Awakened Beta-Cell Function Decreases the Risk of Hypoglycemia in Pregnant Women with Type 1 Diabetes Mellitus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statements
2.2. Study Participants
2.3. Data Collection
2.4. Blood Sample Analyses
2.5. Sample Size
2.6. Statistical Analyses
3. Results
Impact of Clinically Significant Biochemical Hypoglycemia (CSBH) on Maternal and Neonatal Characteristics
4. Discussion
4.1. C-Peptide Concentration in Pregnant Women with Type 1 Diabetes Mellitus
4.2. C-Peptide, Insulin Doses, and Glycemic Control
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Persson, M.; Norman, M.; Hanson, U. Obstetric and perinatal outcomes in type 1 diabetic pregnancies: A large, population-based study. Diabetes Care 2009, 32, 2005–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association. 14. Management of Diabetes in Pregnancy: Standards of Medical Care in Diabetes. Diabetes Care 2021, 44 (Suppl. 1), S200–S210. [Google Scholar] [CrossRef] [PubMed]
- Delmis, J.; Ivanisevic, M.; Horvaticek, M. N-3 PUFA and Pregnancy Preserve C-Peptide in Women with Type 1 Diabetes Mellitus. Pharmaceutics 2021, 4, 2082. [Google Scholar] [CrossRef] [PubMed]
- Ringholm, L.; Pedersen-Bjergaard, U.; Thorsteinsson, B.; Damm, P.; Mathiesen, E.R. Hypoglycaemia during pregnancy in women with type 1 diabetes. Diabet Med. 2012, 29, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Leinonen, P.; Hiilesmaa, V.; Kaaja, R.; Teramo, K.A. Maternal Mortality in Type 1 Diabetes. Diabetes Care 2001, 24, 1501–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evers, I.M.; ter Braak, E.W.; de Valk, H.W.; van Der Schoot, B.; Hanssen, N.; Visser, G.H.A. Risk indicators predictive for severe hypoglycemia during first trimester of type 1 diabetic pregnancy. Diabetes Care 2002, 25, 554–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- IHSG. Glucose Concentrations of Less Than 3.0 mmol/L Should Be Reported in Clinical Trials: A Joint Position Statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care 2017, 40, 155–157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kinsley, B. Achieving better outcomes in pregnancies complicated by type 1 and type 2 diabetes mellitus. Clin. Ther. 2007, 29 (Suppl. D), S153–S160. [Google Scholar] [CrossRef] [PubMed]
