Evolution and New Horizons of Endoscopy in Inflammatory Bowel Diseases
Abstract
:1. Introduction
2. Methods
3. Endoscopy in IBD: Applications
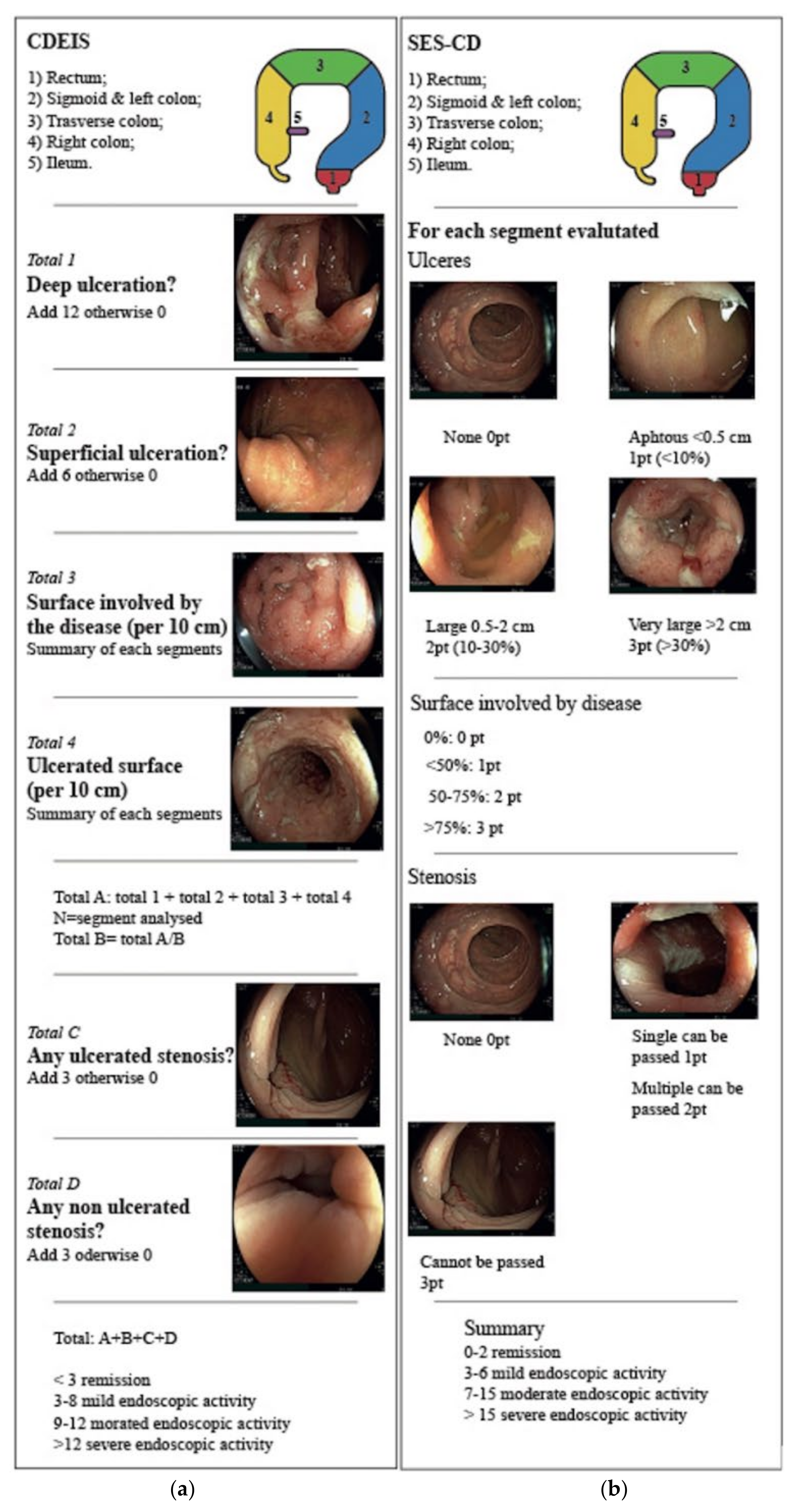
3.1. Endoscopy Activity: Endoscopic Scores
3.1.1. Endoscopic Scores for Crohn’s Disease
3.1.2. Endoscopic Scores for Ulcerative Colitis
3.2. Surveillance Evolution
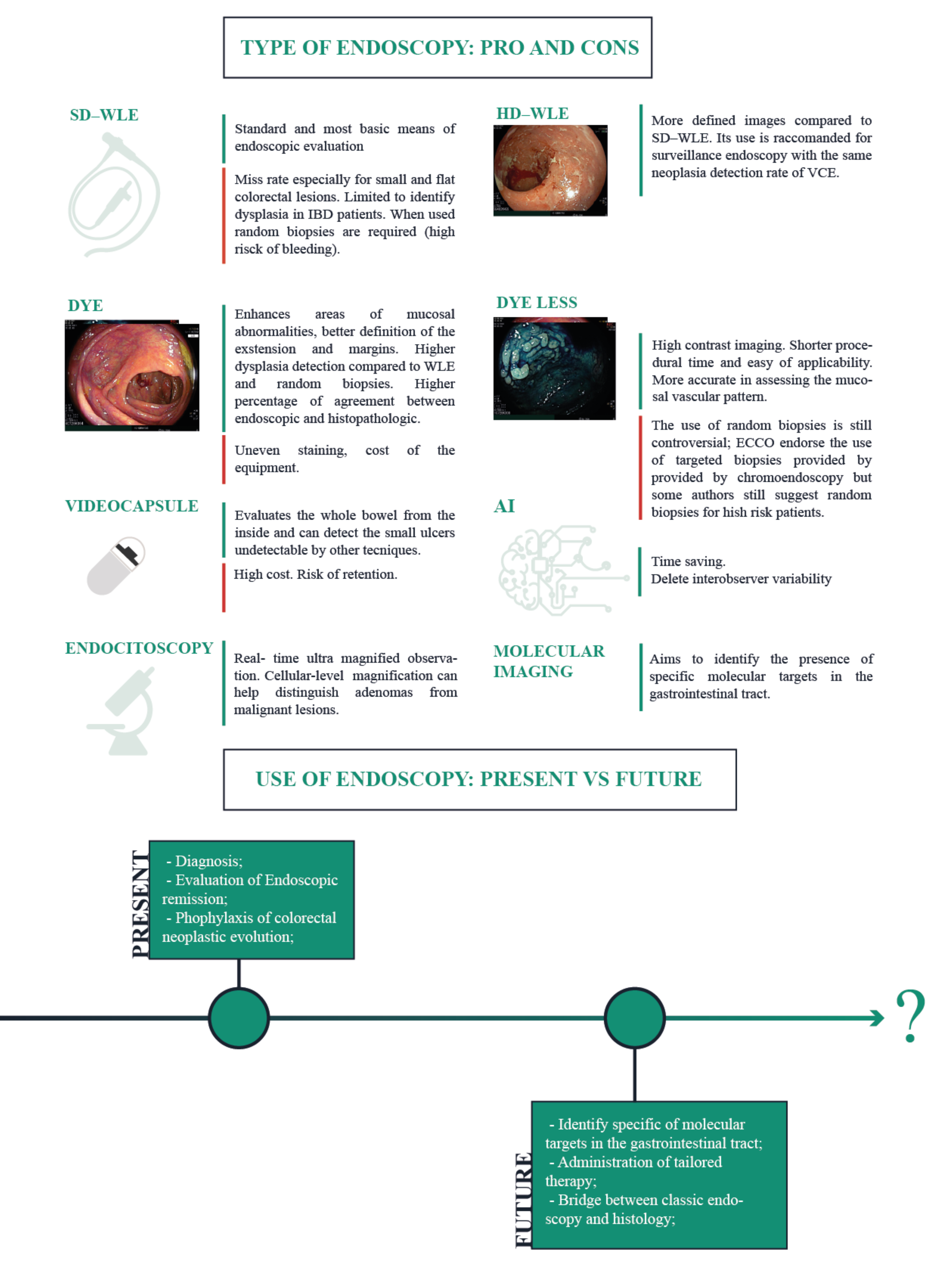
4. Endoscopy in IBD: Techniques
4.1. Chromoendoscopy
4.2. Video Capsule Endoscopy
4.2.1. Risk of Capsule Retention
4.2.2. AI for Capsule Endoscopy
4.3. Molecular Imaging
4.4. Endocytoscopy
4.5. Artificial Intelligence
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Maaser, C.; Sturm, A.; Vavricka, S.R.; Kucharzik, T.; Fiorino, G.; Annese, V.; Calabrese, E.; Baumgart, D.C.; Bettenworth, D.; Borralho Nunes, P.; et al. ECCO-ESGAR Guideline for Diagnostic Assessment in IBD Part 1: Initial diagnosis, monitoring of known IBD, detection of complications. J. Crohn Colitis 2019, 13, 144–164K. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baert, F.; Moortgat, L.; Van Assche, G.; Caenepeel, P.; Vergauwe, P.; De Vos, M.; Stokkers, P.; Hommes, D.; Rutgeerts, P.; Vermeire, S.; et al. Mucosal Healing Predicts Sustained Clinical Remission in Patients with Early-Stage Crohn’s Disease. Gastroenterology 2010, 138, 463–468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kopylov, U.; Klang, E.; Yablecovitch, D.; Lahat, A.; Avidan, B.; Neuman, S.; Levhar, N.; Greener, T.; Rozendorn, N.; Beytelman, A.; et al. Magnetic resonance enterography versus capsule endoscopy activity indices for quantification of small bowel inflammation in Crohn’s disease. Ther. Adv. Gastroenterol. 2016, 9, 655–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, B.; Rubin, D.T. Understanding Endoscopic Disease Activity in IBD: How to Incorporate It into Practice. Curr. Gastroenterol. Rep. 2016, 18, 5. [Google Scholar] [CrossRef]
- Bryant, R.V.; Winer, S.; Spl, T.; Riddell, R.H. Systematic review: Histological remission in inflammatory bowel disease. Is ‘complete’ remission the new treatment paradigm? An IOIBD initiative. J. Crohn Colitis 2014, 8, 1582–1597. [Google Scholar] [CrossRef] [Green Version]
- Turner, D.; Ricciuto, A.; Lewis, A.; D’Amico, F.; Dhaliwal, J.; Griffiths, A.M.; Bettenworth, D.; Sandborn, W.J.; Sands, B.E.; Reinisch, W.; et al. STRIDE-II: An Update on the Selecting Therapeutic Targets in Inflammatory Bowel Disease (STRIDE) Initiative of the International Organization for the Study of IBD (IOIBD): Determining Therapeutic Goals for Treat-to-Target strategies in IBD. Gastroenterology 2021, 160, 1570–1583. [Google Scholar] [CrossRef]
- Rex, D.; Cutler, C.; Lemmel, G.; Rahmani, E.Y.; Clark, D.W.; Helper, D.J.; Lehman, G.A.; Mark, D.G. Colonoscopic miss rates of adenomas determined by back-to-back colonoscopies. Gastroenterology 1997, 112, 24–28. [Google Scholar] [CrossRef]
- Kiesslich, R.; Fritsch, J.; Holtmann, M.; Koehler, H.H.; Stolte, M.; Kanzler, S.; Nafe, B.; Jung, M.; Galle, P.R.; Neurath, M.F. Methylene blue-aided chromoendoscopy for the detection of intraepithelial neoplasia and colon cancer in ulcerative colitis. Gastroenterology 2003, 124, 880–888. [Google Scholar] [CrossRef]
- Van den Broek, F.J.C.; Stokkers, P.C.F.; Reitsma, J.B.; Boltjes, R.P.; Ponsioen, C.Y.; Fockens, P.; Dekker, E. Random Biopsies Taken During Colonoscopic Surveillance of Patients With Longstanding Ulcerative Colitis: Low Yield and Absence of Clinical Consequences. Am. J. Gastroenterol. 2014, 109, 715–722. [Google Scholar] [CrossRef]
