Characteristics and Outcomes of Bloodstream Infections in a Tertiary-Care Pediatric Hematology–Oncology Unit: A 10-Year Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Setting, Study Population, and Design
2.2. Management of BSIs
2.3. Definitions
2.4. Assessment of Antibiotic Therapy
2.5. Microbiology
2.6. Statistical Analysis
3. Results
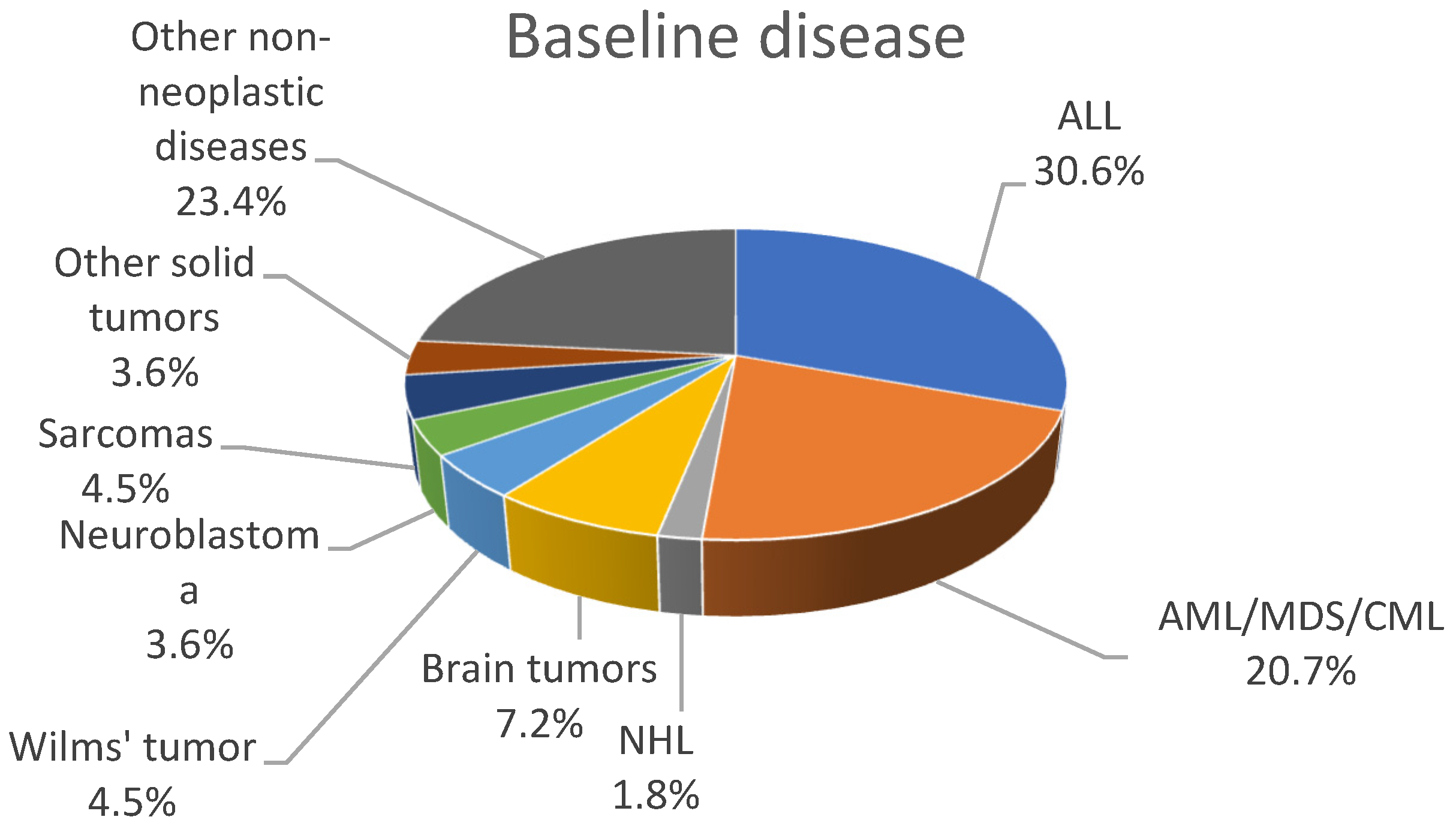
3.1. Demographics and Epidemiology
3.2. Blood Culture Results
3.3. Empirical Antibiotic Treatment
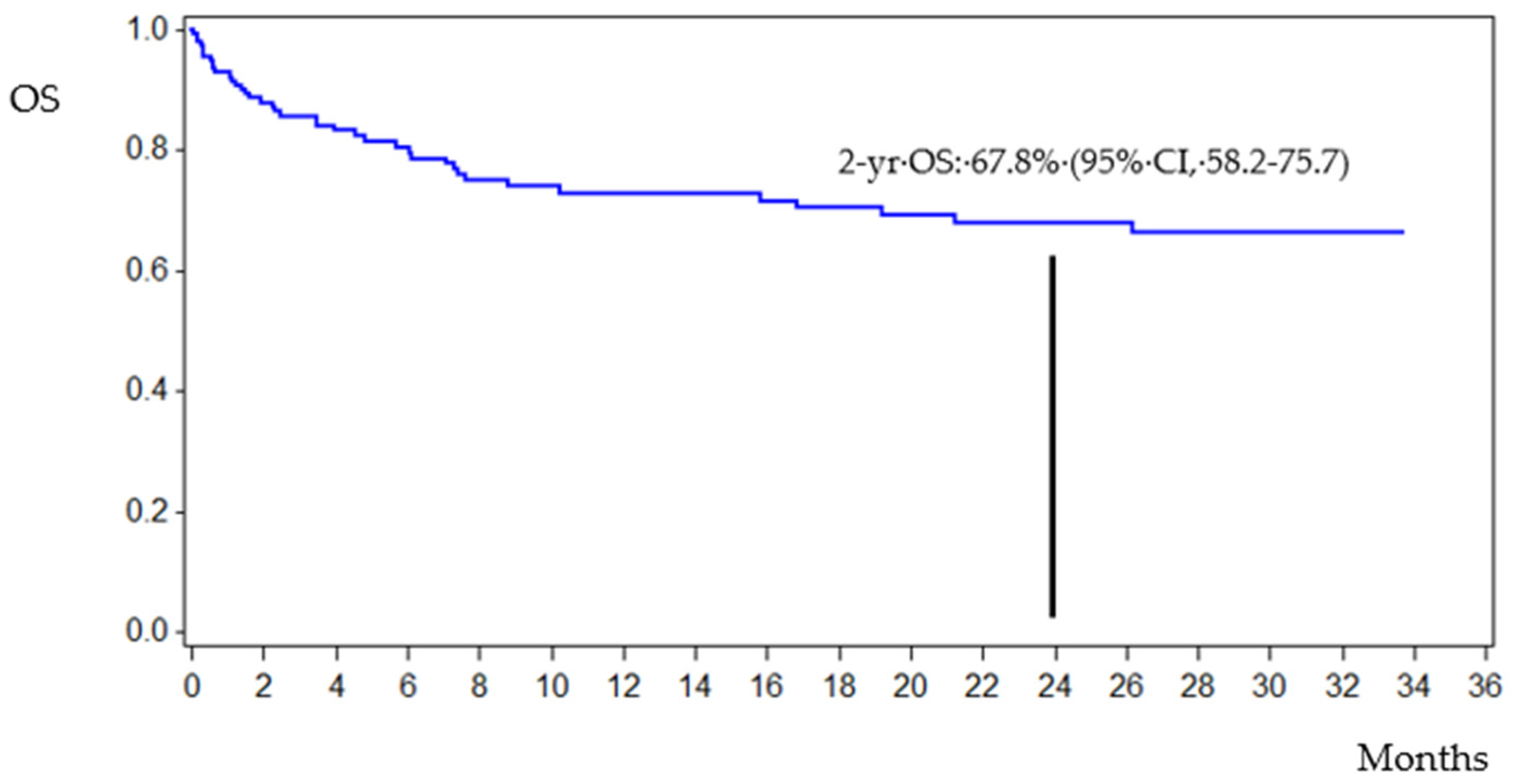
3.4. Patient Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trecarichi, E.M.; Pagano, L.; Candoni, A.; Pastore, D.; Cattaneo, C.; Fanci, R.; Nosari, A.; Caira, M.; Spadea, A.; Busca, A.; et al. Current Epidemiology and Antimicrobial Resistance Data for Bacterial Bloodstream Infections in Patients with Hematologic Malignancies: An Italian Multicentre Prospective Survey. Clin. Microbiol. Infect. 2015, 21, 337–343. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Islas-Muñoz, B.; Volkow-Fernández, P.; Ibanes-Gutiérrez, C.; Villamar-Ramírez, A.; Vilar-Compte, D.; Cornejo-Juárez, P. Bloodstream Infections in Cancer Patients. Risk Factors Associated with Mortality. Int. J. Infect. Dis. 2018, 71, 59–64. [Google Scholar] [CrossRef] [Green Version]
