Incidence of SARS-CoV-2 Infection and Related Mortality by Education Level during Three Phases of the 2020 Pandemic: A Population-Based Cohort Study in Rome
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Study Design and Population
2.3. Outcomes and Follow-Up
2.4. Exposure and Covariates
2.5. Statistical Analysis
3. Results
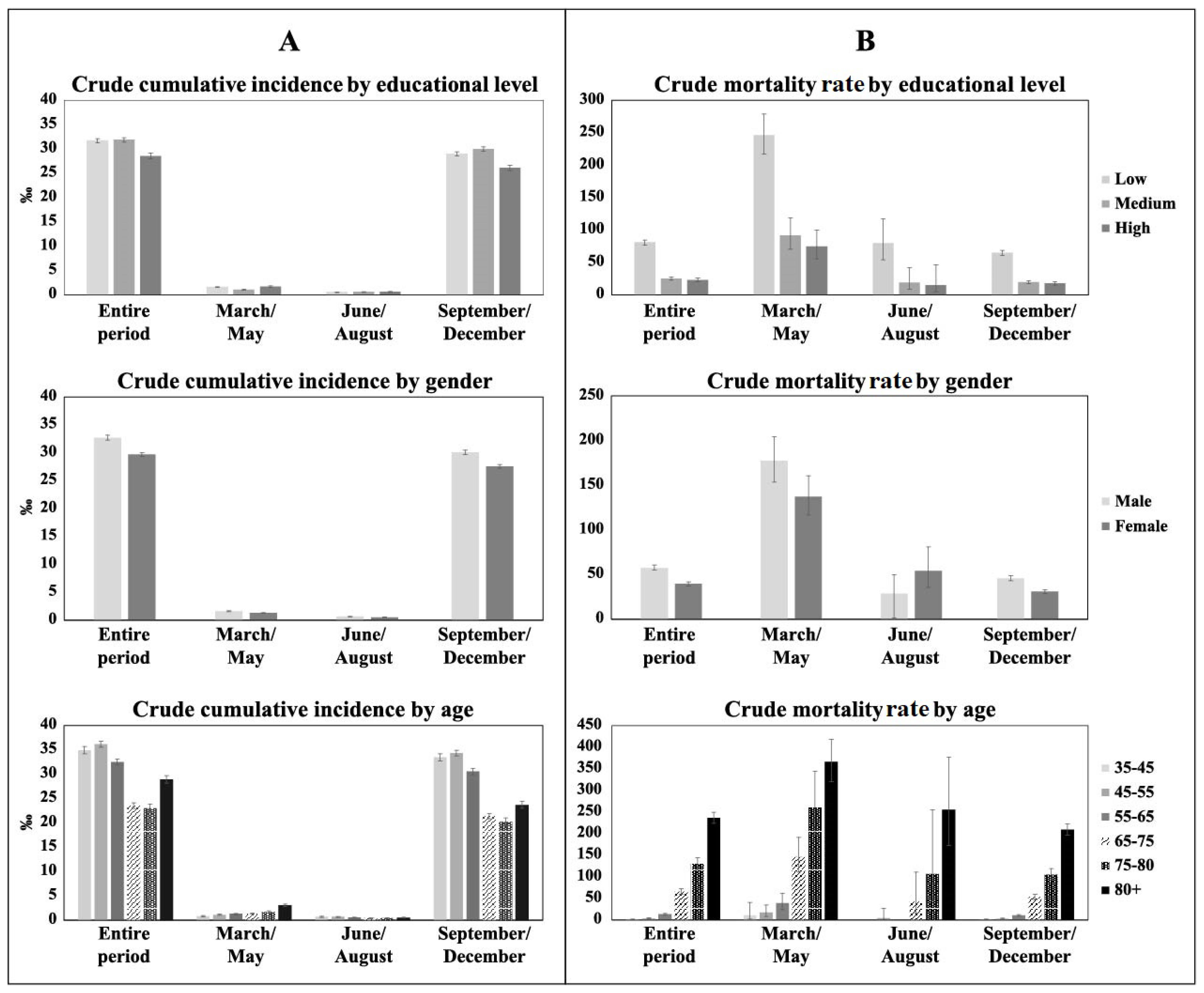
3.1. Incidence and Mortality Rate by Educational Level, Sex, and Age
3.2. Association between Educational Level and SARS-CoV-2 Infection
3.3. Association between Educational Level and Mortality within 30 Days
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. COVID 19 Public Health Emergency of International Concern (PHEIC); WHO: Geneva, Switzerland, 2020; pp. 1–10. [Google Scholar]
- Dessie, Z.G.; Zewotir, T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect. Dis. 2021, 21, 855. [Google Scholar] [CrossRef]
- Oestergaard, L.B.; Schmiegelow, M.D.; Bruun, N.E.; Skov, R.L.; Petersen, A.; Andersen, P.S.; Torppedersen, C. The associations between socioeconomic status and risk of Staphylococcus aureus bacteremia and subsequent endocarditis—A Danish nationwide cohort study. BMC Infect. Dis. 2017, 17, 589. [Google Scholar] [CrossRef] [Green Version]
- Bambra, C.; Riordan, R.; Ford, J.; Matthews, F. The COVID-19 pandemic and health inequalities. J. Epidemiol. Community Health 2020, 74, 964–968. [Google Scholar] [CrossRef]
- Di Girolamo, C.; Bartolini, L.; Caranci, N.; Moro, M.L. Socioeconomic inequalities in overall and covid-19 mortality during the first outbreak peak in emilia-romagna region (Northern Italy). Epidemiol. Prev. 2020, 44, 288–296. [Google Scholar] [CrossRef]
- Ahmed, F.; Ahmed, N.; Pissarides, C.; Stiglitz, J. Why inequality could spread COVID-19. Lancet Public Heal. 2020, 5, e240. [Google Scholar] [CrossRef]
- Wang, Z.; Tang, K. Combating COVID-19: Health equity matters. Nat. Med. 2020, 26, 458. [Google Scholar] [CrossRef]
- Khalatbari-Soltani, S.; Cumming, R.C.; Delpierre, C.; Kelly-Irving, M. Importance of collecting data on socioeconomic determinants from the early stage of the COVID-19 outbreak onwards. J. Epidemiol. Community Health 2020, 74, 620–623. [Google Scholar] [CrossRef]
- Clouston, S.A.P.; Natale, G.; Link, B.G. Socioeconomic inequalities in the spread of coronavirus-19 in the United States: A examination of the emergence of social inequalities. Soc. Sci. Med. 2021, 268, 113554. [Google Scholar] [CrossRef]
