Impact of the Type of Dialysis on Time to Transplantation: Is It Just a Matter of Immunity?
Abstract
1. Introduction
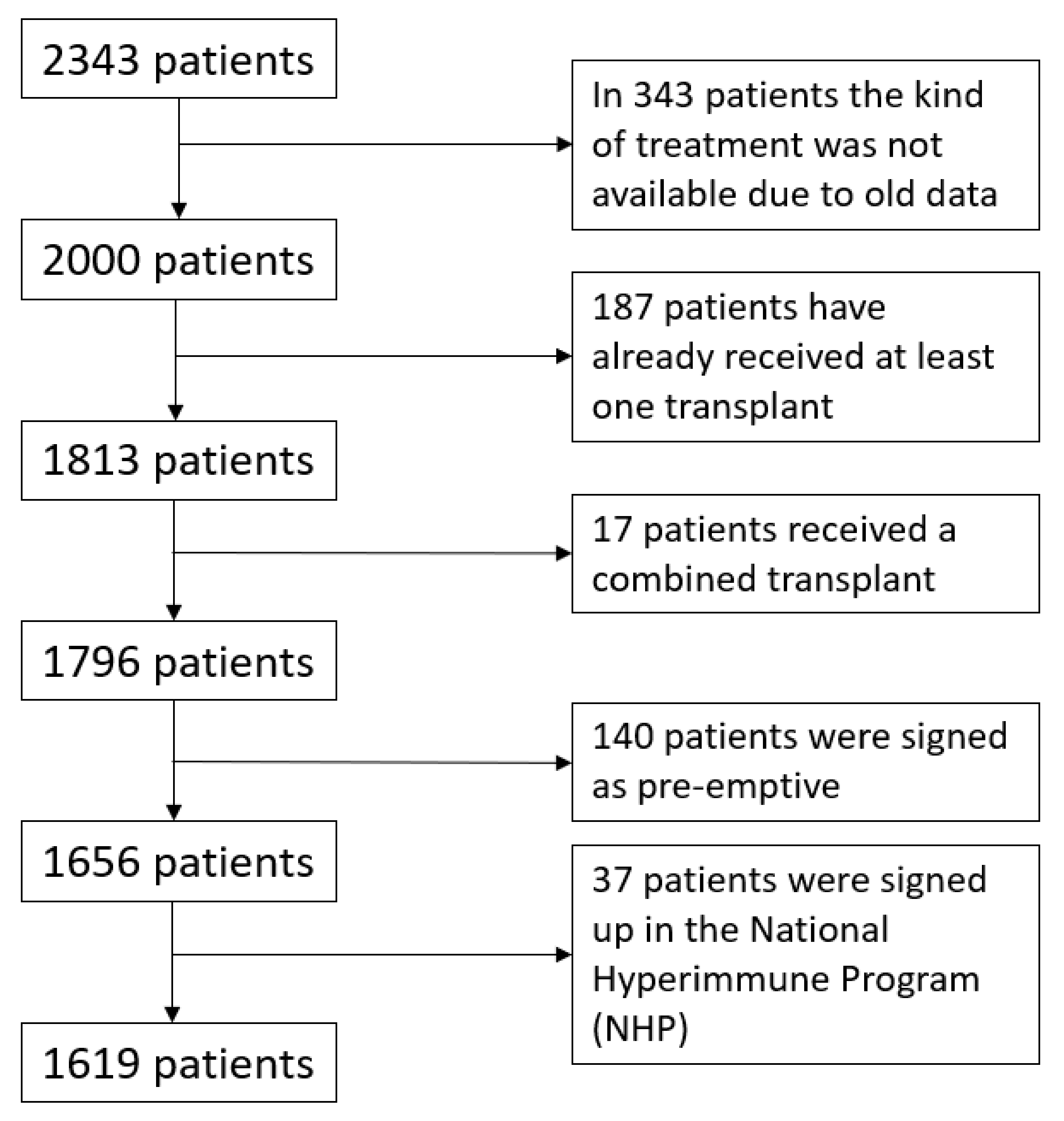
2. Materials and Methods
3. Statistical Analysis
4. Results
4.1. Age
4.2. BMI
4.3. Blood Group
4.4. Diagnosis
5. Discussion
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Desai, A.A.; Bolus, R.; Nissenson, A.; Bolus, S.; Solomon, M.D.; Khawar, O.; Gitlin, M.; Talley, J.; Spiegel, B.M. Identifying best practices in dialysis care: Results of cognitive interviews and a national survey of dialysis providers. Clin. J. Am. Soc. Nephrol. CJASN 2008, 3, 1066–1076. [Google Scholar] [CrossRef]
- Khawar, O.; Kalantar-Zadeh, K.; Lo, W.K.; Johnson, D.; Mehrotra, R. Is the declining use of long-term peritoneal dialysis justified by outcome data? Clin. J. Am. Soc. Nephrol. CJASN 2007, 2, 1317–1328. [Google Scholar] [CrossRef] [PubMed]
- Zaza, G.; Rugiu, C.; Trubian, A.; Granata, S.; Poli, A.; Lupo, A. How has peritoneal dialysis changed over the last 2015, 30 years: Experience of the Verona dialysis center. BMC Nephrol. 2015, 16, 53. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Weinhandl, E.D.; Foley, R.N.; Gilbertson, D.T.; Arneson, T.J.; Snyder, J.J.; Collins, A.J. Propensity-matched mortality comparison of incident hemodialysis and peritoneal dialysis patients. J. Am. Soc. Nephrol. 2010, 21, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Quinn, R.R.; Hux, J.E.; Oliver, M.J.; Austin, P.C.; Tonelli, M.; Laupacis, A. Selection bias explains apparent differential mortality between dialysis modalities. J. Am. Soc. Nephrol. 2011, 22, 1534–1542. [Google Scholar] [CrossRef]
- Yeates, K.; Zhu, N.; Vonesh, E.; Trpeski, L.; Blake, P.; Fenton, S. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol. Dial. Transplant. 2012, 27, 3568–3575. [Google Scholar] [CrossRef] [PubMed]
- Vonesh, E.F.; Snyder, J.J.; Foley, R.N.; Collins, A.J. The differential impact of risk factors on mortality in hemodialysis and peritoneal dialysis. Kidney Int. 2004, 66, 2389–2401. [Google Scholar] [CrossRef]
- Termorshuizen, F.; Korevaar, J.C.; Dekker, F.W.; Van Manen, J.G.; Boeschoten, E.W.; Krediet, R.T. Hemodialysis and peritoneal dialysis: Comparison of adjusted mortality rates according to the duration of dialysis: Analysis of The Netherlands Cooperative Study on the Adequacy of Dialysis 2. J. Am. Soc. Nephrol. 2003, 14, 2851–2860. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, R.A.; Ashby, V.B.; Milford, E.L.; Ojo, A.O.; Ettenger, R.E.; Agodoa, L.Y.; Held, P.J.; Port, F.K. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N. Engl. J. Med. 1999, 341, 1725–1730. [Google Scholar] [CrossRef] [PubMed]