- Silva, M.; Marra, A.R.; Pereira, C.A.P.; Medina-Pestana, J.O.; Camargo, L.F.A. Bloodstream Infection after Kidney Transplantation: Epidemiology, Microbiology, Associated Risk Factors, and Outcome. Transplantation 2010, 90, 581–587. [Google Scholar] [CrossRef]
- Garrido, M.M.; Garrido, R.Q.; Cunha, T.N.; Ehrlich, S.; Martins, I.S. Comparison of Epidemiological, Clinical and Microbiological Characteristics of Bloodstream Infection in Children with Solid Tumours and Haematological Malignancies. Epidemiol. Infect. 2019, 147, e298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Nadal, G.; Puerta-Alcalde, P.; Gudiol, C.; Cardozo, C.; Albasanz-Puig, A.; Marco, F.; Laporte-Amargós, J.; Moreno-García, E.; Domingo-Doménech, E.; Chumbita, M.; et al. Inappropriate Empirical Antibiotic Treatment in High-Risk Neutropenic Patients With Bacteremia in the Era of Multidrug Resistance. Clin. Infect. Dis. 2020, 70, 1068–1074. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Current Trends in the Epidemiology of Nosocomial Bloodstream Infections in Patients with Hematological Malignancies and Solid Neoplasms in Hospitals in the United States. Clin. Infect. Dis. 2003, 36, 1103–1110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Vidal, C.; Cardozo-Espinola, C.; Puerta-Alcalde, P.; Marco, F.; Tellez, A.; Agüero, D.; Romero-Santana, F.; Díaz-Beyá, M.; Giné, E.; Morata, L.; et