Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): A Gender Perspective
Abstract
:1. Introduction
2. “Prodromal” FXTAS
3. FXTAS in Women
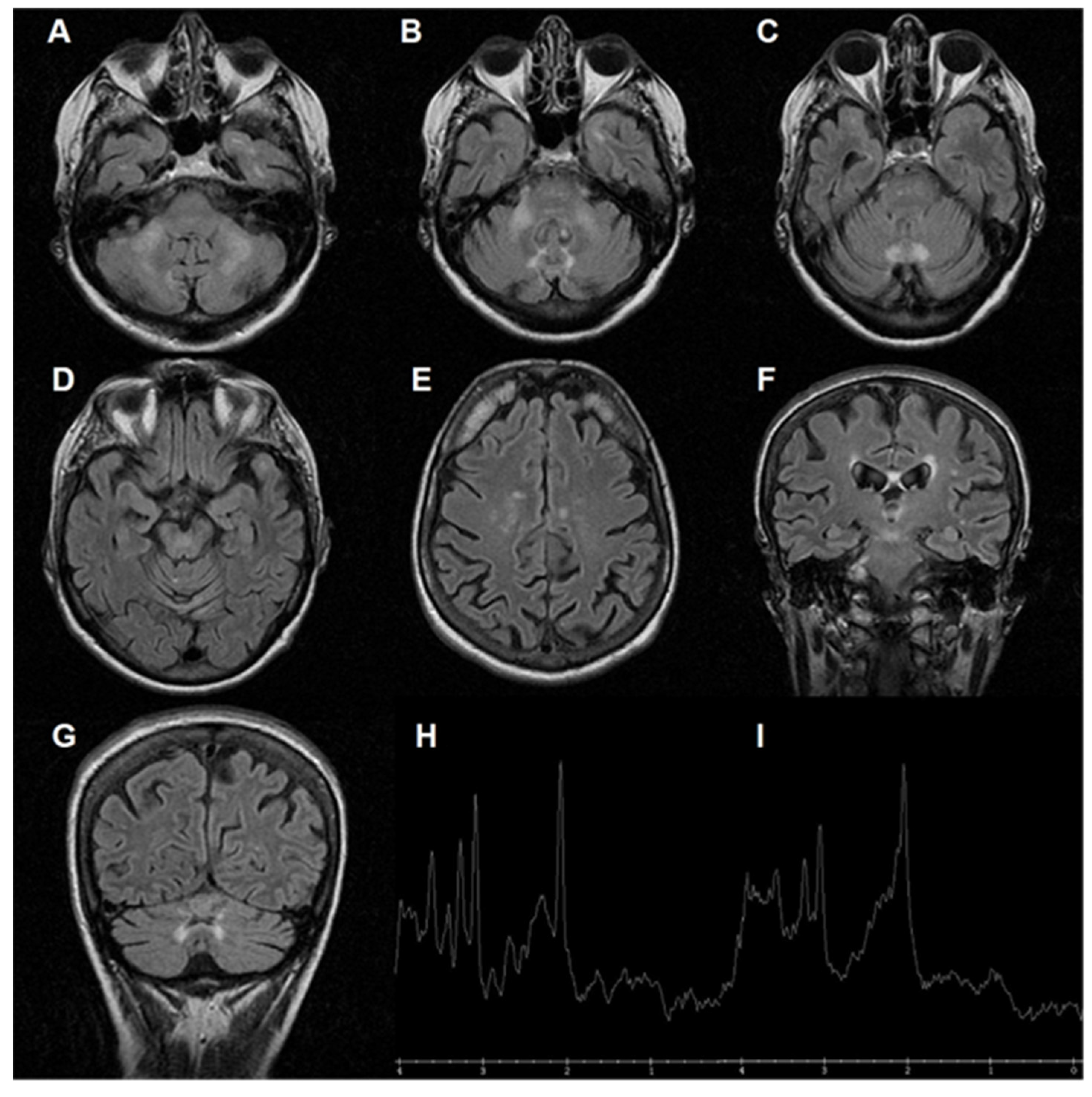
4. Biomarkers, FXTAS Pathophysiology, Mitochondrial Dysfunction, and Potential Drug Targets
5. Management of FXTAS
6. Conclusions
Funding
Conflicts of Interest
References
- Hagerman, P.J.; Hagerman, R.J. Fragile X-associated tremor/ataxia syndrome—An older face of the fragile X gene. Nat. Clin. Pr. Cardiovasc. Med. 2007, 3, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Herring, J.; Richstein, J. Fragile X Premutation Associated Conditions (FXPAC). Front. Pediatr. 2020, 8, 266. [Google Scholar] [CrossRef]
- Krans, A.; Kearse, M.G.; Todd, P.K. Repeat-associated non-AUG translation from antisense CCG repeats in fragile X tremor/ataxia syndrome. Ann. Neurol. 2016, 80, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Cabal-Herrera, A.M.; Tassanakijpanich, N.; Salcedo-Arellano, M.J.; Hagerman, R.J. Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): Pathophysiology and Clinical Implications. Int. J. Mol. Sci. 2020, 21, 4391. [Google Scholar] [CrossRef]
- Salcedo-Arellano, M.J.; Cabal-Herrera, A.M.; Tassanakijpanich, N.; McLennan, Y.A.; Hagerman, R.J. Ataxia as the Major Manifestation of Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): Case Series. Biomedicines 2020, 8, 136. [Google Scholar] [CrossRef] [PubMed]
- Islam, F.; Lee, W. A Case of Generalized Chorea Presenting as an Early Feature of Fragile-X Associated Tremor/Ataxia Syndrome. Mov. Disord. Clin. Pract. 2020, 7, 464–466. [Google Scholar] [CrossRef]
- Filley, C.M.; Brown, M.S.; Onderko, K.; Ray, M.; Bennett, R.E.; Berry-Kravis, E.; Grigsby, J. White matter disease and cognitive impairment in FMR1 premutation carriers. Neurology 2015, 84, 2146–2152. [Google Scholar] [CrossRef] [Green Version]
- Jacquemont, S.; Hagerman, R.J.; Leehey, M.; Grigsby, J.; Zhang, L.; Brunberg, J.A.; Greco, C.; Portes, V.D.; Jardini, T.; Levine, R.; et al. Fragile X premutation tremor/ataxia syndrome: Molecular, clinical, and neuroimaging correlates. Am. J. Hum. Genet. 2003, 72, 869–878. [Google Scholar] [CrossRef] [Green Version]
- Hagerman, R.; Hagerman, P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. 2013, 12, 786–798. [Google Scholar] [CrossRef] [Green Version]
- Orsucci, D.; Raglione, L.M.; Mazzoni, M.; Vista, M. Therapy of episodic ataxias: Case report and review of the literature. Drugs Context 2019, 8, 212576. [Google Scholar] [CrossRef] [Green Version]
- Lu, Q.; Shang, L.; Tian, W.T.; Cao, L.; Zhang, X.; Liu, Q. Complicated paroxysmal kinesigenic dyskinesia associated with SACS mutations. Ann. Transl. Med. 2020, 8, 8. [Google Scholar] [CrossRef] [PubMed]