- Port, F.K.; Wolfe, R.A.; Mauger, E.A.; Berling, D.P.; Jiang, K. Comparison of survival probabilities for dialysis patients vs. cadaveric renal transplant recipients. JAMA 1993, 270, 1339. [Google Scholar] [CrossRef]
- Gaston, R.S.; Danovitch, G.M.; Adams, P.L.; Wynn, J.J.; Merion, R.M.; Deierhoi, M.H.; Metzger, R.A.; Cecka, J.M.; Harmon, W.E.; Leichtman, A.B.; et al. The report of a national conference on the wait list for kidney transplantation. Am. J. Transplant. 2003, 3, 775. [Google Scholar] [CrossRef]
- Held, P.J.; Turenne, M.N.; Liska, D.W.; Zobel, D.L.; Webb, R.L.; Alexander, S.R.; Jones, C. Treatment modality patterns and transplantation among the United States pediatric end-stage renal disease population: A longitudinal study. Clin. Transplant. 1991, 71–85. [Google Scholar] [PubMed]
- Snyder, J.J.; Kasiske, B.L.; Gilbertson, D.T.; Collins, A.J. A comparison of transplant outcomes in peritoneal and hemodialysis patients. Kidney Int. 2002, 62, 1423–1430. [Google Scholar] [CrossRef] [PubMed]
- Sanfilippo, F.P.; Vaughn, W.K.; Peters, T.G.; Shield, C.F.; Adams, P.L.; Lorber, M.I.; Williams, G.M. Factors affecting the waiting time of cadaveric kidney transplant candidates in the USA. JAMA 1992, 267, 247. [Google Scholar] [CrossRef] [PubMed]
- Rigoni, M.; Torri, E.; Nollo, G.; Zarantonello, D.; Laudon, A.; Sottini, L.; Guarrera, G.M.; Brunori, G. Survival and time-to-transplantation of peritoneal dialysis versus hemodialysis for ESRD patients: Competing-risks regression model in a single Italian center experience. J. Nephrol. 2017, 30, 441–447. [Google Scholar] [CrossRef]
- Report CNT. 2019. Available online: http://www.trapianti.salute.gov.it/ (accessed on 11 November 2021).
- Kaplan, E.L.; Meier, P. Nonparametric estimation for incomplete observations. J. Am. Statist. Assoc. 1958, 53, 457–481. [Google Scholar] [CrossRef]
- Bostock, I.C.; Alberu, J.; Arvizu, A.; Hernández-Mendez, E.A.; De-Santiago, A.; Gonzalez-Tableros, N.; López, M.; Castelán, N.; Contreras, A.G.; Morales-Buenrostro, L.E.; et al. Probability of deceased donor kidney transplantation based on % PRA. Transpl. Immunol. 2013, 28, 154–158. [Google Scholar] [CrossRef]