- Lewis, N.M.; Friedrichs, M.; Wagstaff, S.; Sage, K.; LaCross, N.; Bui, D.; McCaffrey, K.; Barbeau, B.; George, A.; Rose, C.; et al. Disparities in COVID-19 Incidence, Hospitalizations, and Testing, by Area-Level Deprivation—Utah, March 3–July 9, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1369–1373. [Google Scholar] [CrossRef]
- Aguilar-Palacio, I.; Maldonado, L.; Malo, S.; Sánchez-Recio, R.; Marcos-Campos, I.; Magallón-Botaya, R.; Rabanaque, M.J. Covid-19 inequalities: Individual and area socioeconomic factors (Aragón, Spain). Int. J. Environ. Res. Public Health 2021, 18, 6607. [Google Scholar] [CrossRef]
- Mari Dell’Olmo, M.; Gotsens, M.; Pasar, M.I.; Rodr, M.; De Olalla, P.G.; Rius, C.; Borrell, C. Socioeconomic Inequalities in COVID-19 in a European Urban Area: Two Waves, Two Patterns. Int. J. Environ. Res. Public Health 2021, 18, 1256. [Google Scholar] [CrossRef]
- Mena, G.E.; Martinez, P.P.; Mahmud, A.S.; Marquet, P.A.; Buckee, C.O.; Santillana, M. Socioeconomic status determines COVID-19 incidence and related mortality in Santiago, Chile. Science 2021, 372, eabg5298. [Google Scholar] [CrossRef]
- Liao, T.F.; De Maio, F. Association of Social and Economic Inequality with Coronavirus Disease 2019 Incidence and Mortality Across US Counties. JAMA Netw. Open 2021, 4, e2034578. [Google Scholar] [CrossRef]
- Ginsburgh, V.; Magerman, G.; Natali, I. COVID-19 and the role of inequality in French regional departments. Eur. J. Health Econ. 2021, 22, 311–327. [Google Scholar] [CrossRef]
- Fielding-Miller, R.K.; Sundaram, M.E.; Brouwer, K. Social determinants of COVID-19 mortality at the county level. PLoS ONE 2020, 15, e0240151. [Google Scholar] [CrossRef]
- Ribeiro, K.B.; Ribeiro, A.F.; de Sousa Mascena Veras, M.A.; De Castro, M.C. Social inequalities and COVID-19 mortality in the city of Saõ Paulo, Brazil. Int. J. Epidemiol. 2021, 50, 732–742. [Google Scholar] [CrossRef]
- Marmot, M.; Allen, J. COVID-19: Exposing and amplifying inequalities. J. Epidemiol. Commun. Health 2020, 74, 681–682. [Google Scholar] [CrossRef]
- Galobardes, B. Indicators of socioeconomic position (part 1). J. Epidemiol. Commun. Health 2006, 60, 7–12. [Google Scholar] [CrossRef] [Green Version]
- Clouston, S.A.P.; Rubin, M.S.; Phelan, J.C.; Link, B.G. A Social History of Disease: Contextualizing the Rise and Fall of Social Inequalities in Cause-Specific Mortality. Demography 2016, 53, 1631–1656. [Google Scholar] [CrossRef]
- Michelozzi, P.; De’Donato, F.; Scortichini, M.; Pezzotti, P.; Stafoggia, M.; De Sario, M.; Costa, G.; Noccioli, F.; Riccardo, F.; Bella, A.; et al. Temporal dynamics in total excess mortality and COVID-19 deaths in Italian cities. BMC Public Health 2020, 20, 1238. [Google Scholar] [CrossRef]