- Moussata, D.; Allez, M.; Cazals-Hatem, D.; Treton, X.; Laharie, D.; Reimund, J.M.; Bertheau, P.; Bourreille, A.; Lavergne-Slove, A.; Brixi, H.; et al. Are random biopsies still useful for the detection of neoplasia in patients with IBD undergoing surveillance colonoscopy with chromoendoscopy? Gut 2018, 67, 616–624. [Google Scholar] [CrossRef]
- Kandiah, K.; Subramaniam, S.; Thayalasekaran, S.; Chedgy, F.J.; Longcroft-Wheaton, G.; Fogg, C.; Brown, J.F.; Smith, S.C.; Iacucci, M.; Bhandari, P. Multicentre randomised controlled trial on virtual chromoendoscopy in the detection of neoplasia during colitis surveillance high-definition colonoscopy (the VIRTUOSO trial). Gut 2021, 70, 1684–1690. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, V.; Mannath, J.; Ragunath, K.; Hawkey, C.J. Meta-analysis: The diagnostic yield of chromoendoscopy for detecting dysplasia in patients with colonic inflammatory bowel disease: Meta-analysis: Chromoendoscopy for IBD surveillance. Aliment. Pharmacol. Ther. 2011, 33, 304–312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soetikno, R.; Subramanian, V.; Kaltenbach, T.; Rouse, R.V.; Sanduleanu, S.; Suzuki, N.; Tanaka, S.; McQuaid, K. The Detection of Nonpolypoid (Flat and Depressed) Colorectal Neoplasms in Patients with Inflammatory Bowel Disease. Gastroenterology 2013, 144, 1349–1352.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neumann, H.; Fujishiro, M.; Wilcox, C.M.; Mönkemüller, K. Present and future perspectives of virtual chromoendoscopy with i-scan and optical enhancement technology: I-scan: Optical enhancement technology. Dig. Endosc. 2014, 26, 43–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacucci, M.; Furfaro, F.; Matsumoto, T.; Uraoka, T.; Smith, S.; Ghosh, S.; Kiesslich, R. Advanced endoscopic techniques in the assessment of inflammatory bowel disease: New technology, new era. Gut 2019, 68, 562–572. [Google Scholar] [CrossRef]
- Dionisio, P.M.; Gurudu, S.R.; Leighton, J.A.; Leontiadis, G.I.; Fleischer, D.E.; Hara, A.K.; Heigh, R.I.; Shiff, A.D.; Sharma, V.K. Capsule Endoscopy Has a Significantly Higher Diagnostic Yield in Patients with Suspected and Established Small-Bowel Crohn’s Disease: A Meta-Analysis. Am. J. Gastroenterol. 2010, 105, 1240–1248. [Google Scholar] [CrossRef]
- Jensen, M.D.; Nathan, T.; Rafaelsen, S.R.; Kjeldsen, J. Diagnostic Accuracy of Capsule Endoscopy for Small Bowel Crohn’s Disease Is Superior to That of MR Enterography or CT Enterography. Clin. Gastroenterol. Hepatol. 2011, 9, 124–129.e1. [Google Scholar] [CrossRef]
- Cave, D.; Legnani, P.; de Franchis, R.; Lewis, B.S. ICCE Consensus for Capsule Retention. Endoscopy 2005, 37, 1065–1067. [Google Scholar] [CrossRef]
- Atreya, R.; Goetz, M. Molecular imaging in gastroenterology. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 704–712. [Google Scholar] [CrossRef]
- Sasajima, K.; Kudo, S.; Inoue, H.; Takeuchi, T.; Kashida, H.; Hidaka, E.; Kawachi, H.; Sakashita, M.; Tanaka, J.; Shiokawa, A. Real-time in vivo virtual histology of colorectal lesions when using the endocytoscopy system. Gastrointest. Endosc. 2006, 63, 1010–1017. [Google Scholar] [CrossRef]
- Mary, J.Y.; Modigliani, R. Development and validation of an endoscopic index of the severity for Crohn’s disease: A prospective multicentre study. Groupe d’Etudes Therapeutiques des Affections Inflammatoires du Tube Digestif (GETAID). Gut 1989, 30, 983–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daperno, M.; D’Haens, G.; Van Assche, G.; Baert, F.; Bulois, P.; Maunoury, V.; Sostegni, R.; Rocca, R.; Pera, A.; Gevers, A.; et al. Development and validation of a new, simplified endoscopic activity score for Crohn’s disease: The SES-CD. Gastrointest. Endosc. 2004, 60, 505–512. [Google Scholar] [CrossRef]
