumoniae group | 7.12 | 8.01 | 0.89 | ||||
| Streptococcus sinensis group | 1.54 | 2.47 | 0.93 | ||||
| Haemophilus parainfluenzae group | 9.14 | 10.25 | 1.12 | ||||
| Granulicatella adiacens group | 1.86 | 3.03 | 1.17 | ||||
| Veillonella parvula group | 0.71 | 1.97 | 1.26 | ||||
| Neisseria_uc | 0.09 | 1.57 | 1.48 | ||||
| Veillonella dispar | 3.81 | 6.18 | 2.37 | ||||
| Neisseria sicca group | 2.78 | 5.42 | 2.64 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimpo, Y.; Nomura, Y.; Sekiya, T.; Arai, C.; Okada, A.; Sogabe, K.; Hanada, N.; Tomonari, H. Effects of the Dental Caries Preventive Procedure on the White Spot Lesions during Orthodontic Treatment—An Open Label Randomized Controlled Trial. J. Clin. Med. 2022, 11, 854. https://doi.org/10.3390/jcm11030854
Shimpo Y, Nomura Y, Sekiya T, Arai C, Okada A, Sogabe K, Hanada N, Tomonari H. Effects of the Dental Caries Preventive Procedure on the White Spot Lesions during Orthodontic Treatment—An Open Label Randomized Controlled Trial. Journal of Clinical Medicine. 2022; 11(3):854. https://doi.org/10.3390/jcm11030854
Chicago/Turabian StyleShimpo, Yudai, Yoshiaki Nomura, Toshiko Sekiya, Chihiro Arai, Ayako Okada, Kaoru Sogabe, Nobuhiro Hanada, and Hiroshi Tomonari. 2022. "Effects of the Dental Caries Preventive Procedure on the White Spot Lesions during Orthodontic Treatment—An Open Label Randomized Controlled Trial" Journal of Clinical Medicine 11, no. 3: 854. https://doi.org/10.3390/jcm11030854
APA StyleShimpo, Y., Nomura, Y., Sekiya, T., Arai, C., Okada, A., Sogabe, K., Hanada, N., & Tomonari, H. (2022). Effects of the Dental Caries Preventive Procedure on the White Spot Lesions during Orthodontic Treatment—An Open Label Randomized Controlled Trial. Journal of Clinical Medicine, 11(3), 854. https://doi.org/10.3390/jcm11030854