al. Risk Factors for Mortality in Patients with Acute Leukemia and Bloodstream Infections in the Era of Multiresistance. PLoS ONE 2018, 13, e0199531. [Google Scholar] [CrossRef]
- Montassier, E.; Batard, E.; Gastinne, T.; Potel, G.; de La Cochetière, M.F. Recent Changes in Bacteremia in Patients with Cancer: A Systematic Review of Epidemiology and Antibiotic Resistance. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 841–850. [Google Scholar] [CrossRef]
- Averbuch, D.; Orasch, C.; Cordonnier, C.; Livermore, D.M.; Mikulska, M.; Viscoli, C.; Gyssens, I.C.; Kern, W.V.; Klyasova, G.; Marchetti, O.; et al. European Guidelines for Empirical Antibacterial Therapy for Febrile Neutropenic Patients in the Era of Growing Resistance: Summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013, 98, 1826–1835. [Google Scholar] [CrossRef] [Green Version]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Segel, G.B.; Halterman, J.S. Neutropenia in Pediatric Practice. Pediatr. Rev. 2008, 29, 12–23, quiz 24. [Google Scholar] [CrossRef] [PubMed]
- EUCAST: Clinical Breakpoints and Dosing of Antibiotics. Available online: https://www.eucast.org/clinical_breakpoints/ (accessed on 23 November 2021).