- Heard, T.T.; Ramgopal, S.; Picker, J.; Lincoln, S.A.; Rotenberg, A.; Kothare, S.V. EEG abnormalities and seizures in genetically diagnosed Fragile X syndrome. Int. J. Dev. Neurosci. 2014, 38, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.; Leavitt, B.; Farzin, F.; Jacquemont, S.; Greco, C.; Brunberg, J.; Tassone, F.; Hessl, D.; Harris, S.; Zhang, L.; et al. Fragile-X–Associated Tremor/Ataxia Syndrome (FXTAS) in Females with the FMR1 Premutation. Am. J. Hum. Genet. 2004, 74, 1051–1056. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Keefe, J.A.; Bang, D.; Robertson, E.E.; Bs, A.B.; Ouyang, B.; Liu, Y.; Pal, G.; Berry-Kravis, E.; Hall, D.A. Prodromal Markers of Upper Limb Deficits in FMR1 Premutation Carriers and Quantitative Outcome Measures for Future Clinical Trials in Fragile X-associated Tremor/Ataxia Syndrome. Mov. Disord. Clin. Pr. 2020, 7, 810–819. [Google Scholar] [CrossRef]
- McKinney, W.S.; Bartolotti, J.; Khemani, P.; Wang, J.Y.; Hagerman, R.J.; Mosconi, M.W. Cerebellar-cortical function and connectivity during sensorimotor behavior in aging FMR1 gene premutation carriers. NeuroImage Clin. 2020, 27, 102332. [Google Scholar] [CrossRef]
- Tabolacci, E.; Pomponi, M.G.; Remondini, L.; Pietrobono, R.; Nobile, V.; Pennacchio, G.; Gurrieri, F.; Neri, G.; Genuardi, M.; Chiurazzi, P. Methylated premutation of the FMR1 gene in three sisters: Correlating CGG expansion and epigenetic inactivation. Eur. J. Hum. Genet. 2020, 28, 567–575. [Google Scholar] [CrossRef]
- Hall, A.D.; Todorova-Koteva, K.; Pandya, S.; Bernard, B.; Ouyang, B.; Walsh, M.; Pounardjian, T.; Deburghraeve, C.; Zhou, L.; Losh, M.; et al. Neurological and endocrine phenotypes of fragile X carrier women. Clin. Genet. 2016, 89, 60–67. [Google Scholar] [CrossRef] [Green Version]
- Schneider, A.; Summers, S.; Tassone, F.; Seritan, A.; Hessl, D.; Hagerman, P.; Hagerman, R. Women with Fragile X–associated Tremor/Ataxia Syndrome. Mov. Disord. Clin. Pr. 2020, 7, 910–919. [Google Scholar] [CrossRef]
- Rosario, R.; Anderson, R. The molecular mechanisms that underlie fragile X-associated premature ovarian insufficiency: Is it RNA or protein based? Mol. Hum. Reprod. 2020, 26, 727–737. [Google Scholar] [CrossRef]
- Salcedo-Arellano, M.J.; Wang, J.Y.; BS, Y.A.M.; Doan, M.; Cabal-Herrera, A.M.; Jimenez, S.; Bs, M.W.W.; Bs, D.S.; Juarez, P.; Tassone, F.; et al. Cerebral Microbleeds in Fragile X–Associated Tremor/Ataxia Syndrome. Mov. Disord. 2021, 36, 1935–1943. [Google Scholar] [CrossRef]
- Zafarullah, M.; Palczewski, G.; Rivera, S.M.; Hessl, D.R.; Tassone, F. Metabolic profiling reveals dysregulated lipid metabolism and potential biomarkers associated with the development and progression of Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS). FASEB J. 2020, 34, 16676–16692. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, D.A.; Nguyen, T.T.A.; Hall, D.A.; Robertson-Dick, E.; Berry-Kravis, E.; Cologna, S.M. Characterization of the Cerebrospinal Fluid Proteome in Patients with Fragile X-Associated Tremor/Ataxia Syndrome. Cerebellum 2021. [Google Scholar] [CrossRef] [PubMed]