- Rutgeerts, P.; Geboes, K.; Vantrappen, G.; Beyls, J.; Kerremans, R.; Hiele, M. Predictability of the postoperative course of Crohn’s disease. Gastroenterology 1990, 99, 956–963. [Google Scholar] [CrossRef]
- Vashist, N.M.; Samaan, M.; Mosli, M.H.; Parker, C.E.; MacDonald, J.K.; Nelson, S.A.; Zou, G.Y.; Feagan, B.G.; Khanna, R.; Jairath, V. Endoscopic scoring indices for evaluation of disease activity in ulcerative colitis. Cochrane Database Syst. Rev. 2018. Cochrane IBD Group. [Google Scholar] [CrossRef]
- Schroeder, K.W.; Tremaine, W.J.; Ilstrup, D.M. Coated Oral 5-Aminosalicylic Acid Therapy for Mildly to Moderately Active Ulcerative Colitis. N. Engl. J. Med. 1987, 317, 1625–1629. [Google Scholar] [CrossRef]
- Di Ruscio, M.; Variola, A.; Vernia, F.; Lunardi, G.; Castelli, P.; Bocus, P.; Geccherle, A. Role of Ulcerative Colitis Endoscopic Index of Severity (UCEIS) versus Mayo Endoscopic Subscore (MES) in Predicting Patients′ Response to Biological Therapy and the Need for Colectomy. Digestion 2021, 102, 534–545. [Google Scholar] [CrossRef]
- Irani, N.R.; Wang, L.M.; Collins, G.S.; Keshav, S.; Travis, S.P.L. Correlation between Endoscopic and Histological Activity in Ulcerative Colitis Using Validated Indices. J. Crohn Colitis 2018, 12, 1151–1157. [Google Scholar] [CrossRef]
- Iacucci, M.; Daperno, M.; Lazarev, M.; Arsenascu, R.; Tontini, G.E.; Akinola, O.; Gui, X.S.; Villanacci, V.; Goetz, M.; Lowerison, M.; et al. Development and reliability of the new endoscopic virtual chromoendoscopy score: The PICaSSO (Paddington International Virtual ChromoendoScopy ScOre) in ulcerative colitis. Gastrointest. Endosc. 2017, 86, 1118–1127.e5. [Google Scholar] [CrossRef]
- Iacucci, M.; Smith, S.C.L.; Bazarova, A.; Shivaji, U.N.; Bhandari, P.; Cannatelli, R.; Daperno, M.; Ferraz, J.; Goetz, M.; Gui, X.; et al. An International Multicenter Real-Life Prospective Study of Electronic Chromoendoscopy Score PICaSSO in Ulcerative Colitis. Gastroenterology 2021, 160, 1558–1569.e8. [Google Scholar] [CrossRef]
- Gupta, R.B.; Harpaz, N.; Itzkowitz, S.; Hossain, S.; Matula, S.; Kornbluth, A.; Bodian, C.; Ullman, T. Histologic Inflammation Is a Risk Factor for Progression to Colorectal Neoplasia in Ulcerative Colitis: A Cohort Study. Gastroenterology 2007, 133, 1099–1105. [Google Scholar] [CrossRef] [Green Version]
- Eaden, J.A. The risk of colorectal cancer in ulcerative colitis: A meta-analysis. Gut 2001, 48, 526–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laine, L.; Kaltenbach, T.; Barkun, A.; McQuaid, K.R.; Subramanian, V.; Soetikno, R. SCENIC International Consensus Statement on Surveillance and Management of Dysplasia in Inflammatory Bowel Disease. Gastroenterology 2015, 148, 639–651.e28. [Google Scholar] [CrossRef] [PubMed]
- Cairns, S.R.; Scholefield, J.H.; Steele, R.J.; Dunlop, M.G.; Thomas, H.J.; Evans, G.D.; Eaden, J.A.; Rutter, M.D.; Atkin, W.P.; Saunders, B.P.; et al. Guidelines for colorectal cancer screening and surveillance in moderate and high risk groups (update from 2002). Gut 2010, 59, 666–689. [Google Scholar] [CrossRef] [PubMed]
- Ponsky, J.L.; Strong, A.T. A History of Flexible Gastrointestinal Endoscopy. Surg. Clin. N. Am. 2020, 100, 971–992. [Google Scholar] [CrossRef]
- Eaden, J.A.; Mayberry, J.F. Guidelines for screening and surveillance of asymptomatic colorectal cancer in patients with inflammatory bowel disease. Gut 2002, 51 (Suppl. 5), v10–v12. [Google Scholar] [CrossRef] [Green Version]