- Peckham, H.; de Gruijter, N.M.; Raine, C.; Radziszewska, A.; Ciurtin, C.; Wedderburn, L.R.; Rosser, E.C.; Webb, K.; Deakin, C.T. Male sex identified by global COVID-19 meta-analysis as a risk factor for death and ITU admission. Nat. Commun. 2020, 11, 6317. [Google Scholar] [CrossRef]
- Rose, T.; Mason, K.; Pennington, A.; McHale, P.; Buchan, I.; Taylor-Robinson, D.; Barr, B. Inequalities in COVID19 mortality related to ethnicity and socioeconomic deprivation. medRxiv 2020. [Google Scholar] [CrossRef]
- Kawachi, I. COVID-19 and the “rediscovery” of health inequities. Int. J. Epidemiol. 2020, 49, 1415–1418. [Google Scholar] [CrossRef]
- Marmot, M.; Allen, J.; Bell, R.; Bloomer, E.; Goldblatt, P. WHO European review of social determinants of health and the health divide. Lancet 2012, 380, 1011–1029. [Google Scholar] [CrossRef]
- Singu, S.; Acharya, A.; Challagundla, K.; Byrareddy, S.N. Impact of Social Determinants of Health on the Emerging COVID-19 Pandemic in the United States. Front. Public Health 2020, 8, 406. [Google Scholar] [CrossRef]
- Brandén, M.; Aradhya, S.; Kolk, M.; Härkönen, J.; Drefahl, S.; Malmberg, B.; Rostila, M.; Cederström, A.; Andersson, G.; Mussino, E. Residential context and COVID-19 mortality among adults aged 70 years and older in Stockholm: A population-based, observational study using individual-level data. Lancet Healtht Longev. 2020, 1, e80–e88. [Google Scholar] [CrossRef]


| Participants | SARS-CoV-2 Infection | CCI ‰ | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| N = 1,538,231 | 100% | N = 47,736 | 100% | 31.00 | 30.80 | 31.30 | |
| EDUCATIONAL LEVEL | 630,745 | 41.00 | 19,999 | 41.90 | |||
| Low | 31.71 | 31.27 | 32.15 | ||||
| Medium | 555,885 | 36.14 | 17,707 | 37.09 | 31.85 | 31.39 | 32.33 |
| High | 351,601 | 22.86 | 10,030 | 21.01 | 28.53 | 27.97 | 29.09 |
| SEX | 684,453 | 44.5 | 22,384 | 46.89 | |||
| Male | 32.70 | 32.28 | 33.13 | ||||
| Female | 853,778 | 55.5 | 25,352 | 53.11 | 29.69 | 29.33 | 30.06 |
| AGE CLASSES | 243,054 | 15.8 | 8487 | 17.78 | |||
| 35–44 | 34.92 | 34.18 | 35.67 | ||||
| 45–54 | 377,845 | 24.56 | 13,653 | 28.60 | 36.13 | 35.53 | 36.74 |
| 55–64 | 340,400 | 22.13 | 11,053 | 23.15 | 32.47 | 31.87 | 33.08 |
| 65–74 | 267,232 | 17.37 | 6276 | 13.15 | 23.48 | 22.91 | 24.07 |
| 75–79 | 115,456 | 7.51 | 2653 | 5.56 | 22.98 | 22.12 | 23.87 |
| 80+ | 194,244 | 12.63 | 5614 | 11.76 | 28.90 | 28.16 | 29.67 |