- Santoro, A.; Franceschini, E.; Meschiari, M.; Menozzi, M.; Zona, S.; Venturelli, C.; Digaetano, M.; Rogati, C.; Guaraldi, G.; Paul, M.; et al. Epidemiology and Risk Factors Associated With Mortality in Consecutive Patients With Bacterial Bloodstream Infection: Impact of MDR and XDR Bacteria. Open Forum Infect. Dis. 2020, 7, ofaa461. [Google Scholar] [CrossRef] [PubMed]
- Gustinetti, G.; Mikulska, M. Bloodstream Infections in Neutropenic Cancer Patients: A Practical Update. Virulence 2016, 7, 280–297. [Google Scholar] [CrossRef] [PubMed]
- Gudiol, C.; Bodro, M.; Simonetti, A.; Tubau, F.; González-Barca, E.; Cisnal, M.; Domingo-Domenech, E.; Jiménez, L.; Carratalà, J. Changing Aetiology, Clinical Features, Antimicrobial Resistance, and Outcomes of Bloodstream Infection in Neutropenic Cancer Patients. Clin. Microbiol. Infect. 2013, 19, 474–479. [Google Scholar] [CrossRef] [Green Version]
- Collin, B.A.; Leather, H.L.; Wingard, J.R.; Ramphal, R. Evolution, Incidence, and Susceptibility of Bacterial Bloodstream Isolates from 519 Bone Marrow Transplant Patients. Clin. Infect. Dis. 2001, 33, 947–953. [Google Scholar] [CrossRef] [Green Version]
- Ramphal, R. Changes in the Etiology of Bacteremia in Febrile Neutropenic Patients and the Susceptibilities of the Currently Isolated Pathogens. Clin. Infect. Dis. 2004, 39 (Suppl. 1), S25–S31. [Google Scholar] [CrossRef] [Green Version]
- Lakshmaiah, K.C.; Malabagi, A.S.; Govindbabu; Shetty, R.; Sinha, M.; Jayashree, R.S. Febrile Neutropenia in Hematological Malignancies: Clinical and Microbiological Profile and Outcome in High Risk Patients. J. Lab. Physicians 2015, 7, 116–120. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Hinedi, K.; Khairallah, H.; Saadeh, B.; Abbasi, S.; Noureen, M.; Raza, S.; Alkhatti, A. Epidemiology and Source of Infection in Patients with Febrile Neutropenia: A Ten-Year Longitudinal Study. J. Infect. Public Health 2019, 12, 364–366. [Google Scholar] [CrossRef]
- Castagnola, E.; Bagnasco, F.; Mesini, A.; Agyeman, P.K.A.; Ammann, R.A.; Carlesse, F.; Santolaya de Pablo, M.E.; Groll, A.H.; Haeusler, G.M.; Lehrnbecher, T.; et al. Antibiotic Resistant Bloodstream Infections in Pediatric Patients Receiving Chemotherapy or Hematopoietic Stem Cell Transplant: Factors Associated with Development of Resistance, Intensive Care Admission and Mortality. Antibiotics 2021, 10, 266. [Google Scholar] [CrossRef]
- Mikulska, M.; Viscoli, C.; Orasch, C.; Livermore, D.M.; Averbuch, D.; Cordonnier, C.; Akova, M. Fourth European Conference on Infections in Leukemia Group (ECIL-4), a joint venture of EBMT, EORTC, ICHS, ELN and ESGICH/ESCMID Aetiology and Resistance in Bacteraemias among Adult and Paediatric Haematology and Cancer Patients. J. Infect. 2014, 68, 321–331. [Google Scholar] [CrossRef]
- Lehrnbecher, T.; Robinson, P.; Fisher, B.; Alexander, S.; Ammann, R.A.; Beauchemin, M.; Carlesse, F.; Groll, A.H.; Haeusler, G.M.; Santolaya, M.; et al. Guideline for the Management of Fever and Neutropenia in Children With Cancer and Hematopoietic Stem-Cell Transplantation Recipients: 2017 Update. J. Clin. Oncol. 2017, 35, 2082–2094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nordmann, P.; Naas, T.; Poirel, L. Global Spread of Carbapenemase-Producing Enterobacteriaceae. Emerg. Infect. Dis. 2011, 17, 1791–1798. [Google Scholar] [CrossRef] [PubMed]
- Blair, J.M.A.; Webber, M.A.; Baylay, A.J.; Ogbolu, D.O.; Piddock, L.J.V. Molecular Mechanisms of Antibiotic Resistance. Nat. Rev. Microbiol. 2015, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Tzouvelekis, L.S.; Markogiannakis, A.; Psichogiou, M.; Tassios, P.T.; Daikos, G.L. Carbapenemases in Klebsiella Pneumoniae and Other Enterobacteriaceae: An Evolving Crisis of Global Dimensions. Clin. Microbiol. Rev. 2012, 25, 682–707. [Google Scholar] [CrossRef] [Green Version]
