Influence of Body Mass Index, Cancer Type and Treatment on Long-Term Metabolic and Liver Outcomes in Childhood Cancer Survivors
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
- −
- BMI classification: normal weight, overweight or obese;
- −
- Anticancer treatments: only chemotherapy (OC), chemotherapy plus radiotherapy (CR), or bone marrow transplantation (BMT);
- −
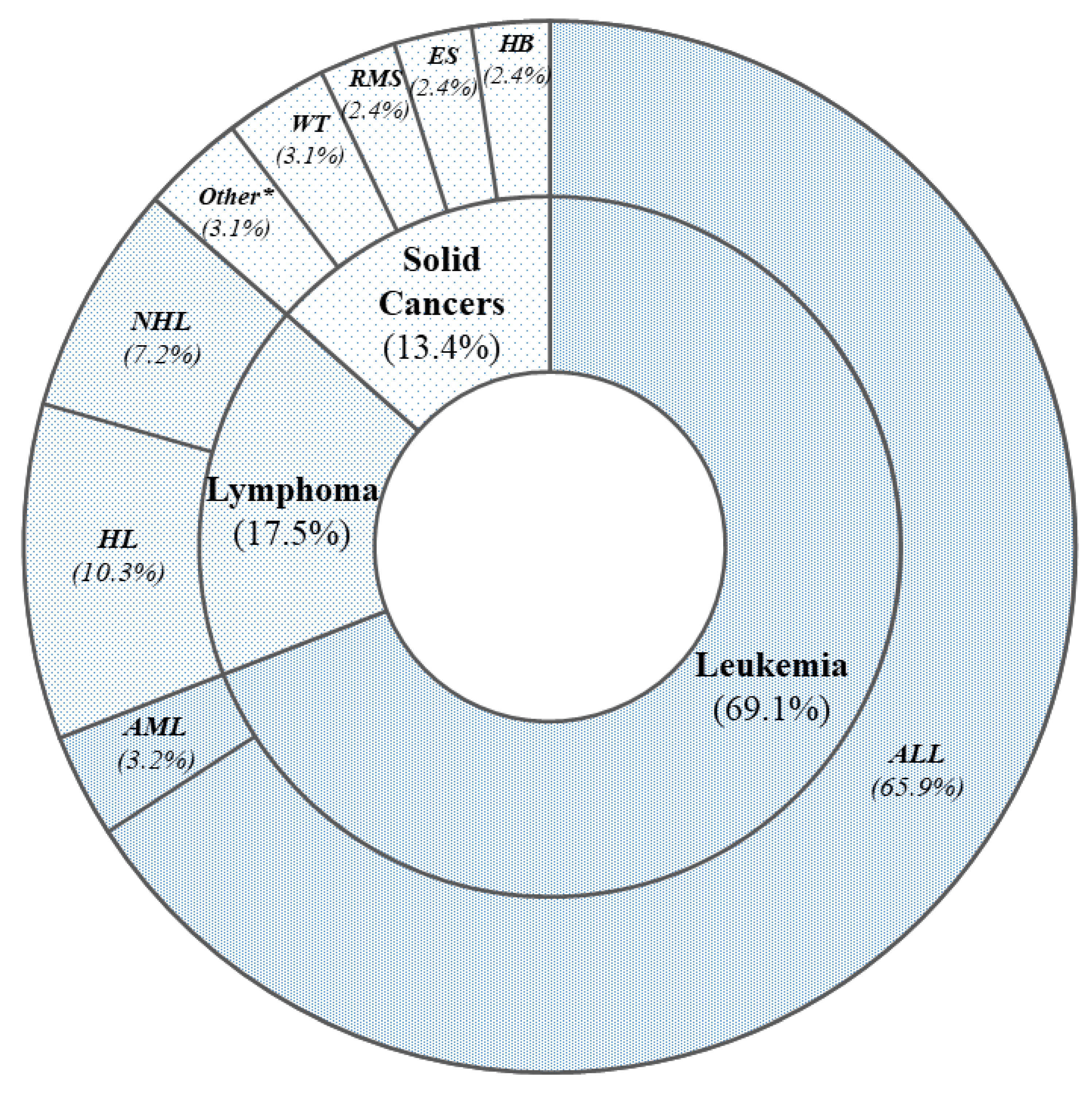
- Type of tumor: leukemia, lymphomas, or solid cancers.
2.2. Metabolic and Biochemical Assay
2.3. Indexes of Insulin Resistance and Insulin Secretion
2.4. NAFLD and Liver Fibrosis
2.5. Visceral Adipose Function and Cardio-Metabolic Risk
- −
- Females: [WC (cm)/36.58 + (1.89 × BMI (kg/m2)] × (triglycerides (mg/dL)/0.81) × (1.52/HDL (mg/dL));
- −
- Males: [WC (cm)/39.68 + (1.88 × BMI (kg/m2)] × (triglycerides (mg/dL)/1.03) × (1.31/HDL (mg/dL)).