- Abecassis, M.; Bartlett, S.T.; Collins, A.J.; Davis, C.L.; Delmonico, F.L.; Friedewald, J.J.; Hays, R.; Howard, A.; Jones, E.; Leichtman, A.B.; et al. Kidney transplantation as primary therapy for end-stage renal disease: A National Kidney Foundation/Kidney Disease Outcomes Quality Initiative (NKF/KDOQITM) conference. Clin. J. Am. Soc. Nephrol. 2008, 3, 471–480. [Google Scholar] [CrossRef]
- Knoll, G.; Cockfield, S.; Blydt-Hansen, T.; Baran, D.; Kiberd, B.; Landsberg, D.; Rush, D.; Cole, E. Canadian Society of transplantation consensus guidelines on eligibility for kidney transplantation. CMAJ 2005, 173, S1–S25. [Google Scholar] [CrossRef]
- Huang, Y.; Samaniego, M. Preemptive kidney transplantation: Has it come of age? Nephrol. Ther. 2012, 8, 428–432. [Google Scholar] [CrossRef]
- Moist, L.M.; Port, F.K.; Orzol, S.M.; Young, E.W.; Ostbye, T.; Wolfe, R.A.; Hulbert-Shearon, T.; Jones, C.A.; Bloembergen, W.E. Predictors of loss of residual renal function among new dialysis patients. J. Am. Soc. Nephrol. 2000, 11, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Heaf, J.G.; Wehberg, S. Relative survival of peritoneal Dialysis and Hemodialysis Patients: Effect of Cohort and Mode of Dialysis Initiation. PLoS ONE 2014, 9, e90119. [Google Scholar] [CrossRef] [PubMed]
- Martins, L.S.; Malheiro, J.; Pedroso, S.; Almeida, M.; Dias, L.; Henriques, A.C.; Silva, D.; Davide, J.; Cabrita, A.; Noronha, I.L.; et al. Pancreas-kidney transplantation: Impact of dialysis modality on the outcome. Transpl. Int. 2015, 28, 972–979. [Google Scholar] [CrossRef] [PubMed]
- López-Oliva, M.O.; Rivas, B.; Pérez-Fernández, E.; Ossorio, M.; Ros, S.; Chica, C.; Aguilar, A.; Bajo, M.A.; Escuin, F.; Hidalgo, L.; et al. Pretransplant peritoneal dialysis relative to hemodialysis improves long-term survival of kidney transplant patients: A single-center observational study. Int. Urol. Nephrol. 2014, 46, 825–832. [Google Scholar] [CrossRef]
- Tang, M.; Li, T.; Liu, H. A comparison of transplant outcomes in peritoneal and hemodialysis patients: A meta-analysis. Blood Purif. 2016, 42, 170–176. [Google Scholar] [CrossRef]
- Dipalma, T.; Fernández-Ruiz, M.; Praga, M.; Polanco, N.; González, E.; Gutiérrez-Solis, E.; Gutiérrez, E.; Andrés, A. Pre-transplant dialysis modality does not influence short- or long-term outcome in kidney transplant recipients: Analysis of paired kidneys from the same deceased donor. Clin. Transplant. 2016, 30, 1097–1107. [Google Scholar] [CrossRef]