- Palmer, J.P.; Fleming, G.A.; Greenbaum, C.J.; Herold, K.C.; Jansa, L.D.; Kolb, H.; Lachin, J.M.; Polonsky, K.S.; Pozzilli, P.; Skyler, J.S.; et al. ADA Workshop Report: C-Peptide Is the Appropriate Outcome Measure for Type 1 Diabetes Clinical Trials to Preserve β-Cell Function. Diabetes 2004, 53, 250–264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, L.R.; Rehfeld, J.F.; Pedersen-Bjergaard, U.; Damm, P.; Mathiesen, E.R. Pregnancy-induced rise in serum C-peptide concentrations in women with type 1 diabetes. Diabetes Care 2009, 32, 1052–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horvaticek, M.; Djelmis, J.; Ivanisevic, M.; Oreskovic, S.; Herman, M. Effect of eicosapentaenoic acid and docosahexaenoic acid supplementation on C-peptide preservation in pregnant women with type-1 diabetes: A randomized placebo-controlled clinical trial. Eur. J. Clin. Nutr. 2017, 71, 968–972. [Google Scholar] [CrossRef] [PubMed]
- Wilder, R.L. Hormones, pregnancy, and autoimmune diseases. Ann. N.Y. Acad. Sci. 1998, 840, 45–50. [Google Scholar] [CrossRef]
- Poole, J.A.; Claman, H.N. Immunology of Pregnancy: Implications for the Mother. Clin. Rev. Allergy Immunol. 2004, 26, 161–170. [Google Scholar] [CrossRef]
- Sorenson, R.; Brelje, T. Adaptation of Islets of Langerhans to Pregnancy: β-Cell Growth, Enhanced Insulin Secretion and the Role of Lactogenic Hormones. Horm. Metab. Res. 1997, 29, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Parsons, J.A.; Brelje, T.C.; Sorenson, R.I. Adaptation of Islets of Langerhans to Pregnancy: Increased Islet Cell Proliferation and Insulin Secretion Correlates with the Onset of Placental Lactogen Secretion. Endocrinology 1992, 130, 1459–1466. [Google Scholar] [PubMed]
- Rieck, S.; Kaestner, K.H. Expansion of b-cell mass in response to pregnancy. Trends Endocrinol. Metab. 2013, 21, 151–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Madsbad, S.; Krarup, T.; Reguer, L.; Faber, O.K.; Binder, C. Effect of strict blood glucose control on residual b-cell Function in insulin-dependent diabetics. Diabetologia 1981, 20, 530–534. [Google Scholar] [CrossRef] [PubMed]
- Ilic, S.; Jovanovic, L.; Wolitzer, A.O. Is the paradoxical first trimester drop in insulin requirement due to an increase in C-peptide concentration in pregnant Type 1 diabetic women? Diabetologia 2000, 43, 1329–1336. [Google Scholar] [PubMed] [Green Version]