| SARS-CoV-2 Infection | Death within 30 Days | CMR ‰ | 95% CI | ||||
|---|---|---|---|---|---|---|---|
| N = 47,736 | 100% | N = 2281 | 100% | 47.78 | 45.86 | 49.79 | |
| EDUCATIONAL LEVEL | 19,999 | 41.90 | 1614 | 70.76 | |||
| Low | 80.70 | 76.86 | 84.74 | ||||
| Medium | 17,707 | 37.09 | 438 | 19.2 | 24.74 | 22.53 | 27.17 |
| High | 10,030 | 21.01 | 229 | 10.04 | 22.83 | 20.06 | 25.99 |
| SEX | 22,384 | 46.89 | 1284 | 56.29 | |||
| Male | 57.36 | 54.31 | 60.59 | ||||
| Female | 25,352 | 53.11 | 997 | 43.71 | 39.32 | 36.96 | 41.84 |
| AGE CLASSES | 8487 | 17.78 | 10 | 0.44 | |||
| 35–44 | 1.18 | 0.63 | 2.19 | ||||
| 45–54 | 13,653 | 28.60 | 48 | 2.1 | 3.52 | 2.65 | 4.67 |
| 55–64 | 11,053 | 23.15 | 142 | 6.23 | 12.85 | 10.90 | 15.14 |
| 65–74 | 6276 | 13.15 | 411 | 18.02 | 65.49 | 59.45 | 72.14 |
| 75–79 | 2653 | 5.56 | 345 | 15.12 | 130.04 | 117.02 | 144.51 |
| 80+ | 5614 | 11.76 | 1325 | 58.09 | 236.03 | 223.64 | 249.08 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Angelici, L.; Sorge, C.; Di Martino, M.; Cappai, G.; Stafoggia, M.; Agabiti, N.; Girardi, E.; Lanini, S.; Nicastri, E.; Davoli, M.; et al. Incidence of SARS-CoV-2 Infection and Related Mortality by Education Level during Three Phases of the 2020 Pandemic: A Population-Based Cohort Study in Rome. J. Clin. Med. 2022, 11, 877. https://doi.org/10.3390/jcm11030877
Angelici L, Sorge C, Di Martino M, Cappai G, Stafoggia M, Agabiti N, Girardi E, Lanini S, Nicastri E, Davoli M, et al. Incidence of SARS-CoV-2 Infection and Related Mortality by Education Level during Three Phases of the 2020 Pandemic: A Population-Based Cohort Study in Rome. Journal of Clinical Medicine. 2022; 11(3):877. https://doi.org/10.3390/jcm11030877
Chicago/Turabian StyleAngelici, Laura, Chiara Sorge, Mirko Di Martino, Giovanna Cappai, Massimo Stafoggia, Nera Agabiti, Enrico Girardi, Simone Lanini, Emanuele Nicastri, Marina Davoli, and et al. 2022. "Incidence of SARS-CoV-2 Infection and Related Mortality by Education Level during Three Phases of the 2020 Pandemic: A Population-Based Cohort Study in Rome" Journal of Clinical Medicine 11, no. 3: 877. https://doi.org/10.3390/jcm11030877
APA StyleAngelici, L., Sorge, C., Di Martino, M., Cappai, G., Stafoggia, M., Agabiti, N., Girardi, E., Lanini, S., Nicastri, E., Davoli, M., & Cesaroni, G. (2022). Incidence of SARS-CoV-2 Infection and Related Mortality by Education Level during Three Phases of the 2020 Pandemic: A Population-Based Cohort Study in Rome. Journal of Clinical Medicine, 11(3), 877. https://doi.org/10.3390/jcm11030877