- Kramer, A.; Jager, K.J.; Fogarty, D.G.; Ravani, P.; Finne, P.; Pérez-Panadés, J.; Prütz, K.G.; Arias, M.; Heaf, J.G.; Wanner, C.; et al. Association between pre-transplant dialysis modality and patient and graft survival after kidney transplantation. Nephrol. Dial. Transplant. 2012, 27, 4473–4480. [Google Scholar] [CrossRef][Green Version]
- Molnar, M.Z.; Mehrotra, R.; Duong, U.; Bunnapradist, S.; Lukowsky, L.R.; Krishnan, M.; Kovesdy, C.P.; Kalantar-Zadeh, K. Dialysis modality and outcomes in kidney transplant recipients. Clin. J. Am. Soc. Nephrol. 2012, 7, 332–341. [Google Scholar] [CrossRef] [PubMed]
- Goldfarb-Rumyantzev, A.S.; Hurdle, J.F.; Scandling, J.D.; Baird, B.C.; Cheung, A.K. The role of pretransplantation renal replacement therapy modality in kidney allograft and recipient survival. Am. J. Kidney Dis. 2005, 46, 537–549. [Google Scholar] [CrossRef] [PubMed]
- Schwenger, V.; Döhler, B.; Morath, C.; Zeier, M.; Opelz, G. The role of pretransplant dialysis modality on renal allograft outcome. Nephrol. Dial. Transplant. 2011, 26, 3761–3766. [Google Scholar] [CrossRef]
- Katznelson, S.; Bhaduri, S.; Cecka, J.M. Clinical aspects of sensitization. Clin. Transpl. 1997, 285–296. [Google Scholar] [PubMed]
- Barama, A.; Oza, U.; Panek, R.; Belitsky, P.; MacDonald, A.S.; Lawen, J.; McAlister, V.; Kiberd, B. Effect of recipient sensitization (peak PRA) on graft outcome in haploidentical living related kidney transplants. Clin. Transplant. 2000, 14, 212–217. [Google Scholar] [CrossRef]
- Gebel, H.M.; Bray, R.A.; Nickerson, P. Pre-transplant assessment of donor-reactive, HLA-specific antibodies in renal transplantation: Contraindication vs. risk. Am. J. Transpl. 2003, 3, 1488–1500. [Google Scholar] [CrossRef]
- Tambur, A.R.; Leventhal, J.R.; Walsh, R.C.; Zitzner, J.R.; Friedewald, J.J. HLA- DQ barrier: Effects on cPRA calculations. Transplantation 2013, 96, 1065–1072. [Google Scholar] [CrossRef] [PubMed]
- Sapir-Pichhadze, R.; Tinckam, K.J.; Laupacis, A.; Logan, A.G.; Beyene, J.; Kim, S.J. Immune sensitization and mortality in Wait-Listed Kidney Transplant Candidates. J. Am. Soc. Nephrol. 2016, 27, 570–578. [Google Scholar] [CrossRef]
- Registry, A. ANZDATA Report 2011 Chapter 8 Transplantation. 2012. Available online: https://www.anzdata.org.au/report/anzdata-35th-annual-report-2012/ (accessed on 11 November 2021).