- Napoli, E.; McLennan, Y.A.; Schneider, A.; Tassone, F.; Hagerman, R.J.; Giulivi, C. Characterization of the Metabolic, Clinical and Neuropsychological Phenotype of Female Carriers of the Premutation in the X-Linked FMR1 Gene. Front. Mol. Biosci. 2020, 7, 578640. [Google Scholar] [CrossRef] [PubMed]
- Gohel, D.; Berguerand, N.C.; Tassone, F.; Singh, R. The emerging molecular mechanisms for mitochondrial dysfunctions in FXTAS. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2020, 1866, 165918. [Google Scholar] [CrossRef] [PubMed]
- NNobile, V.; Palumbo, F.; Lanni, S.; Ghisio, V.; Vitali, A.; Castagnola, M.; Marzano, V.; Maulucci, G.; De Angelis, C.; De Spirito, M.; et al. Altered mitochondrial function in cells carrying a premutation or unmethylated full mutation of the FMR1 gene. Hum. Genet. 2020, 139, 227–245. [Google Scholar] [CrossRef]
- Alvarez-Mora, M.I.; Santos, C.; Carreño-Gago, L.; Madrigal, I.; Tejada, M.I.; Martinez, F.; Izquierdo-Alvarezh, S.; Garcia-Arumibdi, E.; Milaab, M.; Rodriguez-Revenga, L. Role of mitochondrial DNA variants in the development of fragile X-associated tremor/ataxia syndrome. Mitochondrion 2020, 52, 157–162. [Google Scholar] [CrossRef]
- Alvarez-Mora, M.I.; Podlesniy, P.; Gelpi, E.; Hukema, R.; Madrigal, I.; Pagonabarraga, J.; Trullas, R.; Mila, M.; Rodriguez-Revenga, L. Fragile X-associated tremor/ataxia syndrome: Regional decrease of mitochondrial DNA copy number relates to clinical manifestations. Genes Brain Behav. 2019, 18, e12565. [Google Scholar] [CrossRef]
- Wang, J.; Napoli, E.; Kim, K.; McLennan, Y.A.; Hagerman, R.J.; Giulivi, C. Brain Atrophy and White Matter Damage Linked to Peripheral Bioenergetic Deficits in the Neurodegenerative Disease FXTAS. Int. J. Mol. Sci. 2021, 22, 9171. [Google Scholar] [CrossRef]
- Napoli, E.; Flores, A.; Mansuri, Y.; Hagerman, R.J.; Giulivi, C. Sulforaphane improves mitochondrial metabolism in fibroblasts from patients with fragile X-associated tremor and ataxia syndrome. Neurobiol. Dis. 2021, 157, 105427. [Google Scholar] [CrossRef]
- Napoli, E.; Schneider, A.; Wang, J.Y.; Trivedi, A.; Carrillo, N.R.; Tassone, F.; Rogawski, M.; Hagerman, R.J.; Giulivi, C. Allopregnanolone Treatment Improves Plasma Metabolomic Profile Associated with GABA Metabolism in Fragile X-Associated Tremor/Ataxia Syndrome: A Pilot Study. Mol. Neurobiol. 2019, 56, 3702–3713. [Google Scholar] [CrossRef]
- Wang, J.Y.; Trivedi, A.M.; Carrillo, N.R.; Yang, J.; Schneider, A.; Giulivi, C.; Adams, P.; Tassone, F.; Kim, K.; Rivera, S.M.; et al. Open-Label Allopregnanolone Treatment of Men with Fragile X-Associated Tremor/Ataxia Syndrome. Neurotherapeutics 2017, 14, 1073–1083. [Google Scholar] [CrossRef] [PubMed]
