Influence of Photodynamic Therapy on Lichen Sclerosus with Neoplastic Background
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Clinical impact of Photodynamic Therapy
3.1.1. Objective Benefits of Photodynamic Therapy
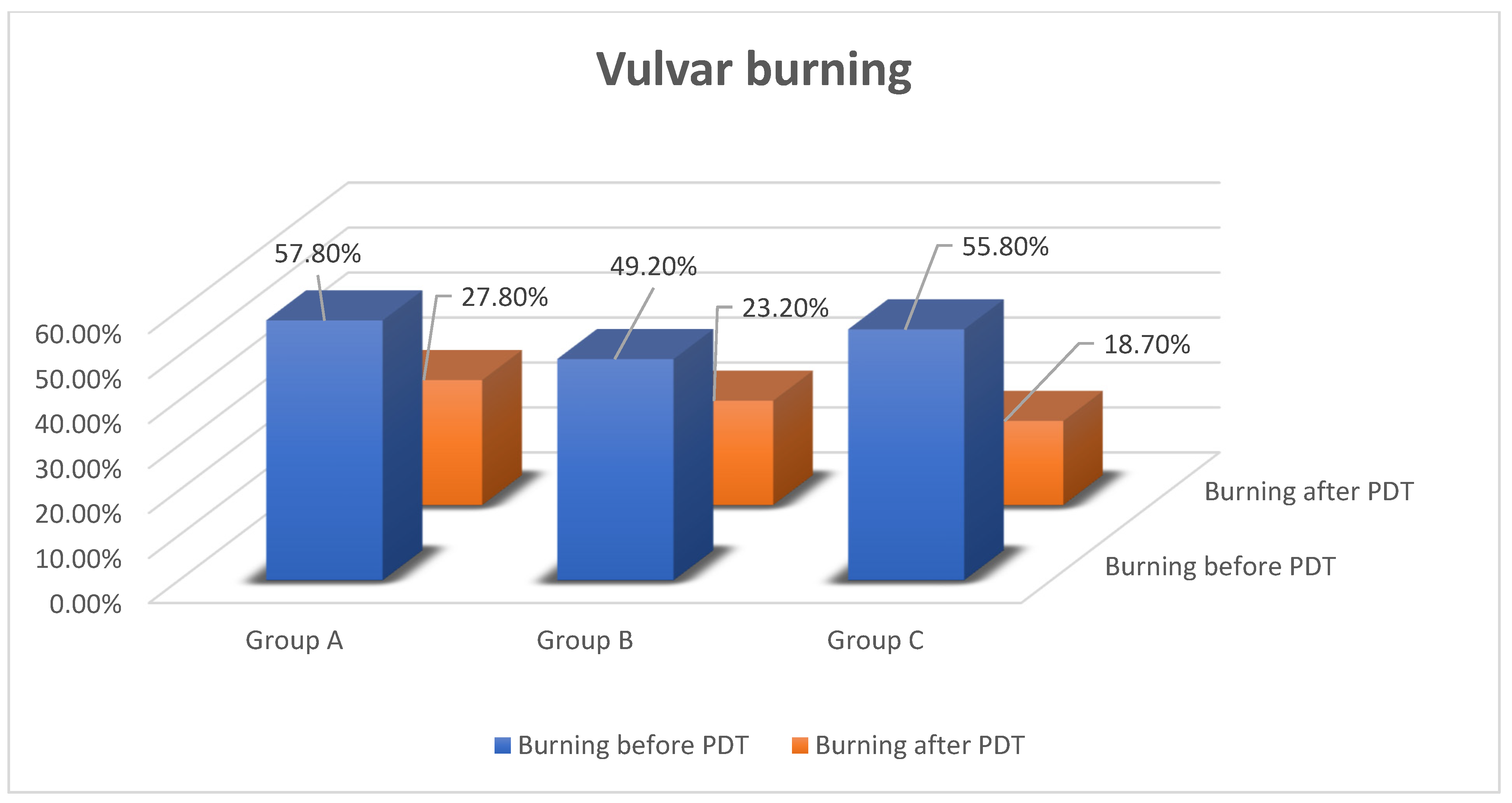
3.1.2. Subjective Benefits of PDT
3.1.3. Quality of Life
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Christensen, A.; Haugsdal, M.; Bowdler, N. Importance of the physical exam and in-office tests in the evaluation of vulvovaginal irritation. Proc. Obstet. Gynecol. 2014, 4, 11. [Google Scholar] [CrossRef]
- Bowen, A.; Vester, A.; Marsden, L.; Florell, S.; Sharp, H.; Summers, P. The role of vulvar skin biopsy in the evaluation of chronic vulvar pain. Am. J. Obstet. Gynecol. 2008, 199, 467.e1–467.e6. [Google Scholar] [CrossRef]
- Perez-Lopez, F.R.; Vieira-Baptista, P. Lichen sclerosus in women: A review. Climacteric 2017, 20, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Micheletti, L.; Preti, M.; Radici, G. Vulvar lichen sclerosus and neoplastic transformation: A retrospective study of 976 cases. J. Low. Genit. Tract Dis. 2016, 20, 180–183. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.; Bradford, J.; Fischer, G. Long-term Management of adult vulvar lichen sclerosus: A prospective cohort study of 507 women. JAMA Dermatol. 2015, 151, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp, C.A.; van den Hondel, K.E.; Korfage, I.J.; Gestem, M.J.; Meijden, W.I. Quality of life in Dutch women with lichen sclerosus. Br. J. Dermatol. 2013, 168, 787–793. [Google Scholar] [CrossRef]
- Singh, N.; Ghatage, P. Etiology, clinical features, and diagnosis of vulvar lichen sclerosus: A scoping review. Obstet. Gynecol. Int. 2020, 2020, 7480754. [Google Scholar] [CrossRef]
- Powell, J.J.; Wojnarowska, F. Lichen sclerosus. Semin. Lancet 1999, 353, 1777–1783. [Google Scholar] [CrossRef]
- Lacarrubba, F.; Pellacani, G.; Verzi, A.E.; Pippione, M.; Micali, G. Extragenital lichen sclerosus: Clinical, dermoscopic, confocal microscopy and histologic correlations. J. Am. Acad. Dermatol. 2015, 72, 50–52. [Google Scholar] [CrossRef]
- Olek-Hrab, K.; Jenerowicz, D.; Osmola-Mańkowska, A.; Polańska, A.; Teresiak-Mikołajczak, E.; Silny, W.; Adamski, Z. Wybrane dermatozy sromu. Ginekol. Pol. 2013, 84, 959–965. [Google Scholar]