- Itzkowitz, S.H.; Present, D.H. Consensus Conference: Colorectal Cancer Screening and Surveillance in Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2005, 11, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Clarke, W.T.; Feuerstein, J.D. Colorectal cancer surveillance in inflammatory bowel disease: Practice guidelines and recent developments. WJG 2019, 25, 4148–4157. [Google Scholar] [CrossRef]
- Graham, D.G.; Banks, M.R. Advances in upper gastrointestinal endoscopy. F1000Research 2015, 4, 1457. [Google Scholar] [CrossRef]
- Subramanian, V.; Ramappa, V.; Telakis, E.; Mannath, J.; Jawhari, A.U.; Hawkey, C.J.; Ragunath, K. Comparison of High Definition with Standard White Light Endoscopy for Detection of Dysplastic Lesions during Surveillance Colonoscopy in Patients with Colonic Inflammatory Bowel Disease. Inflamm. Bowel Dis. 2013, 19, 350–355. [Google Scholar] [CrossRef]
- Kiesslich, R.; von Bergh, M.; Hahn, M.; Hermann, G.; Jung, M. Chromoendoscopy with Indigocarmine Improves the Detection of Adenomatous and Nonadenomatous Lesions in the Colon. Endoscopy 2001, 33, 1001–1006. [Google Scholar] [CrossRef]
- Marion, J.F.; Waye, J.D.; Present, D.H.; Israel, Y.; Bodian, C.; Harpaz, N.; Chapman, M.; Itzkowitz, S.; Steinlauf, A.F.; Abreu, M.T.; et al. Chromoendoscopy-Targeted Biopsies Are Superior to Standard Colonoscopic Surveillance for Detecting Dysplasia in Inflammatory Bowel Disease Patients: A Prospective Endoscopic Trial. Am. J. Gastroenterol. 2008, 103, 2342–2349. [Google Scholar] [CrossRef] [PubMed]
- El-Dallal, M.; Chen, Y.; Lin, Q.; Rakowsky, S.; Sattler, L.; Foromera, J.; Grossberg, L.; Cheifetz, A.S.; Feuerstein, J.D. Meta-analysis of Virtual-based Chromoendoscopy Compared with Dye-spraying Chromoendoscopy Standard and High-definition White Light Endoscopy in Patients with Inflammatory Bowel Disease at Increased Risk of Colon Cancer. Inflamm. Bowel Dis. 2020, 26, 1319–1329. [Google Scholar] [CrossRef] [PubMed]
- Bisschops, R.; Bessissow, T.; Joseph, J.A.; Baert, F.; Ferrante, M.; Ballet, V.; Willekens, H.; Demedts, I.; Geboes, K.; De Hertogh, G.; et al. Chromoendoscopy versus narrow band imaging in UC: A prospective randomised controlled trial. Gut 2018, 67, 1087–1094. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.J.; Braden, B. Indications, stains and techniques in chromoendoscopy. QJM 2013, 106, 117–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bisschops, R.; East, J.E.; Hassan, C.; Hazewinkel, Y.; Kamiński, M.F.; Neumann, H.; Pellisé, M.; Antonelli, G.; Bustamante Balen, M.; Coron, E.; et al. Advanced imaging for detection and differentiation of colorectal neoplasia: European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2019. Endoscopy 2019, 51, 1155–1179. [Google Scholar] [CrossRef] [Green Version]
- Gralnek, I.M.; Defranchis, R.; Seidman, E.; Leighton, J.A.; Legnani, P.; Lewis, B.S. Development of a capsule endoscopy scoring index for small bowel mucosal inflammatory change. Aliment. Pharmacol. Ther. 2007, 27, 146–154. [Google Scholar] [CrossRef]
- Niv, Y.; Ilani, S.; Levi, Z.; Hershkowitz, M.; Niv, E.; Fireman, Z.; O’Donnel, S.; O’Morain, C.; Eliakim, R.; Scapa, E.; et al. Validation of the Capsule Endoscopy Crohn’s Disease Activity Index (CECDAI or Niv score): A multicenter prospective study. Endoscopy 2012, 44, 21–26. [Google Scholar] [CrossRef]
- Pons Beltrán, V.; Nos, P.; Bastida, G.; Argüello, L.; Aguas, M.; Rubín, A.; Pertejo, V.; Sala, T. Evaluation of postsurgical recurrence in Crohn’s disease: A new indication for capsule endoscopy? Gastrointest. Endosc. 2007, 66, 533–540. [Google Scholar] [CrossRef]