2.6. Statistical Analysis
3. Results
3.1. Anthropometric, Clinical, and Metabolic Parameters
3.2. Indexes of Insulin Resistance and Insulin Secretion
3.3. NAFLD and Liver Fibrosis
3.4. Visceral Adipose Function as a Surrogate of Cardio-Metabolic Risk
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Oeffinger, K.C.; Mertens, A.C.; Sklar, C.A.; Yasui, Y.; Fears, T.; Stovall, M.; Vik, T.A.; Inskip, P.D.; Robison, L.L. Obesity in Adult Survivors of Childhood Acute Lymphoblastic Leukemia: A Report from the Childhood Cancer Survivor Study. J. Clin. Oncol. 2003, 21, 1359–1365. [Google Scholar] [CrossRef]
- Jemal, A.; Siegel, R.; Xu, J.; Ward, E. Cancer Statistics, 2010. CA A Cancer J. Clin. 2010, 60, 277–300. [Google Scholar] [CrossRef]
- Smith, S.M.; Link, M.P.; Effinger, K.E. Minding the Gap for Survivors of Childhood Cancer. JAMA Oncol. 2020, 6, 330–332. [Google Scholar] [CrossRef]
- Shapiro, C.L. Cancer Survivorship. N. Engl. J. Med. 2018, 379, 2438–2450. [Google Scholar] [CrossRef]
- Duca, Y.; di Cataldo, A.; Russo, G.; Cannata, E.; Burgio, G.; Compagnone, M.; Alamo, A.; Condorelli, R.A.; la Vignera, S.; Calogero, A.E. Testicular Function of Childhood Cancer Survivors: Who Is Worse? J. Clin. Med. 2019, 8, 2204. [Google Scholar] [CrossRef] [Green Version]
- Tumminia, A.; Milluzzo, A.; Cinti, F.; Parisi, M.; Tata, F.; Frasca, F.; Frittitta, L.; Vigneri, R.; Sciacca, L. Abnormal 1-Hour Post-Load Glycemia during Pregnancy Impairs Post-Partum Metabolic Status: A Single-Center Experience. J. Endocrinol. Investig. 2018, 41, 567–573. [Google Scholar] [CrossRef]
- Milluzzo, A.; Tumminia, A.; Vella, V.; Gianì, F.; Manzella, L.; Frittitta, L.; Belfiore, A.; Vigneri, R.; Sciacca, L. Short-Term Adverse Effects of Anticancer Drugs in Patients with Type 2 Diabetes. J. Chemother. 2019, 31, 150–159. [Google Scholar] [CrossRef]
- Milluzzo, A.; Barchitta, M.; Maugeri, A.; Agodi, A.; Sciacca, L. Body mass index is related to short term retinal worsening in type 2 diabetes patients treated with anticancer drugs. Minerva Endocrinol. 2022. Online ahead of print. [Google Scholar] [CrossRef]
- Milluzzo, A.; Vigneri, P.; Martorana, F.; Vigneri, R.; Sciacca, L. Type 2 Diabetes and Cancer: Problems and Suggestions for Best Patient Management. Explor. Med. 2020, 1, 184–204. [Google Scholar] [CrossRef]
- Barone, R.; Gulisano, M.; Cannata, E.; Padalino, S.; Saia, F.; Maugeri, N.; Pettinato, F.; lo Nigro, L.; Casabona, A.; Russo, G.; et al. Self- and Parent-Reported Psychological Symptoms in Young Cancer Survivors and Control Peers: Results from a Clinical Center. J. Clin. Med. 2020, 9, 3444. [Google Scholar] [CrossRef]
- Van Santen, H.M.; Chemaitilly, W.; Meacham, L.R.; Tonorezos, E.S.; Mostoufi-Moab, S. Endocrine Health in Childhood Cancer Survivors. Pediatr. Clin. N. Am. 2020, 67, 1171–1186. [Google Scholar] [CrossRef] [PubMed]
- Roberts, A.; James, J.; Dhatariya, K.; Agarwal, N.; Brake, J.; Brooks, C.; Castro, E.; Gregory, R.; Higham, C.; Hobley, L.; et al. Management of Hyperglycaemia and Steroid (Glucocorticoid) Therapy: A Guideline from the Joint British Diabetes Societies (JBDS) for Inpatient Care Group. Diabet. Med. 2018, 35, 1011–1017. [Google Scholar] [CrossRef] [PubMed]
- Thatishetty, A.V.; Agresti, N.; O’Brien, C.B. Chemotherapy-Induced Hepatotoxicity. Clin. Liver Dis. 2013, 17, 671–686. [Google Scholar] [CrossRef] [PubMed]