- Faravardeh, A.; Eickhoff, M.; Jackson, S.; Spong, R.; Kukla, A.; Issa, N.; Matas, A.J.; Ibrahim, H.N. Predictors of graft failure and death in elderly kidney transplant recipients. Transplantation 2013, 96, 1089. [Google Scholar] [CrossRef] [PubMed]
- Lim, W.H.; Chapman, J.R.; Wong, G. Peak panel reactive antibody, cancer, graft, and patient outcomes in kidney transplant recipients. Transplantation 2015, 99, 1043–1050. [Google Scholar] [CrossRef]
- Wetmore, J.B.; Peng, Y.; Monda, K.L.; Kats, A.M.; Kim, D.H.; Bradbury, B.D.; Collins, A.J.; Gilbertson, D.T. Trends in anemia management practices in patients receiving hemodialysis and peritoneal dialysis: A retro- spective cohort analysis. Am. J. Nephrol. 2015, 41, 354–361. [Google Scholar] [CrossRef]
- Hoogeveen, E.K.; Aalten, J.; Rothman, K.J.; Roodnat, J.I.; Mallat, M.J.; Borm, G.; Weimar, W.; Hoitsma, A.J.; de Fijter, J.W. Effect of Obesity on the Outcome of Kidney Transplantation: A 20-Year Follow-Up. Transplantation 2011, 91, 869–874. [Google Scholar] [CrossRef]
- Streja, E.; Molnar, M.Z.; Kovesdy, C.P.; Bunnapradist, S.; Jing, J.; Nissenson, A.R.; Mucsi, I.; Danovitch, G.M.; Kalantar-Zadeh, K. Associations of Pretransplant Weight and Muscle Mass with Mortality in Renal Transplant Recipients. Clin. J. Am. Soc. Nephrol. 2011, 6, 1463–1473. [Google Scholar] [CrossRef]
- Chan, W.L.W.; Bosch, J.A.; Jones, D.; McTernan, P.; Philips, A.; Borrows, R. Obesity in kidney transplantation. J. Ren. Nutr. 2014, 24, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Abedini, S.; Holme, I.; März, W.; Weihrauch, G.; Fellström, B.; Jardine, A.; Cole, E.; Maes, B.; Neumayer, H.H.; Grønhagen-Riska, C.; et al. Inflammation in Renal Transplantation. Clin. J. Am. Soc. Nephrol. 2009, 4, 1246–1254. [Google Scholar] [CrossRef] [PubMed]
- Kaisar, M.O.; Armstrong, K.; Hawley, C.; Campbell, S.; Mudge, D.; Johnson, D.W.; Prins, J.B.; Isbel, N.M. Adiponectin is associated with cardiovascular disease in male renal transplant recipients: Baseline results from the LANDMARK 2 study. BMC Nephrol. 2009, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Winkelmayer, W.C.; Schaeffner, E.S.; Chandraker, A.; Kramar, R.; Rumpold, H.; Sunder-Plassmann, G.; Födinger, M. A J-shaped association between high- sensitivity C-reactive protein and mortality in kidney transplant recipients. Transplant. Int. 2007, 20, 505–511. [Google Scholar] [CrossRef]
- Chanouzas, D.; Ng, K.P.; Fallouh, B.; Baharani, J. What influ- ences patient choice of treatment modality at the pre-dialysis stage? Nephrol. Dial. Transpl. 2012, 27, 1542–1547. [Google Scholar] [CrossRef]