- Guindi, M. Indeterminate colitis. J. Clin. Pathol. 2004, 57, 1233–1244. [Google Scholar] [CrossRef]
- Eliakim, R. The Impact of Wireless Capsule Endoscopy on Gastrointestinal Diseases. South. Med. J. 2007, 100, 235–236. [Google Scholar] [CrossRef]
- Mehdizadeh, S.; Chen, G.; Enayati, P.; Cheng, D.W.; Han, N.J.; Shaye, O.A.; Ippoliti, A.; Vasiliauskas, E.A.; Lo, S.K.; Papadakis, K.A. Diagnostic yield of capsule endoscopy in ulcerative colitis and inflammatory bowel disease of unclassified type (IBDU). Endoscopy 2007, 40, 30–35. [Google Scholar] [CrossRef]
- Maunoury, V.; Savoye, G.; Bourreille, A.; Bouhnik, Y.; Jarry, M.; Sacher-Huvelin, S.; Ben Soussan, E.; Lerebours, E.; Galmiche, J.P.; Colombel, J.F. Value of wireless capsule endoscopy in patients with indeterminate colitis (inflammatory bowel disease type unclassified). Inflamm. Bowel Dis. 2007, 13, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Gurudu, S.R.; De Petris, G.; Sharma, V.K.; Shiff, A.D.; Heigh, R.I.; Fleischer, D.E.; Post, J.; Erickson, P.; Leighton, J.A. Retention of the capsule endoscope: A single-center experience of 1000 capsule endoscopy procedures. Gastrointest. Endosc. 2008, 68, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Saurin, J.-C.; Delvaux, M.; Gaudin, J.-L.; Fassler, I.; Villarejo, J.; Vahedi, K.; Bitoun, A.; Canard, J.-M.; Souquet, J.C.; Ponchon, T.; et al. Diagnostic Value of Endoscopic Capsule in Patients with Obscure Digestive Bleeding: Blinded Comparison with Video Push-Enteroscopy. Endoscopy 2003, 35, 576–584. [Google Scholar] [CrossRef] [PubMed]
- Eliakim, R. Video capsule endoscopy of the small bowel. Curr. Opin. Gastroenterol. 2013, 29, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Caunedo-Álvarez, Á.; Romero-Vazquez, J.; Herrerias-Gutierrez, J.M. Patency© and agile© capsules. WJG 2008, 14, 5269. [Google Scholar] [CrossRef]
- Lewis, B.S.; Eisen, G.M.; Friedman, S. A Pooled Analysis to Evaluate Results of Capsule Endoscopy Trials. Endoscopy 2005, 37, 960–965. [Google Scholar] [CrossRef]
- Aoki, T.; Yamada, A.; Aoyama, K.; Saito, H.; Tsuboi, A.; Nakada, A.; Niikura, R.; Fujishiro, M.; Oka, S.; Ishihara, S.; et al. Automatic detection of erosions and ulcerations in wireless capsule endoscopy images based on a deep convolutional neural network. Gastrointest. Endosc. 2019, 89, 357–363.e2. [Google Scholar] [CrossRef]
- Klang, E.; Barash, Y.; Margalit, R.Y.; Soffer, S.; Shimon, O.; Albshesh, A.; Ben-Horin, S.; Amitai, M.M.; Eliakim, R.; Kopylov, U. Deep learning algorithms for automated detection of Crohn’s disease ulcers by video capsule endoscopy. Gastrointest. Endosc. 2020, 91, 606–613.e2. [Google Scholar] [CrossRef]
- Aoki, T.; Yamada, A.; Aoyama, K.; Saito, H.; Fujisawa, G.; Odawara, N.; Kondo, R.; Tsuboi, A.; Ishibashi, R.; Nakada, A.; et al. Clinical usefulness of a deep learning-based system as the first screening on small-bowel capsule endoscopy reading. Dig. Endosc. 2020, 32, 585–591. [Google Scholar] [CrossRef]
- Goetz, M.; Wang, T.D. Molecular Imaging in Gastrointestinal Endoscopy. Gastroenterology 2010, 138, 828–833.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.Y.; Myung, S.J. Optical Molecular Imaging for Diagnosing Intestinal Diseases. Clin. Endosc. 2013, 46, 620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atreya, R.; Neumann, H.; Neufert, C.; Waldner, M.J.; Billmeier, U.; Zopf, Y.; Willma, M.; App, C.; Münster, T.; Kessler, H.; et al. In vivo imaging using fluorescent antibodies to tumor necrosis factor predicts therapeutic response in Crohn’s disease. Nat. Med. 2014, 20, 313–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rath, T.; Bojarski, C.; Neurath, M.F.; Atreya, R. Molecular imaging of mucosal α4β7 integrin expression with the fluorescent anti-adhesion antibody vedolizumab in Crohn’s disease. Gastrointest. Endosc. 2017, 86, 406–408. [Google Scholar] [CrossRef]