- Hagerman, R.; Hagerman, P. Fragile X-associated tremor/ataxia syndrome: Pathophysiology and management. Curr. Opin. Neurol. 2021, 34, 541–546. [Google Scholar] [CrossRef] [PubMed]
- Lapostolle, A.; Delion, T.; Arnaud, S.; Manceau, P.; Degos, B. Thrombocytopenia and agranulocytosis in a FXTAS choreic patient treated with tetrabenazine. Neurol. Sci. 2021, 42, 3475–3477. [Google Scholar] [CrossRef] [PubMed]
- Haify, S.N.; Buijsen, R.A.M.; Verwegen, L.; Haify, S.N.; Buijsen, R.A.; Verwegen, L.; Severijnen, L.A.W.; de Boer, H.; Boumeester, V.; Monshouwer, R.; et al. Small molecule 1a reduces FMRpolyG-mediated toxicity in in vitro and in vivo models for FMR1 premutation. Hum. Mol. Genet. 2021, 30, 1632–1648. [Google Scholar] [CrossRef]
- Derbis, M.; Kul, E.; Niewiadomska, D.; Sekrecki, M.; Piasecka, A.; Taylor, K.; Hukema, R.K.; Stork, O.; Sobczak, K. Short antisense oligonucleotides alleviate the pleiotropic toxicity of RNA harboring expanded CGG repeats. Nat. Commun. 2021, 12, 1265. [Google Scholar] [CrossRef]
- Higuchi, Y.; Ando, M.; Yoshimura, A.; Hakotani, S.; Koba, Y.; Sakiyama, Y.; Hiramatsu, Y.; Tashiro, Y.; Maki, Y.; Hashiguchi, A.; et al. Prevalence of Fragile X-Associated Tremor/Ataxia Syndrome in Patients with Cerebellar Ataxia in Japan. Cerebellum 2021. [Google Scholar] [CrossRef]
- Almansour, A.; Ishiura, H.; Mitsui, J.; Matsukawa, T.; Matsukawa, M.K.; Shimizu, H.; Sugiyama, A.; Toda, T.; Tsuji, S. Frequency of FMR1 Premutation Alleles in Patients with Undiagnosed Cerebellar Ataxia and Multiple System Atrophy in the Japanese Population. Cerebellum 2021. [Google Scholar] [CrossRef]
- Martin, E.M.; Zhu, Y.; Kraan, C.M.; Kumar, K.R.; Godler, D.E.; Field, M. Men with FMR1 premutation alleles of less than 71 CGG repeats have low risk of being affected with fragile X-associated tremor/ataxia syndrome (FXTAS). J. Med. Genet. 2021, 107758. [Google Scholar] [CrossRef]
- Salcedo-Arellano, M.J.; Dufour, B.; McLennan, Y.; Martinez-Cerdeno, V.; Hagerman, R. Fragile X syndrome and associated disorders: Clinical aspects and pathology. Neurobiol. Dis. 2020, 136, 104740. [Google Scholar] [CrossRef]
- Storey, E.; Bui, M.Q.; Stimpson, P.; Tassone, F.; Atkinson, A.; Loesch, D.Z. Relationships between motor scores and cognitive functioning in FMR1 female premutation X carriers indicate early involvement of cerebello-cerebral pathways. Cerebellum Ataxia 2021, 8, 15. [Google Scholar] [CrossRef]