- Fistarol, S.; Itin, P. Diagnosis and treatment of lichen sclerosus. Am. J. Clin. Dermatol. 2013, 14, 27–47. [Google Scholar] [CrossRef] [PubMed]
- Aslanian, F.; Marques, M.; Matos, H.; Pontes, L.; Porto, L.; Azevedo, L.; Filgueira, A. HLA markers in familial lichen sclerosus. JDDG J. Dtsch. Dermatol. Ges. 2006, 4, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Olejek, A.; Stęplewska, K.; Gabriel, A.; Kozak-Darmas, I.; Jarek, A.; Kellas-Ślęczka, S.; Bydliński, F.; Sieroń-Stołtny, K.; Horak, S.; Chełmicki, A.; et al. Efficacy of photodynamic therapy in vulvar lichen sclerosus treatment based on immunohistochemical analysis of CD34, CD44, myelin basic protein and Ki67 antibodies. Int. J. Gynecol. Cancer 2010, 20, 879–887. [Google Scholar] [CrossRef] [PubMed]
- Olejek, A.; Kozak-Darmas, I.; Olszak-Wąsik, K. Ocena stężenia przeciwciał przeciwtarczycowych, miana przeciwciał przeciwjądrowych oraz obecności przeciwciał przeciwko Borrelia burgdorferi w grupie pacjentek leczonych terapią fotodynamiczną z powodu liszaja twardzinowego sromu. Prz. Menopauzalny 2012, 4, 275–280. [Google Scholar]
- Edmonds, E.; Mavin, S.; Francis, N.; Ho-Yen, D.; Bunker, C. Borrelia burgdorferi is not associated with genital lichen sclerosus in men. Br. J. Dermatol. 2009, 160, 459–460. [Google Scholar] [CrossRef] [PubMed]
- Hald, A.; Blaakaer, J. The possible role of human papillomavirus infection in the development of lichen sclerosus. Int. J. Dermatol. 2018, 57, 139–146. [Google Scholar] [CrossRef]
- Wakeham, K.; Kavanagh, K.; Cuschieri, K.; Millan, D.; Pollock, K.G.; Bell, S.; Burton, K.; Reed, N.S.; Graham, S.V. HPV status and favourable outcome in vulvar squamous cancer. Int. J. Cancer 2017, 140, 1134–1146. [Google Scholar] [CrossRef]
- Vilmer, C.; Cavelier-Balloy, B.; Nogues, C.; Trassard, M.; Le Doussal, V. Analysis of laterations adjacent to invasive vulvar carcinoma and their relationship with the associated carcinoma: A study of 67 cases. Eur. J. Gynaecol. Oncol. 1998, 19, 25–31. [Google Scholar]
- Hoang, L.H.; Park, K.J.; Soslow, R.A.; Murali, R. Squamous precursor lesions of the vulva: Current classification and diagnostic challenges. Pathology 2016, 48, 291–302. [Google Scholar] [CrossRef]
- Sadalla, J.C.; Lourenco, S.V.; Sotto, M.N.; Baracat, E.C.; Carvalho, J.P. Claudin and p53 expression in vulvar lichen sclerosus and squamous-cell carcinoma. J. Clin. Pathol. 2011, 64, 853–857. [Google Scholar] [CrossRef]