- Iacucci, M.; Grisan, E.; Labarile, N.; Nardone, O.; Smith, S.C.; Jeffery, L.; Ghosh, S.; Buda, A. P397 Response to biologics in IBD patients assessed by Computerized image analysis of Probe Based Confocal Laser Endomicroscopy with molecular labeling. J. Crohn Colitis 2021, 15 (Suppl. 1), S406–S407. [Google Scholar] [CrossRef]
- Prenzel, F.; Uhlig, H.H. Frequency of indeterminate colitis in children and adults with IBD—A metaanalysis. J. Crohn Colitis 2009, 3, 277–281. [Google Scholar] [CrossRef]
- Yantiss, R.K.; Das, K.M.; Farraye, F.A.; Odze, R.D. Alterations in the Immunohistochemical Expression of Das-1 and CG-3 in Colonic Mucosal Biopsy Specimens Helps Distinguish Ulcerative Colitis from Crohn Disease and from Other Forms of Colitis. Am. J. Surg. Pathol. 2008, 32, 844–850. [Google Scholar] [CrossRef]
- Mitsunaga, M.; Kosaka, N.; Choyke, P.L.; Young, M.R.; Dextras, C.R.; Saud, S.M.; Colburn, N.H.; Sakabe, M.; Nagano, T.; Asanuma, D.; et al. Fluorescence endoscopic detection of murine colitis-associated colon cancer by topically applied enzymatically rapid-activatable probe. Gut 2013, 62, 1179–1186. [Google Scholar] [CrossRef]
- Kwon, R.S.; Wong Kee Song, L.M.; Adler, D.G.; Conway, J.D.; Diehl, D.L.; Farraye, F.A.; Kantsevoy, S.V.; Kaul, V.; Kethu, S.R.; Mamula, P.; et al. Endocytoscopy. Gastrointest. Endosc. 2009, 70, 610–613. [Google Scholar] [CrossRef]
- Iacucci, M.; Jeffery, L.; Acharjee, A.; Nardone, O.M.; Zardo, D.; Smith, S.C.L.; Bazarova, A.; Cannatelli, R.; Shivaji, U.N.; Williams, J.; et al. Ultra-high Magnification Endocytoscopy and Molecular Markers for Defining Endoscopic and Histologic Remission in Ulcerative Colitis—An Exploratory Study to Define Deep Remission. Inflamm. Bowel Dis. 2021, 27, 1719–1730. [Google Scholar] [CrossRef]
- Takishima, K.; Maeda, Y.; Ogata, N.; Misawa, M.; Mori, Y.; Homma, M.; Nemoto, T.; Miyata, Y.; Akimoto, Y.; Mochida, K.; et al. Beyond complete endoscopic healing: Goblet appearance using an endocytoscope to predict future sustained clinical remission in ulcerative colitis. Dig. Endosc. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kudo, S.; Misawa, M.; Mori, Y.; Hotta, K.; Ohtsuka, K.; Ikematsu, H.; Saito, Y.; Takeda, K.; Nakamura, H.; Ichimasa, K.; et al. Artificial Intelligence-assisted System Improves Endoscopic Identification of Colorectal Neoplasms. Clin. Gastroenterol. Hepatol. 2020, 18, 1874–1881.e2. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Kudo, S.; Misawa, M.; Saito, Y.; Ikematsu, H.; Hotta, K.; Ohtsuka, K.; Urushibara, F.; Kataoka, S.; Ogawa, Y.; et al. Real-Time Use of Artificial Intelligence in Identification of Diminutive Polyps during Colonoscopy: A Prospective Study. Ann. Intern. Med. 2018, 169, 357. [Google Scholar] [CrossRef]
- Fukunaga, S.; Kusaba, Y.; Tsuruta, O. Use of Endocytoscopy for Ulcerative Colitis Surveillance: A Case Study. Gastroenterology 2020, 158, e1–e2. [Google Scholar] [CrossRef]