- Frei, U.; Noeldeke, J.; Machold-Fabrizii, V.A.; Arbogast, H.; Margreiter, R.; Fricke, L.; Voiculescu, A.; Kliem, V.; Ebel, H.; Albert, U.; et al. Prospective age- matching in elderly kidney transplant recipients—A 5-year analysis of Eurotransplant Senior Program. Am. J. Transplant. 2008, 8, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Neri, F.; Furian, L.; Cavallin, F.; Ravaioli, M.; Silvestre, C.; Donato, P.; La Manna, G.; Pinna, A.D.; Rigotti, P. How does age affect the outcome of kidney transplantation in elderly recipients? Clin. Transplant. 2017, 31, e13036. [Google Scholar] [CrossRef]
- United States Renal Data System. 2020 USRDS Annual Data Report: Epidemiology of Kidney Disease in the United States; National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2020. [Google Scholar]
- Chang, P.; Gill, J.; Dong, J.; Rose, C.; Yan, H.; Landsberg, D.; Cole, E.H.; Gill, J.S. Living donor age and kidney allograft half-life: Implications for living donor paired exchange programs. Clin. J. Am. Soc. Nephrol. 2012, 7, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Phelan, P.J.; O’Kelly, P.; O’Neill, D.; Little, D.; Hickey, D.; Keogan, M.; Walshe, J.; Magee, C.; Conlon, P.J. Analysis of waiting times on Irish renal Transplant list. Clin. Transpl. 2010, 24, 381–385. [Google Scholar] [CrossRef]
- Ng, M.S.Y.; Ullah, S.; Wilson, G.; McDonald, S.; Sypek, M.; Mallett, A.J. ABO blood group relationships to kidney transplant recipient and graft outcomes. PLoS ONE 2020, 15, e0236396. [Google Scholar] [CrossRef] [PubMed]


| Population | Hemodialysis | Peritoneal Dialysis | p Value | |
|---|---|---|---|---|
| No. of patients | 1619 | 1347 (83.2%) | 271 (16.8%) | |
Sex
| 1053 (65%) 565 (35%) | 898 (66.7%) 449 (33.3%) | 155 (57.2%) 116 (42.8%) | 0.002 * |
| Age (years) | 61.9 ± 12.4 | 61.9 ± 12.3 | 62.2 ± 12.5 | ns |
Blood group
| 651 (40.2%) 179 (11.1%) 76 (4.7%) 705 (43.5%) | 533 (39.6%) 150 (11.1%) 54 (4%) 605 (44.9%) | 118 (43.5%) 29 (10.7%) 22 (8.1%) 100 (36.9%) | 0.007 * |
| BMI | 24.1 ± 3.5 | 23.9 ± 3.5 | 24.7 ± 3.4 | 0.001 * |
| Diabetes | 214 (13.2%) | 182 (13.5%) | 32 (11.8%) | ns |
| Hypertension | 554 (34.2%) | 452 (33.6%) | 102 (37.6%) | ns |
| Cardiovascular disease | 79 (4.9%) | 56 (4.2%) | 23 (8.5%) | ns |
| Neoplastic disease | 18 (1.1%) | 15 (1.1%) | 3 (1.1%) | ns |
Nephropathy
| 433 (26.7%) 169 (10.4%) 161 (9.9%) 328 (20.3%) 153 (9.4%) 374 (23.1%) | 366 (27.2%) 138 (10.2%) 127 (9.4%) 284 (21.1%) 126 (9.3%) 306 (22.7%) | 67 (24.7%) 31 (11.4%) 34 (12.5%) 44 (16.2%) 27 (10%) 68 (25.1%) | ns |
| Prior time of dialysis until listing for transplantation Med [IQR] | 354.5 [108.6] days | 383 [118.3] days | 225 [271.8] days | ns |