- Raspollini, M.R.; Asirelli, G.; Taddei, G.L. The role of angiogenesis and COX-2 expression in the evolution of vulvar lichen sclerosus to squamous cell carcinoma of the vulva. Gynecol. Oncol. 2007, 106, 567–571. [Google Scholar] [CrossRef]
- Trietsch, M.D.; Nooij, L.S.; Gaarenstroom, K.N.; van Poelgeest, M.I.E. Genetic and epigenetic changes in vulvar squamous cell carcinoma and its precursor lesions: A review of the current literature. Gynecol. Oncol. 2015, 136, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Khunnarong, J.; Tangjitgamol, S.; Srijaipracharoen, S. Other gynecologic pathology in endometrial cancer patients. Asian Pac. J. Cancer Prev. 2016, 17, 713–717. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Han, J.H.; Choi, H.-K.; Kim, S.J. Topical TRPM8 agonist (Icilin) relieved vulva pruritus originating from lichen sclerosus et atrophicus. Acta Derm. Venereol. 2012, 92, 449–581. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.D.; Chen, C.-Y.; Huang, H.J.; Wang, T.Y.; Teng, D.; Huang, S.H.; Lai, C.H.; Chen, M.C. Increased risk of second primary malignancies following uterine cancer: A population-based study in Taiwan over a 30-year period. BMC Cancer 2015, 15, 393. [Google Scholar] [CrossRef]
- Borghi, A.; Corazza, M. Novel therapeutic approaches and targets for treatment of vulvar lichen sclerosus. Curr. Pharm. Biotechnol. 2021, 22, 99–114. [Google Scholar] [CrossRef]
- Mautz, T.; Krapf, J.; Goldstein, A. Topical corticosteroids in the treatment of vulvar lichen sclerosus: A review of pharmacokinetics and recommended dosing frequencies. Sex. Med. Rev. 2021, 10, 42–52. [Google Scholar] [CrossRef]
- Krapf, J.; Mitchell, L.; Holton, M.; Goldstein, A. Review Vulvar Lichen Sclerosus: Current Perspectives. Int. J. Women’s Health 2020, 12, 11–20. [Google Scholar] [CrossRef]
- Biniszkiewicz, T.; Olejek, A.; Kozak-Darmas, I.; Sieroń, A. Therapeutic effects of 5-ALA-induced photodynamic therapy in vulvar lichen sclerosus. Photodiagn. Photodyn. Ther. 2005, 2, 157–160. [Google Scholar] [CrossRef]
- Szeimies, R.M.; Landthaler, M.; Karrer, S. Non-oncologic indications for ALA-PDT. J. Dermatol. Treat. 2002, 13, 13–18. [Google Scholar] [CrossRef]
- Neill, S.M.; Lewis, F.M.; Tatnall, F.M.; Cox, N.H. British Association of Dermatologists’ guidelines for the management of lichen sclerosus 2010. Br. J. Dermatol. 2010, 163, 672–682. [Google Scholar] [CrossRef] [PubMed]