- Bossuyt, P.; Nakase, H.; Vermeire, S.; de Hertogh, G.; Eelbode, T.; Ferrante, M.; Hasegawa, T.; Willekens, H.; Ikemoto, Y.; Makino, T.; et al. Automatic, computer-aided determination of endoscopic and histological inflammation in patients with mild to moderate ulcerative colitis based on red density. Gut 2020, 69, 1778–1786. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, K.; Ohtsuka, K.; Fujii, T.; Negi, M.; Suzuki, K.; Shimizu, H.; Oshima, S.; Akiyama, S.; Motobayashi, M.; Nagahori, M.; et al. Development and Validation of a Deep Neural Network for Accurate Evaluation of Endoscopic Images from Patients with Ulcerative Colitis. Gastroenterology 2020, 158, 2150–2157. [Google Scholar] [CrossRef]
- Takenaka, K.; Fujii, T.; Kawamoto, A.; Suzuki, K.; Shimizu, H.; Maeyashiki, C.; Yamaji, O.; Motobayashi, M.; Igarashi, A.; Hanazawa, R.; et al. Deep neural network for video colonoscopy of ulcerative colitis: A cross-sectional study. Lancet Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef]
- Byrne, M.; East, J.; Iacucci, M.; Panaccione, R.; Kalapala, R.; Duvvur, N.; Rughwani, H.; Singh, A.; Henkel, M.; Berry, S.; et al. DOP13 Artificial Intelligence (AI) in endoscopy—Deep learning for detection and scoring of Ulcerative Colitis (UC) disease activity under multiple scoring systems. J. Crohn Colitis 2021, 15 (Suppl. 1), S051–S052. [Google Scholar] [CrossRef]
- Gottlieb, K.; Requa, J.; Karnes, W.; Chandra Gudivada, R.; Shen, J.; Rael, E.; Arora, V.; Dao, T.; Ninh, A.; McGill, J. Central Reading of Ulcerative Colitis Clinical Trial Videos Using Neural Networks. Gastroenterology 2021, 160, 710–719.e2. [Google Scholar] [CrossRef] [PubMed]
- Maeda, Y.; Kudo, S.; Ogata, N.; Misawa, M.; Iacucci, M.; Homma, M.; Nemoto, T.; Takishima, K.; Mochida, K.; Miyachi, H.; et al. Evaluation in real-time use of artificial intelligence during colonoscopy to predict relapse of ulcerative colitis: A prospective study. Gastrointest. Endosc. 2021. [Google Scholar] [CrossRef]

| Rutgeerts Grade | Endoscopic Finding |
|---|---|
| i0 | Absence of lesions in the terminal ileum |
| i1 | Up to 5 anastomotic aphtous lesions in the terminal ileum |
| i2 | Over 5 aphtous lesions with unaffected mucosa between lesions, skip areas of larger lesions or ulcers no larger than 10 mm limited to the ileo-colonic anastomosis |
| i3 | Diffuse aphtous ileal flogosis with inflamed mucosa between aphtae |
| i4 | Diffuse inflammation and associated larger lesions: ulcers larger than 10 mm, cobble/nodules or narrowing/stenosis |
| Descriptor | Score | Definition |
|---|---|---|
| Vascular pattern | Normal (0) Patchy obliteration (1) Obliterated (2) | Normal vascular pattern with arborization of capillaries clearly defined or with blurring or patchy loss of capillary margins Patchy obliteration of vascular pattern Complete obliteration of vascular pattern |
| Bleeding | None (0) Mucosal (1) Luminal mild (2) Luminal moderator severe (3) | No visible blood Some spots or streaks of coagulated blood on the surface of the mucosa ahead of the scope, which can be washed away Some free liquid blood in the lumen Frank blood in the lumen ahead of endoscope or visible oozing from mucosa after washing intraluminal blood or visible oozing from a hemorrhagic mucosa |
| Erosions and ulcers | None (0) Erosions (1) Superficial ulcer (2) Deep ulcer (3) | Normal mucosa, no visible erosions or ulcers Tiny (≤5 mm) defects in the mucosa, which are discrete fibrin-covered ulcers in comparison with erosions, but remain superficial Larger (>5 mm) defects in the mucosa, which are discrete fibrin-covered ulcers in comparison with erosions, but remain superficial Deeper excavated defects in the mucosa, with a slightly raised edge |
| Lesion | Score | Definition |
|---|---|---|