- Buzzetti, E.; Pinzani, M.; Tsochatzis, E.A. The Multiple-Hit Pathogenesis of Non-Alcoholic Fatty Liver Disease (NAFLD). Metabolism 2016, 65, 1038–1048. [Google Scholar] [CrossRef]
- Ross, R.; Neeland, I.J.; Yamashita, S.; Shai, I.; Seidell, J.; Magni, P.; Santos, R.D.; Arsenault, B.; Cuevas, A.; Hu, F.B.; et al. Waist Circumference as a Vital Sign in Clinical Practice: A Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat. Rev. Endocrinol. 2020, 16, 177–189. [Google Scholar] [CrossRef]
- Valerio, G.; Maffeis, C.; Saggese, G.; Ambruzzi, M.A.; Balsamo, A.; Bellone, S.; Bergamini, M.; Bernasconi, S.; Bona, G.; Calcaterra, V.; et al. Diagnosis, Treatment and Prevention of Pediatric Obesity: Consensus Position Statement of the Italian Society for Pediatric Endocrinology and Diabetology and the Italian Society of Pediatrics. Ital. J. Pediatr. 2018, 44, 88. [Google Scholar] [CrossRef] [Green Version]
- Zimmet, P.; Alberti, K.G.M.; Kaufman, F.; Tajima, N.; Silink, M.; Arslanian, S.; Wong, G.; Bennett, P.; Shaw, J.; Caprio, S. The Metabolic Syndrome in Children and Adolescents? An IDF Consensus Report. Pediatr. Diabetes 2007, 8, 299–306. [Google Scholar] [CrossRef]
- Williams, B.; Mancia, G.; Spiering, W.; Agabiti Rosei, E.; Azizi, M.; Burnier, M.; Clement, D.L.; Coca, A.; de Simone, G.; Dominiczak, A.; et al. 2018 ESC/ESH Guidelines for the Management of Arterial Hypertension. Eur. Heart J. 2018, 39, 3021–3104. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA J. Am. Med. Assoc. 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Stand. Med. Care Diabetes—2021 Diabetes Care 2021, 44, S15–S33. [Google Scholar] [CrossRef]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the Concentration of Low-Density Lipoprotein Cholesterol in Plasma, without Use of the Preparative Ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Wallace, T.M.; Levy, J.C.; Matthews, D.R. Use and Abuse of HOMA Modeling. Diabetes Care 2004, 27, 1487–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McAuley, K.A.; Williams, S.M.; Mann, J.I.; Walker, R.J.; Lewis-Barned, N.J.; Temple, L.A.; Duncan, A.W. Diagnosing Insulin Resistance in the General Population. Diabetes Care 2001, 24, 460–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katz, A.; Nambi, S.S.; Mather, K.; Baron, A.D.; Follmann, D.A.; Sullivan, G.; Quon, M.J. Quantitative Insulin Sensitivity Check Index: A Simple, Accurate Method for Assessing Insulin Sensitivity In Humans. J. Clin. Endocrinol. Metab. 2000, 85, 2402–2410. [Google Scholar] [CrossRef]
- Reaven, G.M. HOMA-Beta in the UKPDS and ADOPT. Is the Natural History of Type 2 Diabetes Characterised by a Progressive and Inexorable Loss of Insulin Secretory Function? Maybe? Maybe Not? Diabetes Vasc. Dis. Res. 2009, 6, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Kim, D.; Kim, H.J.; Lee, C.-H.; Yang, J.I.; Kim, W.; Kim, Y.J.; Yoon, J.-H.; Cho, S.-H.; Sung, M.-W.; et al. Hepatic Steatosis Index: A Simple Screening Tool Reflecting Nonalcoholic Fatty Liver Disease. Dig. Liver Dis. 2010, 42, 503–508. [Google Scholar] [CrossRef]
- Castera, L. Non-invasive Tests for Liver Fibrosis in NAFLD: Creating Pathways between Primary Healthcare and Liver Clinics. Liver Int. 2020, 40, 77–81. [Google Scholar] [CrossRef] [Green Version]