- Wines, N.; Willsteed, E. Menopause and the skin. Australas. J. Dermatol. 2001, 42, 149–160. [Google Scholar] [CrossRef]
- Renaud-Vilmer, C.; Cavelier-Balloy, B.; Porcher, R.; Dobertret, L. Vulvar lichen sclerosus. Arch. Dermatol. 2004, 6, 140. [Google Scholar] [CrossRef] [PubMed]
- Cruickshank, M.E.; Hay, I. The management of vulval skin disorders. RCOG GreenTop Guidelines 2011, 58, 1–23. [Google Scholar]
- Mitra, A.; Stables, G.I. Topical photodynamic therapy for non-cancerous skin conditions (Review). Photodiagn. Photodyn. Ther. 2006, 3, 116–127. [Google Scholar] [CrossRef]
- Buggiani, G.; Troiano, M.; Rossi, R.; Lotti, T. Photodynamic therapy: Off-label and alternative use in dermatological practice. Photodiagn. Photodyn. Ther. 2008, 5, 134–138. [Google Scholar] [CrossRef] [PubMed]
- Prodromidou, A.; Chatziioannou, E.; Daskalakis, G.; Stergios, K.; Pergialiotis, V. Photodynamic Therapy for vulvar lichen sclerosus—A systematic review. J. Low. Genit. Tract Dis. 2018, 22, 58–65. [Google Scholar] [CrossRef]
- Li, Z.; Wang, Y.; Wang, J.; Li, S.; Xiao, Z.; Feng, Y.; Gu, J.; Li, J.; Peng, X.; Li, C.; et al. Evaluation of the efficacy of 5-aminolevulinic acid photodynamic therapy for the treatment of vulvar lichen sclerosus. Photodiagn. Photodyn. Ther. 2020, 29, 101596. [Google Scholar] [CrossRef] [PubMed]
- Pragya, A.N. Dermatosis associated with menopause. J. Midlife Health 2014, 5, 168–175. [Google Scholar]
- Lagerstedt, M.; Huotari-Orava, R.; Nyberg, R.; Maenpaa, J.U.; Snellman, E.; Laasanen, S.-L. Reduction in ERRα is associated with lichen sclerosus and vulvar squamous cell carcinoma. Gynecol. Oncol. 2015, 139, 536–540. [Google Scholar] [CrossRef]
- Higgins, C.A.; Cruickshank, M.E. A population-based case-control study of aetiological factors associated with vulval lichen sclerosus. J. Obstet. Gynaecol. 2012, 32, 271–275. [Google Scholar] [CrossRef] [PubMed]
- Soergel, P.; Hillemanns, P. Photodynamic therapy for intraepithelial neoplasia of the lower genital tract (Review). Photodiagn. Photodyn. Ther. 2010, 7, 10–14. [Google Scholar] [CrossRef]
- Tribbia, G.; Pezzica, E.; Buzzi, A.; Sonzogni, A.; Crescini, C. Epithelial changes associated with invasive epidermoid carcinoma of the vulva. Minerva Ginecol. 1995, 47, 531–534. [Google Scholar] [PubMed]