- Gumpel, R.C. Intensive therapy preserves insulin secretion. Ann. Intern. Med. 1998, 129, 913–914. [Google Scholar] [CrossRef]
- Djelmis, J.; Ivaniševic, M.; Desoye, G.; van Poppel, M.; Berberovic, E.; Soldo, D.; Oreskovic, S. Higher Cord Blood Levels of Fatty Acids in Pregnant Women with Type 1 Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2018, 103, 2620–2629. [Google Scholar] [CrossRef] [PubMed]

| CSBH− (n = 30) | CSBH+ (n = 54) | p | |
|---|---|---|---|
| Maternal characteristics in 1st trimester | |||
| Maternal age (years) | 29.8 ± 5.3 | 29.3 ± 6.3 | 0.693 |
| Maternal height (cm) | 166.3 ± 7.4 | 166.7 ± 6.9 | 0.798 |
| Maternal pre-pregnancy weight (kg) | 63.5 ± 10.9 | 63.8 ± 8.0 | 0.804 |
| Pre-pregnancy body mass index (kg/m2) | 22.8 ± 2.9 | 22.9 ± 2.8 | 0.755 |
| Gestational weight gain (kg) | 13.3 ± 4.4 | 13.5 ± 4.8 | 0.858 |
| Duration of type 1 diabetes mellitus (years) | 10.1 ± 6.6 | 13.5 ± 7.1 | 0.032 |
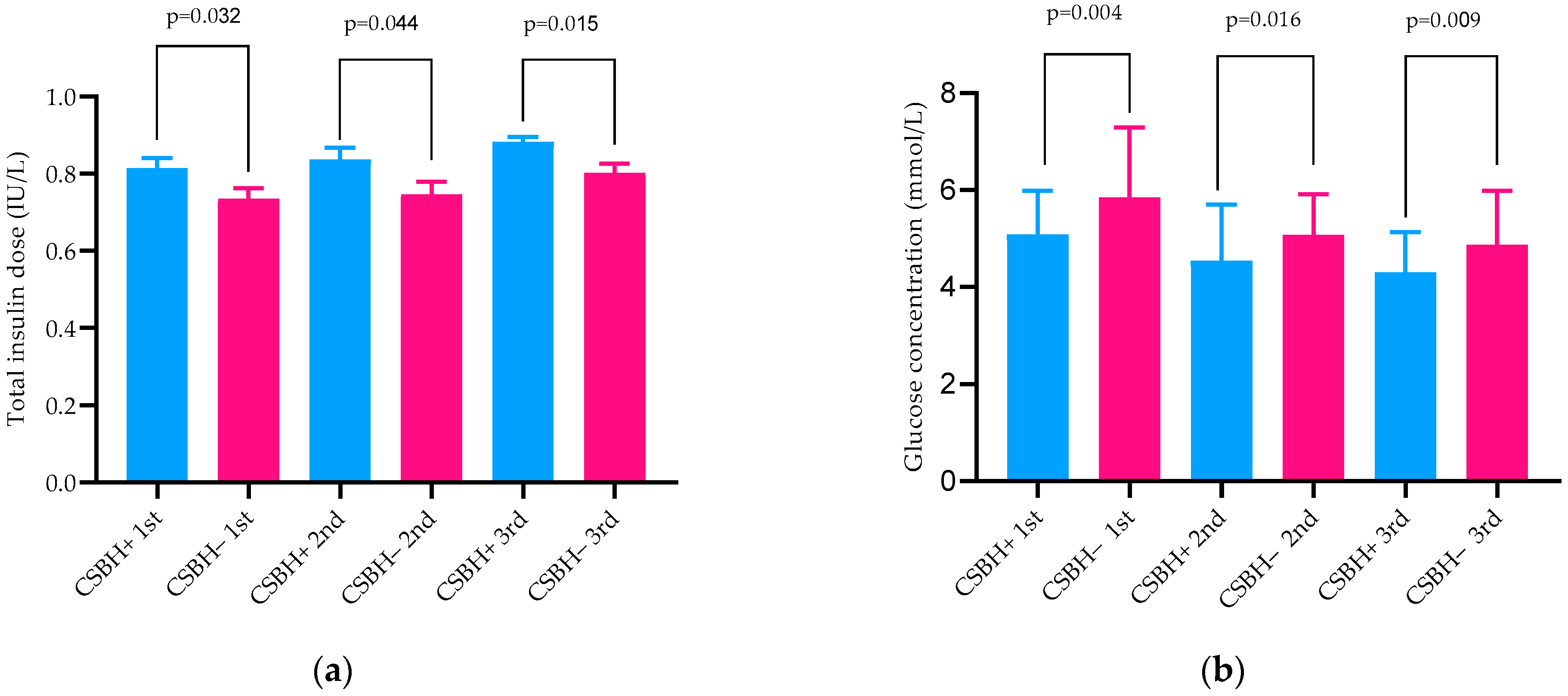
| Total insulin dose (IU/kg) 1st trimester | 0.74 ± 0.15 | 0.82 ± 0.18 | 0.044 |
| Total insulin dose (IU/kg) 2nd trimester | 0.75 ± 0.18 | 0.84 ± 0.21 | 0.045 |
| Total insulin dose (IU/kg) 3rd trimester | 0.80 ± 0.13 | 0.89 ± 0.17 | 0.015 |
| Maternal vein blood (serum and plasma) measurements in 1st trimester of pregnancy | |||
| HbA1c % (mmol/mol) | 6.8 ± 1.3 ** (51) | 6.7 ± 1.0 ** (50) | 0.296 |
| Fasting C-peptide (pmol/L) | 180.0 * (90.0–230.0) | 50.0 (30.0–70.0) | 0.005 |
| Fasting glucose (mmol/L) | 5.1 ± 1.9 | 5.0 ± 2.0 | 0.870 |
| Mean glucose concentration (mmol/L) | 5.9 ± 1.4 | 5.1 ± 0.9 | 0.004 |
| Maternal vein blood (serum and plasma) measurements in 2nd trimester of pregnancy | |||
| HbA1c % (mmol/mol) | 5.9 ± 0.5 (41) | 5.5 ± 0.6 (37) | 0.004 |
| Fasting C-peptide (pmol/L) | 130 (80–220) | 60 (30–90) | 0.004 |
| Fasting glucose (mmol/L) | 5.0 ± 1.8 | 4.4 ± 1.8 | 0.165 |
| Mean glucose concentration (mmol/L) | 5.9 ± 1.4 | 5.1 ± 0.9 | 0.016 |
| Maternal vein blood (serum and plasma) measurements in 3rd trimester of pregnancy | |||
| HbA1c % (mmol/mol) | 6.9 ± 0.7 ** (52) | 5.8 ± 0.8 ** (40) | 0.142 |
| Fasting C-peptide (pmol/L) | 210 (130–240) * | 60 (30–90) | 0.001 |
| Fasting glucose (mmol/L) | 5.1 ± 1.4 | 4.8 ±1.8 | 0.508 |