- Xiao, G.; Zhu, S.; Xiao, X.; Yan, L.; Yang, J.; Wu, G. Comparison of Laboratory Tests, Ultrasound, or Magnetic Resonance Elastography to Detect Fibrosis in Patients with Nonalcoholic Fatty Liver Disease: A Meta-analysis. Hepatology 2017, 66, 1486–1501. [Google Scholar] [CrossRef]
- Mahady, S.E.; Macaskill, P.; Craig, J.C.; Wong, G.L.H.; Chu, W.C.W.; Chan, H.L.Y.; George, J.; Wong, V.W.S. Diagnostic Accuracy of Noninvasive Fibrosis Scores in a Population of Individuals With a Low Prevalence of Fibrosis. Clin. Gastroenterol. Hepatol. 2017, 15, 1453–1460. [Google Scholar] [CrossRef] [Green Version]
- Angulo, P.; Hui, J.M.; Marchesini, G.; Bugianesi, E.; George, J.; Farrell, G.C.; Enders, F.; Saksena, S.; Burt, A.D.; Bida, J.P.; et al. The NAFLD Fibrosis Score: A Noninvasive System That Identifies Liver Fibrosis in Patients with NAFLD. Hepatology 2007, 45, 846–854. [Google Scholar] [CrossRef]
- Shah, A.G.; Lydecker, A.; Murray, K.; Tetri, B.N.; Contos, M.J.; Sanyal, A.J. Comparison of Noninvasive Markers of Fibrosis in Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2009, 7, 1104–1112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, M.C.; Pizzolanti, G.; Torregrossa, V.; Misiano, G.; Milano, S.; Giordano, C. Visceral Adiposity Index (VAI) Is Predictive of an Altered Adipokine Profile in Patients with Type 2 Diabetes. PLoS ONE 2014, 9, e91969. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.C.; Giordano, C.; Galia, M.; Criscimanna, A.; Vitabile, S.; Midiri, M.; Galluzzo, A. Visceral Adiposity Index: A Reliable Indicator of Visceral Fat Function Associated with Cardiometabolic Risk. Diabetes Care 2010, 33, 920–922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, M.C.; Giordano, C. Visceral Adiposity Index: An Indicator of Adipose Tissue Dysfunction. Int. J. Endocrinol. 2014, 2014, 730827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, M.C.; Giordano, C.; Pitrone, M.; Galluzzo, A. Cut-off Points of the Visceral Adiposity Index (VAI) Identifying a Visceral Adipose Dysfunction Associated with Cardiometabolic Risk in a Caucasian Sicilian Population. Lipids Health Dis. 2011, 10, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Waas, M.; Neggers, S.J.C.M.M.; Pieters, R.; van den Heuvel-Eibrink, M.M. Components of the Metabolic Syndrome in 500 Adult Long-Term Survivors of Childhood Cancer. Ann. Oncol. 2010, 21, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, P.; Kapoor, G.; Jain, S.; Jain, S.; Sharma, A. Obesity and Sarcopenia in Survivors of Childhood Acute Lymphoblastic Leukemia. Indian Pediatr. 2021, 58, 436–440. [Google Scholar] [CrossRef]
- Gunn, H.M.; Emilsson, H.; Gabriel, M.; Maguire, A.M.; Steinbeck, K.S. Metabolic Health in Childhood Cancer Survivors: A Longitudinal Study in a Long-Term Follow-Up Clinic. J. Adolesc. Young Adult Oncol. 2016, 5, 24–30. [Google Scholar] [CrossRef]
- Indagine Istat. Aspetti Della Vita Quotidiana; Istituto Nazionale di Statistica: Rome, Italy, 2013. [Google Scholar]
- Talvensaari, K.K.; Lanning, M.; Tapanainen, P.; Knip, M. Long-Term Survivors of Childhood Cancer Have an Increased Risk of Manifesting the Metabolic Syndrome. J. Clin. Endocrinol. Metab. 1996, 81, 3051–3055. [Google Scholar] [CrossRef] [Green Version]
- Guler, E.; Col, N.; Buyukcelik, M.; Balat, A. Prevalence of Hypertension Determined by Ambulatory Blood Pressure Monitoring (ABPM) and Body Composition in Long-Term Survivors of Childhood Cancer. Pediatr. Hematol. Oncol. 2018, 35, 1–10. [Google Scholar] [CrossRef]