- Hart, W.R.; Norris, H.J.; Helwig, E.B. Relation of lichen sclerosus et atrophicus of the vulva to development of carcinoma. Obstet. Gynecol. 1975, 45, 369–377. [Google Scholar] [PubMed]
- Halonen, P.; Jakobsson, M.; Heikinheimo, O.; Riska, A.; Gissler, M.; Pukkala, E. Lichen sclerosus and risk of cancer. Int. J. Cancer 2017, 140, 1998–2002. [Google Scholar] [CrossRef]
- Mac Bride, M.; Rhodes, D.J.; Shuster, L.T. Vulvovaginal atrophy. Mayo Clin. Proc. 2010, 85, 87–94. [Google Scholar] [CrossRef]
- Gao, X.H.; Barnardo, M.C.M.N.; Winsey, S.; Ahmad, T.; Cook, J.; Agudelo, J.D.; Zhai, N.; Powell, J.J.; Vuggle, S.V.; Wojnarowska, F. The association between HLA DR, DQ antigens, and vulval lichen sclerosus in the UK: HLA DRB1*12 and its associated DRB1*12/DQB1*0301/04/09/010 haplotype confers susceptibility to vulval lichen sclerosus, and HLA DRB1*0301/04 and its associated DRB1*0301/04/DQB1*0201/02/03 haplotype protects from vulval lichen sclerosus. J. Investig. Dermatol. 2005, 125, 895–899. [Google Scholar]
- Van de Nieuwenhof, H.P.; van der Avoort, I.A.M.; de Hullu, J.A. Review of squamous premalignant vulvar lesions. Crit. Rev. Oncol. Hematol. 2008, 68, 131–156. [Google Scholar] [CrossRef]
- Maździarz, A.; Osuch, B.; Kowalska, M.; Nalewczyńska, A. Photodynamic therapy in the treatment of vulvar lichen sclerosus. Photodiagn. Photodyn. Ther. 2017, 19, 135–139. [Google Scholar] [CrossRef]
- Criscuolo, A.; Schipani, C.; Cannizzaro, M.; Messinese, S.; Chimenti, S.; Piccione, E.; Saraceno, R. New therapeutic approaches in the treatment of anogenital lichen sclerosus: Does photodynamic therapy represent a novel option? G. Ital. Dermatol. Venereol. Organo Uff. Soc. Ital. Dermatol. Sifilogr. 2017, 152, 117–121. [Google Scholar] [CrossRef]
- Liu, J.; Hao, J.; Wang, Y.; Liu, Y.; Xu, T. Clinical and dermoscopic assessment of vulvar lichen sclerosus after 5-aminolevulinic acid Photodynamic Therapy: A prospective study. Photodiagn. Photodyn. Ther. 2021, 33, 102109. [Google Scholar] [CrossRef] [PubMed]
- Sheinis, M.; Selk, A. Development of the adult vulvar lichen sclerosus severity scale—A Delphi Consensus Exercise for Item Generation. J. Low. Genit. Tract Dis. 2018, 22, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Olejek, A.; Sieroń-Stołtny, K.; Kozak-Darmas, I.; Kellas-Ślęczka, S.; Stęplewska, K.; Biniszkiewicz, T.; Birkner, B.; Zamłyński, J.; Nowak, L.; Sieroń, A. Terapia fotodynamiczna w leczeniu liszaja twardzinowego sromu. Prz. Menopauzalny 2009, 5, 257–260. [Google Scholar]