| Vascular pattern | 0 | Normal, clear vascular pattern |
| 1 | Partially visible vascular pattern | |
| 2 | Complete loss of vascular patter | |
| Granularity | 0 | Normal, smooth and glistening |
| 1 | Fine | |
| 2 | Coarse | |
| Ulceration | 0 | Normal, no erosion or ulcer |
| 1 | Erosions or pinpoint ulcerations | |
| 2 | Numerous shallow ulcers with mucopus | |
| 3 | Deep, excavated ulcerations | |
| 4 | Diffusely ulcerated with >30% involvement | |
| Bleeding friability | 0 | Normal, no bleeding, no fraibility |
| 1 | Friable, Bleeding to light touch | |
| 2 | Spontaneous bleeding | |
| Grading of SAES and GAES (4-point scale) | 0 | Normal/quiescent: visible vascular pattern with no bleeding, erosions, ulcers, or friability |
| 1 | Mild: eritherma, decreased or loss of vascular pattern, fine granularity, but no fraibility or spontaneous bleeding | |
| 2 | Moderate: fraibility with bleeding to light touch, coarse granularity, erosions, or pintpoint ulcerations | |
| 3 | Severe: spontaneous bleeding or gross ulcers | |
| GAES VAS 10-cm scale | (0) (10) | |
| Normal Extremely severe |
| Name | Formula | Notes |
|---|---|---|
| Lewis Score | [(Villous parameter × extent × descriptor) + (Ulcer parameter × extent × size)] for tertile 1, 2 or 3 + (Stenosis number × ulcerated × traversed). | The total time of video capsule progression among the bowel is divided in three tertiles, and the score is calculated as the most severe tertile score plus stenosis <135 clinically insignificance 135–790 mild >790 moderate to severe damage |
| CECDAI or NIV | A. Inflammation score 0 = None 1 = Mild to moderate edema/hyperemia/denudation 2 = Severe edema/hyperemia/denudation 3 = Bleeding, exudate, aphthae, erosion, small ulcer (<0.5 cm) 4 = Moderate ulcer (0.5–2cm), pseudo polyp 5 = Large ulcer (>2cm) B. Extent of disease score 0 = No disease –normal examination 1 = Focal disease (single segment is involved) 2 = Patchy disease (2–3 segments are involved) 3 = Diffuse disease (more than 3 segments are involved) C. Stricture score 0 = None 1 = Single-passed 2 = Multiple-passed 3 = Obstruction (non-passage) Segmental score (proximal or distal) = (A × B) +C Total score =proximal ([A × B] + C) +distal ([A × B] + C) CEDCAI = proximal ([A × B] + C) + distal ([A × B] + C). | The score is included in the interval 0 (no damage) to 26 (severe inflammation). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Parigi, T.L.; Mastrorocco, E.; Da Rio, L.; Allocca, M.; D’Amico, F.; Zilli, A.; Fiorino, G.; Danese, S.; Furfaro, F. Evolution and New Horizons of Endoscopy in Inflammatory Bowel Diseases. J. Clin. Med. 2022, 11, 872. https://doi.org/10.3390/jcm11030872
Parigi TL, Mastrorocco E, Da Rio L, Allocca M, D’Amico F, Zilli A, Fiorino G, Danese S, Furfaro F. Evolution and New Horizons of Endoscopy in Inflammatory Bowel Diseases. Journal of Clinical Medicine. 2022; 11(3):872. https://doi.org/10.3390/jcm11030872
Chicago/Turabian StyleParigi, Tommaso Lorenzo, Elisabetta Mastrorocco, Leonardo Da Rio, Mariangela Allocca, Ferdinando D’Amico, Alessandra Zilli, Gionata Fiorino, Silvio Danese, and Federica Furfaro. 2022. "Evolution and New Horizons of Endoscopy in Inflammatory Bowel Diseases" Journal of Clinical Medicine 11, no. 3: 872. https://doi.org/10.3390/jcm11030872
APA StyleParigi, T. L., Mastrorocco, E., Da Rio, L., Allocca, M., D’Amico, F., Zilli, A., Fiorino, G., Danese, S., & Furfaro, F. (2022). Evolution and New Horizons of Endoscopy in Inflammatory Bowel Diseases. Journal of Clinical Medicine, 11(3), 872. https://doi.org/10.3390/jcm11030872