- Friedman, D.N.; Tonorezos, E.S.; Cohen, P. Diabetes and Metabolic Syndrome in Survivors of Childhood Cancer. Horm. Res. Paediatr. 2019, 91, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Meacham, L.R.; Sklar, C.A.; Li, S.; Liu, Q.; Gimpel, N.; Yasui, Y.; Whitton, J.A.; Stovall, M.; Robison, L.L.; Oeffinger, K.C. Diabetes Mellitus in Long-Term Survivors of Childhood Cancer. Arch. Intern. Med. 2009, 169, 1381–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davis, N.L.; Tolfrey, K.; Jenney, M.; Elson, R.; Stewart, C.; Moss, A.D.; Cornish, J.M.; Stevens, M.C.G.; Crowne, E.C. Combined Resistance and Aerobic Exercise Intervention Improves Fitness, Insulin Resistance and Quality of Life in Survivors of Childhood Haemopoietic Stem Cell Transplantation with Total Body Irradiation. Pediatr. Blood Cancer 2020, 67, e28687. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.E.; Wakefield, C.E.; Cohn, R.J. Nutritional Interventions for Survivors of Childhood Cancer. Cochrane Database Syst. Rev. 2016, 22, CD009678. [Google Scholar] [CrossRef] [Green Version]
- Giordano, P.; Muggeo, P.; Delvecchio, M.; Carbonara, S.; Romano, A.; Altomare, M.; Ricci, G.; Valente, F.; Zito, A.; Scicchitano, P.; et al. Endothelial Dysfunction and Cardiovascular Risk Factors in Childhood Acute Lymphoblastic Leukemia Survivors. Int. J. Cardiol. 2017, 228, 621–627. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Tsutsumi, T.; Nakano, D.; Torimura, T. MAFLD: Renovation of Clinical Practice and Disease Awareness of Fatty Liver. Hepatol. Res. 2021. Online ahead of print. [Google Scholar] [CrossRef]
- Lonardo, A.; Mantovani, A.; Lugari, S.; Targher, G. Epidemiology and Pathophysiology of the Association between NAFLD and Metabolically Healthy or Metabolically Unhealthy Obesity. Ann. Hepatol. 2020, 19, 359–366. [Google Scholar] [CrossRef]
- Kim, H.; Lee, D.S.; An, T.H.; Park, H.-J.; Kim, W.K.; Bae, K.-H.; Oh, K.-J. Metabolic Spectrum of Liver Failure in Type 2 Diabetes and Obesity: From NAFLD to NASH to HCC. Int. J. Mol. Sci. 2021, 22, 4495. [Google Scholar] [CrossRef]
- Strasser, S.I.; Sullivan, K.M.; Myerson, D.; Spurgeon, C.L.; Storer, B.; Schoch, H.G.; Murakami, C.S.; McDonald, G.B. Cirrhosis of the Liver in Long-Term Marrow Transplant Survivors. Blood 1999, 93, 3259–3266. [Google Scholar] [CrossRef]


| Demographic and Anthropometric Features | |
|---|---|
| Male gender (%) | 52.4 |
| Age at recruitment (years) | 20.4 ± 6.7 |
| Age at cancer diagnosis (years) | 7.3 ± 4.6 |
| Follow-up since cancer diagnosis (years) | 14.3 ± 6.8 |
| Off-therapy period (years) | 11.9 ± 6.8 |
| BMI (kg/m2) | 24.0 ± 4.6 |
| Normal Weight (%) | 62.7 |
| Overweight (%) | 26.2 |
| Obese (%) | 11.1 |
| WC (cm) | 88.4 ± 13.8 |
| High WC (%) | |
| NCEP ATP III criteria | 27.8 |
| IDF criteria | 53.2 |
| Physical exercise (%) | 53.2 |
| Cigarette smoke (%) | 17.5 |
| Blood pressure | |
| SBP (mmHg) | 115.3 ± 14.6 |
| DBP (mmHg) | 68.3 ± 10.8 |
| Metabolic syndrome (%) | 4.0 |
| Glucose and lipids metabolism | |
| Glycemia (mg/dL) | 86.2 ± 8.1 |
| Insulinemia (μU/mL) | 8.4 (7.4–10.3) |
| HbA1c (%) | 5.3 (4.9–5.4) |
| DM (%) | 0.0 |
| IFG (%) | 4.0 |
| Cholesterol total (mg/dL) | 168.0 ± 29.6 |
| HDL (mg/dL) | 49.8 ± 11.3 |
| LDL (mg/dL) | 94.3 ± 26.0 |
| Triglycerides (mg/dL) | 94.2 ± 68.4 |