- Durante-Mangoni, E.; Andini, R.; Zampino, R. Management of Carbapenem-Resistant Enterobacteriaceae Infections. Clin. Microbiol. Infect. 2019, 25, 943–950. [Google Scholar] [CrossRef]
- Elshamy, A.A.; Aboshanab, K.M. A Review on Bacterial Resistance to Carbapenems: Epidemiology, Detection and Treatment Options. Future Sci. OA 2020, 6, FSO438. [Google Scholar] [CrossRef] [Green Version]
- Knight, T.; Glaser, D.W.; Ching, N.; Melish, M. Antibiotic Susceptibility of Bloodstream Isolates in a Pediatric Oncology Population: The Case for Ongoing Unit-Specific Surveillance. J. Pediatric. Hematol. Oncol. 2019, 41, e271–e276. [Google Scholar] [CrossRef]
- Caselli, D.; Cesaro, S.; Ziino, O.; Zanazzo, G.; Manicone, R.; Livadiotti, S.; Cellini, M.; Frenos, S.; Milano, G.M.; Cappelli, B.; et al. Multidrug Resistant Pseudomonas Aeruginosa Infection in Children Undergoing Chemotherapy and Hematopoietic Stem Cell Transplantation. Haematologica 2010, 95, 1612–1615. [Google Scholar] [CrossRef]
- Tamma, P.D.; Turnbull, A.E.; Harris, A.D.; Milstone, A.M.; Hsu, A.J.; Cosgrove, S.E. Less Is More: Combination Antibiotic Therapy for the Treatment of Gram-Negative Bacteremia in Pediatric Patients. JAMA Pediatr. 2013, 167, 903–910. [Google Scholar] [CrossRef] [Green Version]
- Antoniadou, A.; Giamarellou, H. Fever of Unknown Origin in Febrile Leukopenia. Infect Dis. Clin. N. Am. 2007, 21, 1055–1090. [Google Scholar] [CrossRef]
- Holland, T.; Fowler, V.G.; Shelburne, S.A. Invasive Gram-Positive Bacterial Infection in Cancer Patients. Clin. Infect. Dis. 2014, 59 (Suppl. 5), S331–S334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blennow, O.; Ljungman, P. The Challenge of Antibiotic Resistance in Haematology Patients. Br. J. Haematol. 2016, 172, 497–511. [Google Scholar] [CrossRef] [PubMed]
- Mokart, D.; Slehofer, G.; Lambert, J.; Sannini, A.; Chow-Chine, L.; Brun, J.-P.; Berger, P.; Duran, S.; Faucher, M.; Blache, J.-L.; et al. De-Escalation of Antimicrobial Treatment in Neutropenic Patients with Severe Sepsis: Results from an Observational Study. Intensive Care Med. 2014, 40, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Routsi, C.; Gkoufa, A.; Arvaniti, K.; Kokkoris, S.; Tourtoglou, A.; Theodorou, V.; Vemvetsou, A.; Kassianidis, G.; Amerikanou, A.; Paramythiotou, E.; et al. De-Escalation of Antimicrobial Therapy in ICU Settings with High Prevalence of Multidrug-Resistant Bacteria: A Multicentre Prospective Observational Cohort Study in Patients with Sepsis or Septic Shock. J. Antimicrob. Chemother. 2020, 75, 3665–3674. [Google Scholar] [CrossRef] [PubMed]
- Meryk, A.; Kropshofer, G.; Bargehr, C.; Knoll, M.; Hetzer, B.; Lass-Flörl, C.; Crazzolara, R. Which Type of Empiric Antibiotic Therapy Is Appropriate? A 20-Year Retrospective Study of Bloodstream Infections in Childhood Cancer. Infect. Dis. Ther. 2021, 10, 789–800. [Google Scholar] [CrossRef]
- Carrara, E.; Pfeffer, I.; Zusman, O.; Leibovici, L.; Paul, M. Determinants of Inappropriate Empirical Antibiotic Treatment: Systematic Review and Meta-Analysis. Int. J. Antimicrob. Agents 2018, 51, 548–553. [Google Scholar] [CrossRef]
| Clinical Characteristics | Episodes (N = 154), No. (%) |
|---|---|
| Peak temperature at the beginning of BSI | |
| Median (range) | 38.5 °C (38.0–40.3) |
| Mean (SD) | 38.74 (0.69) |
| N. obs | 144 |
| No. of neutrophils at BSI onset, cells/µL | |
| Median (range) | 180 (0.0–43120.0) |
| <500 | 83 (64.8) |