| Mean glucose concentration (mmol/L) | 5.3 ± 1.0 | 4.6 ± 1.3 | 0.009 |
| CSBH− (n = 30) | CSBH+ (n = 54) | p | |
|---|---|---|---|
| Gestational age at delivery (weeks) | 38.5 ± 0.7 | 38.3 ± 1.1 | 0.488 |
| Birth weight (g) | 3573.0 ± 542.1 | 3449.3 ± 421.2 | 0.231 |
| Birth length (cm) | 49.6 ± 2.1 | 49.0 ± 1.8 | 0.178 |
| Ponderal index | 2.9 ± 0.2 | 2.9 ± 0.3 | 0.822 |
| Fetal macrosomia >4000 g n Yes/No (%) | 7/23 (23.3/76.7) | 5/49 (9.3/90.7) | 0.071 |
| Apgar score at 1 min | 9.7 ± 1.0 | 9.9 ± 0.4 | 0.217 |
| Apgar score at 5 min | 9.8 ± 0.5 | 9.9 ± 0.2 | 0.157 |
| Umbilical vein serum measurements | |||
| C-peptide (pmol/L) | 580.0 (340.0–1100.0) | 850.0 (580.0–1250.0) | 0.056 |
| Umbilical vein glucose mmo/L | 4.7 ± 1.5 | 4.6 ± 1.4 | 0.428 |
| IR HOMA 2 | 1.9 (0.9–2.8) | 2.1 (1.4–2.9) | 0.492 |
| CSBH+ | Duration of T1DM | |
|---|---|---|
| DurationT1DM | 0.224 * | |
| Mean glucose concentration in 1st trimester of pregnancy | −0.375 ** | −0.073 |
| Mean glucose concentration in 2nd trimester of pregnancy | −0.256 * | −0.028 |
| Mean glucose concentration in 3rd trimester of pregnancy | −0.387 ** | −0.115 |
| C-peptide in 1st trimester of pregnancy | −0.331 ** | −0.552 ** |
| C-peptide in 2nd trimester of pregnancy | −0.332 ** | −0.564 ** |
| C-peptide in 3rd trimester of pregnancy | −0.314 ** | −0.546 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Delmis, J.; Ivanisevic, M. Awakened Beta-Cell Function Decreases the Risk of Hypoglycemia in Pregnant Women with Type 1 Diabetes Mellitus. J. Clin. Med. 2022, 11, 1050. https://doi.org/10.3390/jcm11041050
Delmis J, Ivanisevic M. Awakened Beta-Cell Function Decreases the Risk of Hypoglycemia in Pregnant Women with Type 1 Diabetes Mellitus. Journal of Clinical Medicine. 2022; 11(4):1050. https://doi.org/10.3390/jcm11041050
Chicago/Turabian StyleDelmis, Josip, and Marina Ivanisevic. 2022. "Awakened Beta-Cell Function Decreases the Risk of Hypoglycemia in Pregnant Women with Type 1 Diabetes Mellitus" Journal of Clinical Medicine 11, no. 4: 1050. https://doi.org/10.3390/jcm11041050
APA StyleDelmis, J., & Ivanisevic, M. (2022). Awakened Beta-Cell Function Decreases the Risk of Hypoglycemia in Pregnant Women with Type 1 Diabetes Mellitus. Journal of Clinical Medicine, 11(4), 1050. https://doi.org/10.3390/jcm11041050