| Insulin resistance and secretion | |
| HOMA-IR | 1.7 (1.7–2.2) |
| HOMA-IR > 2.5 (%) | 24.1 |
| HOMA-β | 135.2 (91.4–242.7) |
| McAuley Index | 8.5 (6.7–9.0) |
| McAuley Index < 5.8 (%) | 12.0 |
| QUICKI | 0.4 (0.3–0.4) |
| QUICKI < 0.339 (%) | 31.0 |
| Visceral adipose function | |
| VAI | 2.3 (1.9–4.3) |
| Above normal VAI (%) | 37.1 |
| Liver steatosis and fibrosis | |
| AST (U/L) | 21.3 ± 13.1 |
| ALT (U/L) | 23.5 ± 19.1 |
| HSI | 34.7 ± 6.9 |
| HSI ≥ 36 (%) | 41.0 |
| FIB-4 | 0.4 ± 0.2 |
| FIB-4 > 3.25 (%) | 0.0 |
| NFS | −3.5 ± 1.1 |
| NFS < −1.455 (%) | 98.6 |
| NFS > 0.676 (%) | 0.0 |
| Normal Weight (n = 79) | Overweight (n = 33) | Obese (n = 14) | p * | OR | |
|---|---|---|---|---|---|
| Demographic and anthropometric features | |||||
| Male gender (%) | 47.5 | 51.5 | 78.6 | 0.10 | |
| Age at recruitment (years) | 20.7 ± 7.0 | 21.4 ± 5.4 | 23.8 ± 8.6 | 0.30 | |
| Age at cancer diagnosis (years) | 6.7 ± 4.7 | 7.6 ± 4.6 | 6.5 ± 4.0 | 0.56 | |
| Follow-up since cancer diagnosis (years) | 14.0 ± 6.7 | 13.8 ± 5.6 | 17.3 ± 9.3 | 0.21 | |
| Off-therapy period (years) | 11.9 ± 6.7 | 11.4 ± 6.03 | 14.5 ± 10.1 | 0.32 | |
| BMI (kg/m2) | 21.1 ± 2.5 | 27.4 ± 1.5 | 32.7 ± 2.1 | <0.001 | |
| WC (cm) | 80.8 ± 9.0 | 95.7 ± 6.0 | 114.2 ± 7.1 | <0.001 | |
| High WC (%) | |||||
| NCEP ATP III criteria | 8.8 | 42.4 | 100.0 | <0.001 | 11.8 |
| IDF criteria | 31.2 | 84.8 | 100.0 | <0.001 | 10.2 |
| Physical exercise (%) | 58.2 | 45.5 | 42.9 | 0.33 | |
| Cigarette smoke (%) | 21.5 | 6.1 | 21.4 | 0.13 | |
| Blood pressure | |||||
| SBP (mmHg) | 111 ± 11.7 | 117.9 ± 13.2 | 133.8 ±17.4 | 0.001 | |
| DBP (mmHg) | 66 ± 8.8 | 69.3 ± 8.3 | 79.4 ± 17.6 | 0.001 | |
| Metabolic syndrome (%) | 1.2 | 0.0 | 28.6 | 0.01 | |
| Glucose and lipids metabolism | |||||
| Glycemia (mg/dL) | 85.2 ± 7.2 | 86.4 ± 9.2 | 89.4 ± 8.9 | 0.46 | |
| DM (%) | 0.0 | 0.0 | 0.0 | 1.00 | |
| IFG (%) | 1.2 | 6.1 | 14.3 | 0.05 | 3.1 |
| Cholesterol total (mg/dL) | 164.0 ± 29.5 | 169.5 ± 35.6 | 177.4 ± 17.4 | 0.33 | |
| HDL (mg/dL) | 51.6 ± 11.5 | 48.1 ± 10.5 | 45 ± 12.4 | 0.07 | |
| LDL (mg/dL) | 95.0 ± 26.6 | 90.1 ± 30.2 | 102.3 ± 12.5 | 0.99 | |
| Triglycerides (mg/dL) | 90.1 ± 64.1 | 76.8 ± 44.3 | 125.6 ± 96.2 | 0.14 | |
| Insulin resistance and secretion | |||||
| HOMA-IR >2.5 (%) | 30.8 | 10.0 | 33.3 | 0.80 | |
| McAuley Index < 5.8% | 16.7 | 0.0 | 20.0 | 0.83 | |
| QUICKI < 0.339 (%) | 30.8 | 20.0 | 50.0 | 0.63 | |
| Visceral adipose function | |||||
| Above normal VAI (%) | 36.8 | 18.2 | 80.0 | 0.05 | |
| Liver steatosis and fibrosis | |||||
| AST (U/L) | 19.8 ± 14.5 | 21.3 ± 10.7 | 26.4 ± 10.7 | 0.13 | |
| ALT (U/L) | 19.3 ± 15.9 | 29.3 ± 25.1 | 30.2 ± 15.7 | 0.02 | |
| HSI | 30.4 ± 4.6 | 39.0 ± 4.1 | 42.8 ± 5.5 | <0.001 | |
| HSI ≥ 36 (%) | 10.4 | 76.2 | 92.9 | <0.001 | 34.1 |
| FIB-4 | 0.4 ± 0.2 | 0.3 ± 0.2 | 0.5 ± 0.3 | 0.63 | |
| FIB-4 > 3.25 (%) | 0.0 | 0.0 | 0.0 | 1.00 | |
| NFS | −3.8 ± 1.0 | −3.8 ± 0.9 | −2.4 ± 0.9 | 0.001 | |
| NFS < −1.455 (%) | 100 | 100 | 92.9 | 0.99 | |
| NFS > 0.676 (%) | 0.0 | 0.0 | 0.0 | 1.00 |
| Leukemia (n = 87) | Lymphomas (n = 22) | Solid Cancers (n = 17) | p * | |
|---|---|---|---|---|
| Demographic and anthropometric features | ||||
| Male gender (%) | 55.2 | 54.5 | 25.0 | 0.08 |
| Age at recruitment (years) | 21.1 ± 7.2 | 23.0 ± 6.8 | 19.8 ± 4.3 | 0.33 |
| Age at cancer diagnosis (years) | 6.1 ± 4.3 | 10.6 ± 3.8 | 6.8 ± 5.0 | <0.001 |