- Osiecka, B.J.; Jurczyszyn, K.; Nockowski, P.; Murawski, M.; Ziółkowski, P. Photodynamic therapy with green light for the treatment of vulvar lichen sclerosus–preliminary results. Photodiagn. Photodyn. Ther. 2017, 17, 185–187. [Google Scholar] [CrossRef]
- Tampa, M.; Sarbu, M.; Matei, C.; Mitran, C.; Mitran, M.; Caruntu, C.; Constantin, C.; Neagu, M.; Georgescu, S. Photodynamic therapy: A hot topic in dermato-oncology (Review). Oncol. Lett. 2019, 17, 4058–4093. [Google Scholar] [CrossRef]
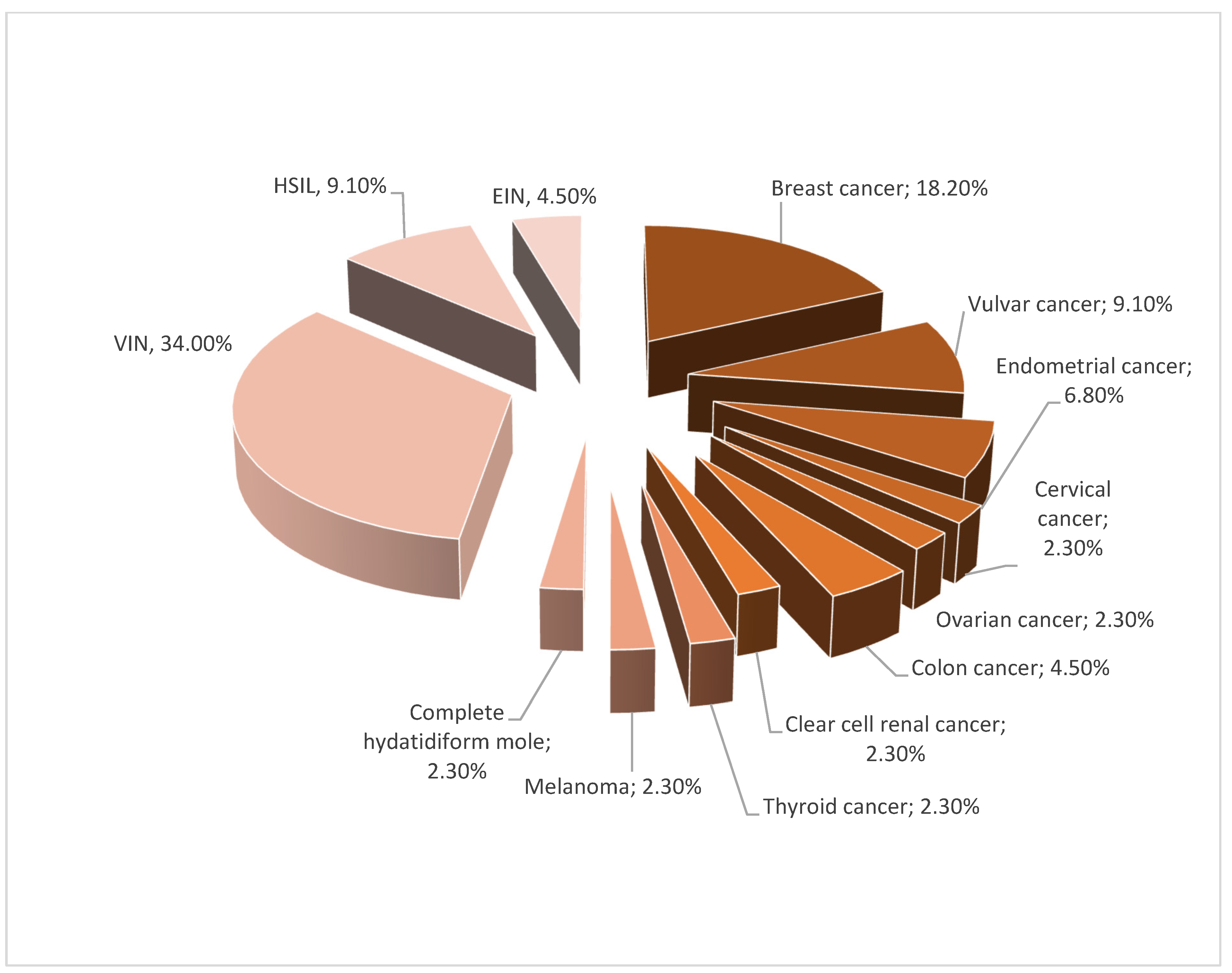




| Inclusion Criteria | Exclusion Criteria | |
|---|---|---|
| 1. | Lichen sclerosus histopathologically confirmed |
|
| 2. | Recurrence of vulvar symptoms more than 3 months after locally conservative therapy:
|
|
| 3. | No use of PDT before |
|
| 4. | Age above 18 years |
|
| 5. | Conscious agreement for using PDT |
|
| 6. | Previous diagnosis of neoplastic disease or neoplasia |
|
| Features | Points | |
|---|---|---|
| 1. | Vulva with no macroscopic changes | 0 |
| 2. | Reddening | |
| Present | 1 | |
| Absent | 0 | |
| 3. | Atrophy | |
| Narrowing of atrium of vagina | 1 | |
| Labia asymmetry | 1 | |
| Low degree of atrophy (labia shrunk less than 1/2) | 2 | |
| Medium degree of atrophy (labia shrunk more than 1/2) | 3 | |
| High degree of atrophy (atrophy of all vulva) | 4 | |
| 4. | Leukoplakia | |
| Absent | 0 | |
| Single focus of leukoplakia | 1 | |
| Low degree (less than ½ of vulva) | 2 | |
| Medium degree (more than ½ of vulva) | 3 | |
| High degree (all vulva affected) | 4 | |
| All vulva + groin affected | 5 | |
| 5. | Erosion | |
| Absent | 0 | |
| Low degree (less than ½ of vulva) | 1 | |
| High degree (more than ½ of vulva) | 2 | |
| 6. | Ulcers | |
| Absent | 0 | |
| Low degree (less than ½ of vulva) | 1 | |
| High degree (more than ½ of vulva) | 2 | |
| 7. | Hyperkeratosis | |
| Absent | 0 | |
| Less than ½ of vulva | 1 | |
| More than ½ of vulva | 2 | |
| 8. | Ruptures | |
| Absent | 0 | |
| Present | 1 | |
| 9. | Excoriation | |
| Absent | 0 | |
| Present | 1 | |
| 10. | Subcutaneous hemorrhages | |
| Absent | 0 | |
| Present | 2 | |
| 11. | Wounds | |
| Absent | 0 | |
| Single presentation | 3 | |
| Numerous | 5 | |
| 12. | Edema of vulva | |