| N. obs | 128 |
| Days of neutropenia before BSIs | |
| Median (range) | 4 (6–240) |
| N. obs | 92 |
| Duration of total neutropenia | |
| Median (range) | 17 (2.0–343.0) |
| Septic shock | 17 (11.0) |
| Presence of central venous catheter | 144 (93.5) |
| Antibiotic prophylaxis | 48 (31.2) |
| Antifungal prophylaxis | 99 (64.3) |
| Antiviral prophylaxis | 61 (39.6) |
| Gram-Positive | Episodes (N = 97), No. (%) |
|---|---|
| Multidrug-resistant | 2 (2.1) |
| Staphylococcus aureus | 10 (10.3) |
| CoNS | 61 (62.9) |
| Staphylococcus epidermidis | 29 |
| Staphylococcus haemolyticus | 7 |
| Other staphylococcus species | |
| S. hominis | 14 |
| S. warneri | 6 |
| S. lentus | 1 |
| S. saprophyticus | 1 |
| S. simulans | 1 |
| S. xylosus | 1 |
| S. capitis | 1 |
| Streptococcus mitis | 8 (8.2) |
| Streptococcus salivarius | 3 (3.1) |
| Streptococcus oralis | 2 (2.1) |
| Enterococcus faecalis | 1 (1.0) |
| Enterococcus faecium | 2 (2.1) |
| Other spp. | 10 (10.3) |
| Brevibacterium spp. | 1 |
| Aerococcus viridans | 1 |
| Rothia | 1 |
| Corynebacterium spp. | 2 |
| Micrococcus luteus | 5 |
| Gram-Negative | Episodes (N = 64), No. (%) |
| Multidrug resistant | 12 (18.8) |
| Escherichia coli | 30 (46.9) |
| Klebsiella spp. | 12 (18.8) |
| Pseudomonas aeruginosa | 14 (21.9) |
| Enterobacter spp. | 4 (6.3) |
| Other spp. | 4 (6.3) |
| Acinetobacter spp. | 1 |
| Achromobacter xilososidans | 1 |
| Capnocytophafa spp. | 1 |
| G-unspecified | 1 |
| Fungi | Episodes (N = 6), No. (%) |
| Candida parapsilosis | 4 (66.7) |
| Fusarium spp. | 1 (16.7) |
| Geotrichum capitatum | 1 (16.7) |
| GN Organisms | In Vitro Antibiotic Resistance No. (%) | ||||
|---|---|---|---|---|---|
| Third- and Fourth-Generation Cephalosporins (%) | Semisynthetic Penicillins/β-Lactamase Inhibitors (%) | Aminoglycosides (%) | Carbapenems (%) | Fluoroquinolones (%) | |
| E. coli (N = 30) | 9 (30) | 6 (20.7; 1 missing) | 8 (26.7) | 0 | 16 (53.3) |
| K. pneumoniae (N = 12) | 3 (25) | 5 (41.7) | 2 (16.7) | 0 | 4 (33.3) |
| P. aeruginosa (N = 9; 5 missing) | 1 (11.1) | 1 (7.1) | 0 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mattei, D.; Baretta, V.; Mazzariol, A.; Maccacaro, L.; Balter, R.; Zaccaron, A.; Bonetti, E.; Chinello, M.; Vitale, V.; Caddeo, G.; et al. Characteristics and Outcomes of Bloodstream Infections in a Tertiary-Care Pediatric Hematology–Oncology Unit: A 10-Year Study. J. Clin. Med. 2022, 11, 880. https://doi.org/10.3390/jcm11030880
Mattei D, Baretta V, Mazzariol A, Maccacaro L, Balter R, Zaccaron A, Bonetti E, Chinello M, Vitale V, Caddeo G, et al. Characteristics and Outcomes of Bloodstream Infections in a Tertiary-Care Pediatric Hematology–Oncology Unit: A 10-Year Study. Journal of Clinical Medicine. 2022; 11(3):880. https://doi.org/10.3390/jcm11030880
Chicago/Turabian StyleMattei, Davide, Valentina Baretta, Annarita Mazzariol, Laura Maccacaro, Rita Balter, Ada Zaccaron, Elisa Bonetti, Matteo Chinello, Virginia Vitale, Giulia Caddeo, and et al. 2022. "Characteristics and Outcomes of Bloodstream Infections in a Tertiary-Care Pediatric Hematology–Oncology Unit: A 10-Year Study" Journal of Clinical Medicine 11, no. 3: 880. https://doi.org/10.3390/jcm11030880
APA StyleMattei, D., Baretta, V., Mazzariol, A., Maccacaro, L., Balter, R., Zaccaron, A., Bonetti, E., Chinello, M., Vitale, V., Caddeo, G., Esposto, M. P., Pezzella, V., Gibellini, D., Tridello, G., & Cesaro, S. (2022). Characteristics and Outcomes of Bloodstream Infections in a Tertiary-Care Pediatric Hematology–Oncology Unit: A 10-Year Study. Journal of Clinical Medicine, 11(3), 880. https://doi.org/10.3390/jcm11030880