| Follow-up since cancer diagnosis (years) | 15.0 ± 7.3 | 12.1 ± 5.9 | 13.1 ± 4.3 | 0.16 |
| Off-therapy period (years) | 12.4 ± 7.5 | 11.1 ± 5.9 | 11.1 ± 5.9 | 0.64 |
| BMI (kg/m2) | 23.5 ± 4.6 | 25.5 ± 5.5 | 24.4 ± 3.6 | 0.16 |
| WC (cm) | 86.9 ± 13.6 | 94.3 ± 16.0 | 88.5 ± 9.5 | 0.19 |
| High WC (%) | ||||
| NCEP ATP III criteria | 21.8 | 40.9 | 43.8 | 0.04 |
| IDF criteria | 47.1 | 59.1 | 75.0 | 0.04 |
| Physical exercise (%) | 51.2 | 72.7 | 31.2 | 0.03 |
| Cigarette smoke (%) | 17.4 | 9.1 | 31.2 | 0.20 |
| Blood pressure | ||||
| SBP (mmHg) | 115.4 ± 15.2 | 118.8 ± 14.9 | 111.2 ± 9.7 | 0.63 |
| DBP (mmHg) | 67.6 ± 10.6 | 72.7 ± 12.6 | 67.2 ± 8.4 | 0.37 |
| Metabolic syndrome (%) | 4.6 | 4.5 | 0.0 | 0.63 |
| Glucose and lipids metabolism | ||||
| Glycemia (mg/dL) | 86.8 ± 8.4 | 84.2 ± 7.7 | 85.5 ± 6.9 | 0.56 |
| Insulinemia (μU/mL) | 8.0 (7.0–9.1) | 13.9 (7.7–19.2) | 9.4 (8.5–9.6) | 0.24 |
| HbA1c (%) | 5.2 (4.9–5.5) | 5.1 (4.4–5.3) | 5.3 (5–5.5) | 0.97 |
| DM (%) | 0.0 | 0.0 | 0.0 | 1.00 |
| IFG (%) | 4.6 | 4.6 | 0.0 | 0.62 |
| Cholesterol total (mg/dL) | 169.5 ± 29.2 | 161.0 ± 33.5 | 162.3 ± 24.3 | 0.67 |
| HDL (mg/dL) | 49.3 ± 10.7 | 45.6 ± 11.8 | 54.9 ± 13.4 | 0.18 |
| LDL (mg/dL) | 94.3 ± 65.4 | 124.3 ± 97.9 | 61.6 ± 24.0 | 0.67 |
| Triglycerides (mg/dL) | 97.2 ± 24.2 | 74.1 ± 38.1 | 96.1 ± 25.8 | 0.41 |
| Insulin resistance and secretion | ||||
| HOMA-IR | 1.7 (1.6–1.8) | 2.7 (1–5–4.2) | 2.2 (2.0–2.2) | 0.65 |
| HOMA-IR >2.5 (%) | 25.0 | 42.9 | 0.0 | 0.37 |
| HOMA-β | 135.2 (88.2–192.6) | 249.9 (151.3–325.1) | 102.6 (90–119.8) | 0.27 |
| McAuley Index | 8.7 (7.7–9.0) | 5.4 (4.7–8.7) | 8.4 (6.9–9.2) | 0.55 |
| McAuley Index < 5.8% | 6.7 | 40.0 | 0.0 | 0.89 |
| QUICKI | 0.4 (0.3–0.4) | 0.3 (0.3–0.4) | 0.4 (0.3–0.3) | 0.72 |
| QUICKI < 0.339 (%) | 25.0 | 57.1 | 20.0 | 0.90 |
| Visceral adipose function | ||||
| VAI | 2.1 (1.9–3.8) | 5.9 (2.4–12.3) | 1.3 (0.9–2.2) | 0.04 |
| Above normal VAI (%) | 40.9 | 60.0 | 14.3 | 0.32 |
| Liver steatosis and fibrosis | ||||
| AST (U/L) | 22.3 ± 15.9 | 21.4 ± 5.2 | 17.4 ± 6.4 | 0.44 |
| ALT (U/L) | 25.7 ± 22. 3 | 22.0 ± 12.5 | 15.3 ± 7.8 | 0.11 |
| HSI | 34.7 ± 7.0 | 35.3 ± 7.5 | 33.4 ± 5.5 | 0.79 |
| HSI ≥ 36 (%) | 42.3 | 38.9 | 33.3 | 0.65 |
| FIB-4 | 0.4 ± 0.2 | 0.5 ± 0.1 | 0.4 ± 0.1 | 0.58 |
| FIB-4 > 3.25 (%) | 0.0 | 0.0 | 0.0 | 1.00 |
| NFS | −3.7 ± 1.0 | −2.9 ± 1.0 | −3.5 ± 1.5 | 0.14 |
| NFS < −1.455 (%) | 100.0 | 93.8 | 100.0 | 0.99 |
| NFS > 0.676 (%) | 0.0 | 0.0 | 0.0 | 1.00 |
| Chemotherapy (n = 90) | Chemo-Radio (n = 25) | Transplant (n = 11) | p * | |
|---|---|---|---|---|
| Demographic and anthropometric features | ||||
| Male gender (%) | 57.1 | 36 | 45.5 | 0.15 |
| Age at recruitment (years) | 20.4 ± 6.5 | 24.2 ± 6.0 | 21.7 ± 9.3 | 0.03 |
| Age at cancer diagnosis (years) | 6.2 ± 4.2 | 9.0 ± 5.0 | 8.0 ± 5.4 | 0.02 |
| Follow-up since cancer diagnosis (years) | 14.1 ± 6.1 | 15.1 ± 7.5 | 13.6 ± 10.3 | 0.79 |
| Off-therapy period (years) | 12.1 ± 6.5 | 13.2 ± 7.0 | 9.2 ± 10.1 | 0.28 |
| BMI (kg/m2) | 24.1 ± 4.8 | 23.9 ± 4.2 | 23.8 ± 4.6 | 0.72 |
| WC (cm) | 88.6 ± 14.0 | 88.7 ± 11.8 | 86.4 ± 16.9 | 0.48 |
| High WC (%) | ||||
| NCEP ATP III criteria | 26.4 | 36.0 | 18.2 | 0.89 |
| IDF criteria | 49.5 | 60.0 | 63.6 | 0.39 |
| Physical exercise (%) | 52.2 | 60.0 | 45.5 | 0.68 |
| Cigarette smoke (%) | 16.7 | 24.0 | 9.1 | 0.51 |