| HCV IgG | 61 (3.8%) | 58 (4.3%) | 3 (1.1%) | ns |
| HBsAb | 901 (55.6%) | 753 (55.9%) | 148 (54.6%) | ns |
| HbcAb | 220 (13.6%) | 186 (13.8%) | 34 (12.5%) | ns |
| CMV IgG | 1037 (64.1%) | 839 (62.3%) | 198 (73.1%) | ns |
PRA max (%)
| 477 (29.5%) 472 (29.1%) 258 (15.9%) 157 (9.7%) | 350 (26%) 395 (29.3%)230 (17.1%) 145 (10.8%) | 127 (46.9%) 77 (28.4%) 28 (10.3%) 12 (4.4%) |
| Source | LogWorth | p Value | |
|---|---|---|---|
| PRAmax | 17.382 |  | 0.00000 |
| Blood group | 13.148 | 0.00000 | |
| Type of dialysis | 4.808 | 0.00002 | |
| Antibodies Number | 4.080 | 0.00008 | |
| Age | 1.332 | 0.04654 | |
| CMV IgG | 0.611 | 0.24496 | |
| Diagnosis | 0.200 | 0.63037 | |
| BMI | 0.187 | 0.65032 | |
| Sex | 0.037 | 0.91757 | |
| HBsAb | 0.023 | 0.94883 |
| Source | LogWorth | p Value | |
|---|---|---|---|
| Type of dialysis | 2.831 |  | 0.00148 |
| Blood group | 2.759 | 0.00174 | |
| BMI | 1.733 | 0.01848 | |
| Age | 0.989 | 0.10251 | |
| HBsAb | 0.959 | 0.11001 | |
| CMV IgG | 0.249 | 0.56355 |
| Source | LogWorth | p Value | |
|---|---|---|---|
| Age | 2.771 |  | 0.00169 |
| Type of dialysis | 1.985 | 0.01034 | |
| BMI | 0.812 | 0.15432 | |
| HBsAb | 0.784 | 0.16447 | |
| Blood Group | 0.407 | 0.39161 | |
| CMV IgG | 0.197 | 0.63481 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righini, M.; Capelli, I.; Busutti, M.; Raimondi, C.; Comai, G.; Donati, G.; Cappuccilli, M.L.; Ravaioli, M.; Chieco, P.; La Manna, G. Impact of the Type of Dialysis on Time to Transplantation: Is It Just a Matter of Immunity? J. Clin. Med. 2022, 11, 1054. https://doi.org/10.3390/jcm11041054
Righini M, Capelli I, Busutti M, Raimondi C, Comai G, Donati G, Cappuccilli ML, Ravaioli M, Chieco P, La Manna G. Impact of the Type of Dialysis on Time to Transplantation: Is It Just a Matter of Immunity? Journal of Clinical Medicine. 2022; 11(4):1054. https://doi.org/10.3390/jcm11041054
Chicago/Turabian StyleRighini, Matteo, Irene Capelli, Marco Busutti, Concettina Raimondi, Giorgia Comai, Gabriele Donati, Maria Laura Cappuccilli, Matteo Ravaioli, Pasquale Chieco, and Gaetano La Manna. 2022. "Impact of the Type of Dialysis on Time to Transplantation: Is It Just a Matter of Immunity?" Journal of Clinical Medicine 11, no. 4: 1054. https://doi.org/10.3390/jcm11041054
APA StyleRighini, M., Capelli, I., Busutti, M., Raimondi, C., Comai, G., Donati, G., Cappuccilli, M. L., Ravaioli, M., Chieco, P., & La Manna, G. (2022). Impact of the Type of Dialysis on Time to Transplantation: Is It Just a Matter of Immunity? Journal of Clinical Medicine, 11(4), 1054. https://doi.org/10.3390/jcm11041054