- Mailick, M.R.; Hong, J.; Movaghar, A.; Mailick, M.R.; Hong, J.; Movaghar, A.; DaWalt, L.; Berry-Kravis, E.M.; Brilliant, M.H.; Boero, J.; et al. Mild Neurological Signs in FMR1 Premutation Women in an Unselected Community-Based Cohort. Mov. Disord. 2021, 36, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Klusek, J.; Fairchild, A.; Moser, C.; Mailick, M.R.; Thurman, A.J.; Abbeduto, L. Family history of FXTAS is associated with age-related cognitive-linguistic decline among mothers with the FMR1 premutation. J. Neurodev. Disord. 2022, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Loesch, D.Z.; Tassone, F.; Atkinson, A.; Stimpson, P.; Trost, N.; Pountney, D.L.; Storey, E. Differential Progression of Motor Dysfunction Between Male and Female Fragile X Premutation Carriers Reveals Novel Aspects of Sex-Specific Neural Involvement. Front. Mol. Biosci. 2021, 7, 577246. [Google Scholar] [CrossRef] [PubMed]
- Ros-Castelló, V.; Latorre, A.; Álvarez-Linera, J.; Martinez-Castrillo, J.C.; Bhatia, K.P.; Pareés, I. Dystonia in a Female Fragile X Premutation Carrier. Mov. Disord. Clin. Pract. 2021, 8, 797–799. [Google Scholar] [CrossRef]
- Schwartzer, J.J.; Garcia-Arocena, D.; Jamal, A.; Izadi, A.; Willemsen, R.; Berman, R.F. Allopregnanolone Improves Locomotor Activity and Arousal in the Aged CGG Knock-in Mouse Model of Fragile X-Associated Tremor/Ataxia Syndrome. Front Neurosci. 2021, 5, 752973. [Google Scholar] [CrossRef]
| Males | Females | Refs. | |
|---|---|---|---|
| Tremor, ataxia, cerebellar signs | very common | very common | [1,2] |
| Gonadal failure | very rare (not reported) | very common | [2] |
| Cognitive impairment | very common | common (milder than in males) | [1,4] |
| Psychiatric issues | common | very common | [43] |
| Atypical features (e.g., dystonia) | very rare | rare | [44] |
| Middle Cerebellar Peduncle Sign | very common | rare | [18,39] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orsucci, D.; Lorenzetti, L.; Baldinotti, F.; Rossi, A.; Vitolo, E.; Gheri, F.L.; Napolitano, A.; Tintori, G.; Vista, M. Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): A Gender Perspective. J. Clin. Med. 2022, 11, 1002. https://doi.org/10.3390/jcm11041002
Orsucci D, Lorenzetti L, Baldinotti F, Rossi A, Vitolo E, Gheri FL, Napolitano A, Tintori G, Vista M. Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): A Gender Perspective. Journal of Clinical Medicine. 2022; 11(4):1002. https://doi.org/10.3390/jcm11041002
Chicago/Turabian StyleOrsucci, Daniele, Lucia Lorenzetti, Fulvia Baldinotti, Andrea Rossi, Edoardo Vitolo, Fabio Luigi Gheri, Alessandro Napolitano, Giancarlo Tintori, and Marco Vista. 2022. "Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): A Gender Perspective" Journal of Clinical Medicine 11, no. 4: 1002. https://doi.org/10.3390/jcm11041002
APA StyleOrsucci, D., Lorenzetti, L., Baldinotti, F., Rossi, A., Vitolo, E., Gheri, F. L., Napolitano, A., Tintori, G., & Vista, M. (2022). Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS): A Gender Perspective. Journal of Clinical Medicine, 11(4), 1002. https://doi.org/10.3390/jcm11041002