| Blood pressure | ||||
| SBP (mmHg) | 116.4 ± 15.1 | 113.9 ± 12.7 | 109.9 ± 13.5 | 0.02 |
| DBP (mmHg) | 68.5 ± 11.3 | 67.5 ± 9.5 | 69.1 ± 10.3 | 0.57 |
| Metabolic syndrome (%) | 4.4 | 0.0 | 9.1 | 0.96 |
| Glucose and lipids metabolism | ||||
| Glycemia (mg/dL) | 86.6 ± 7.7 | 86.9 ± 8.0 | 81.9 ± 11.0 | 0.31 |
| Insulinemia (μU/mL) | 8.7 (7.9–9.8) | 6 (5.8–13.0) | 17.7 (6.4–29.0) | 0.07 |
| HbA1c (%) | 5.3 (4.9–5.5) | 5.3 (4.2–5.4) | 5.1 (4.8–5.3) | 0.45 |
| DM (%) | 0.0 | 0.0 | 0.0 | 1.00 |
| IFG (%) | 3.3 | 4.0 | 9.1 | 0.56 |
| Cholesterol total (mg/dL) | 168.6 ± 32.4 | 158.2 ± 25.0 | 179.1 ± 21.2 | 0.66 |
| HDL (mg/dL) | 49.5 ± 10.5 | 53.3 ± 13.0 | 46.0 ± 13.8 | 0.74 |
| LDL (mg/dL) | 90.9 ± 72.2 | 76.6 ± 25.4 | 129.4 ± 85.9 | 0.21 |
| Triglycerides (mg/dL) | 90.6 ± 28.4 | 99.9 ± 27.0 | 100.9 ± 18.4 | 0.20 |
| Insulin resistance and secretion | ||||
| HOMA-IR | 1.9 (1.7–2.2) | 1.3 (1.3–2.9) | 3.4 (1.3–5.4) | 0.01 |
| HOMA-IR > 2.5 (%) | 13.6 | 33.3 | 75.0 | 0.03 |
| HOMA-β | 136.3 (98.9–224.7) | 77.1 (69.6–167.8) | 462.2 (121.3–803.1) | 0.08 |
| McAuley Index | 8.5 (6.9–9.0) | 8.6 (6–8.8) | 6.7 (3.8–9.6) | 0.10 |
| McAuley Index < 5.8% | 11.1 | 0.0 | 25.0 | 0.99 |
| QUICKI | 0.3 (0.3–0.4) | 0.4 (0.3–0.4) | 0.3 (0.3–0.4) | 0.07 |
| QUICKI < 0.339 (%) | 22.7 | 33.3 | 75.0 | 0.05 |
| Visceral adipose function | ||||
| VAI | 2.1 (1.7–5.0) | 3.1 (1.8–3.5) | 8.4 (1.8–15.0) | 0.14 |
| Above normal VAI (%) | 30.4 | 42.9 | 60.0 | 0.13 |
| Liver steatosis and fibrosis | ||||
| AST (U/L) | 21.0 ± 14.3 | 18.8 ± 4.2 | 29.9 ± 16.1 | 0.23 |
| ALT (U/L) | 22.9 ± 17.1 | 19.4 ± 7.4 | 34.9 ± 37.1 | 0.10 |
| HSI | 34.5 ± 7.2 | 34.1 ± 6.1 | 37.5 ± 6.0 | 0.35 |
| HSI ≥ 36 (%) | 41.4 | 33.3 | 57.1 | 0.49 |
| FIB-4 | 0.4 ± 0.2 | 0.4 ± 0.2 | 0.5 ± 0.4 | 0.9 |
| FIB-4 > 3.25 (%) | 0.0 | 0.0 | 0.0 | 1.00 |
| NFS | −3.5 ± 1.1 | −3.5 ± 0.9 | −3.4 ± 1.3 | 0.70 |
| NFS < −1.455 (%) | 100.0 | 100.0 | 85.7 | 0.99 |
| NFS > 0.676 (%) | 0.0 | 0.0 | 0.0 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Milluzzo, A.; Manuella, L.; Cannata, E.; Russo, G.; La Vignera, S.; Purrello, F.; Di Cataldo, A.; Sciacca, L. Influence of Body Mass Index, Cancer Type and Treatment on Long-Term Metabolic and Liver Outcomes in Childhood Cancer Survivors. J. Clin. Med. 2022, 11, 878. https://doi.org/10.3390/jcm11030878
Milluzzo A, Manuella L, Cannata E, Russo G, La Vignera S, Purrello F, Di Cataldo A, Sciacca L. Influence of Body Mass Index, Cancer Type and Treatment on Long-Term Metabolic and Liver Outcomes in Childhood Cancer Survivors. Journal of Clinical Medicine. 2022; 11(3):878. https://doi.org/10.3390/jcm11030878
Chicago/Turabian StyleMilluzzo, Agostino, Lucia Manuella, Emanuela Cannata, Giovanna Russo, Sandro La Vignera, Francesco Purrello, Andrea Di Cataldo, and Laura Sciacca. 2022. "Influence of Body Mass Index, Cancer Type and Treatment on Long-Term Metabolic and Liver Outcomes in Childhood Cancer Survivors" Journal of Clinical Medicine 11, no. 3: 878. https://doi.org/10.3390/jcm11030878
APA StyleMilluzzo, A., Manuella, L., Cannata, E., Russo, G., La Vignera, S., Purrello, F., Di Cataldo, A., & Sciacca, L. (2022). Influence of Body Mass Index, Cancer Type and Treatment on Long-Term Metabolic and Liver Outcomes in Childhood Cancer Survivors. Journal of Clinical Medicine, 11(3), 878. https://doi.org/10.3390/jcm11030878