| Absent | 0 | |
| Present | 3 | |
| Localizations of Vulvar Changes in Lichen Sclerosus | Points | |
|---|---|---|
| 1. | Vulva with no macroscopic changes | 0 |
| 2. | Localization of urethra | |
| Present | 1 | |
| Absent | 0 | |
| 3. | Clitoris | |
| Present | 1 | |
| Absent | 0 | |
| 4. | Vulvar vestibule | |
| Present | 1 | |
| Absent | 0 | |
| 5. | Labia minora | |
| Both-sided | 2 | |
| One-sided | 1 | |
| Absent | 0 | |
| 6. | Labia majora | |
| Both-sided | 2 | |
| One-sided | 1 | |
| Absent | 0 | |
| 7. | Perianal localization | |
| Present | 1 | |
| Absent | 0 | |
| 8. | Inguinal localization | |
| Both-sided | 2 | |
| One-sided | 1 | |
| Absent | 0 | |
| 9. | Totally affected vulva | |
| Present | 10 | |
| Absent | 0 | |
| Grade | Satisfaction of Patients after PDT |
|---|---|
| A | 70–100% improvement after PDT |
| B | 50–70% improvement after PDT |
| C | 50% improvement after PDT |
| D | 30% improvement after PDT |
| E | 10% improvement after PDT |
| N | No changes after PDT |
| S | Symptoms are more intense than before PDT |
| Affected Anus before PDT | Affected Anus after PDT | Affected Inguinal Area before PDT | Affected Inguinal Area after PDT | All Vulva Affected before PDT | All Vulva Affected after PDT | |
|---|---|---|---|---|---|---|
| Group A | 15.6% | 9.4% | 6.3% | 9.1% | 31.3% | 9.4% |
| Group B | 12.7% | 2.8% | 5.6% | 5.6% | 25.4% | 4.2% |
| Group C | 23.6% | 9.1% | 9.1% | 6.3% | 27.3% | 5.5% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bizoń, M.; Maślińska, D.; Sawicki, W. Influence of Photodynamic Therapy on Lichen Sclerosus with Neoplastic Background. J. Clin. Med. 2022, 11, 1100. https://doi.org/10.3390/jcm11041100
Bizoń M, Maślińska D, Sawicki W. Influence of Photodynamic Therapy on Lichen Sclerosus with Neoplastic Background. Journal of Clinical Medicine. 2022; 11(4):1100. https://doi.org/10.3390/jcm11041100
Chicago/Turabian StyleBizoń, Magdalena, Danuta Maślińska, and Włodzimierz Sawicki. 2022. "Influence of Photodynamic Therapy on Lichen Sclerosus with Neoplastic Background" Journal of Clinical Medicine 11, no. 4: 1100. https://doi.org/10.3390/jcm11041100
APA StyleBizoń, M., Maślińska, D., & Sawicki, W. (2022). Influence of Photodynamic Therapy on Lichen Sclerosus with Neoplastic Background. Journal of Clinical Medicine, 11(4), 1100. https://doi.org/10.3390/jcm11041100





