Severe Thrombotic Thrombocytopenic Purpura (TTP) with Organ Failure in Critically Ill Patients
Abstract
:1. Introduction
2. Epidemiology of TTP in ICU
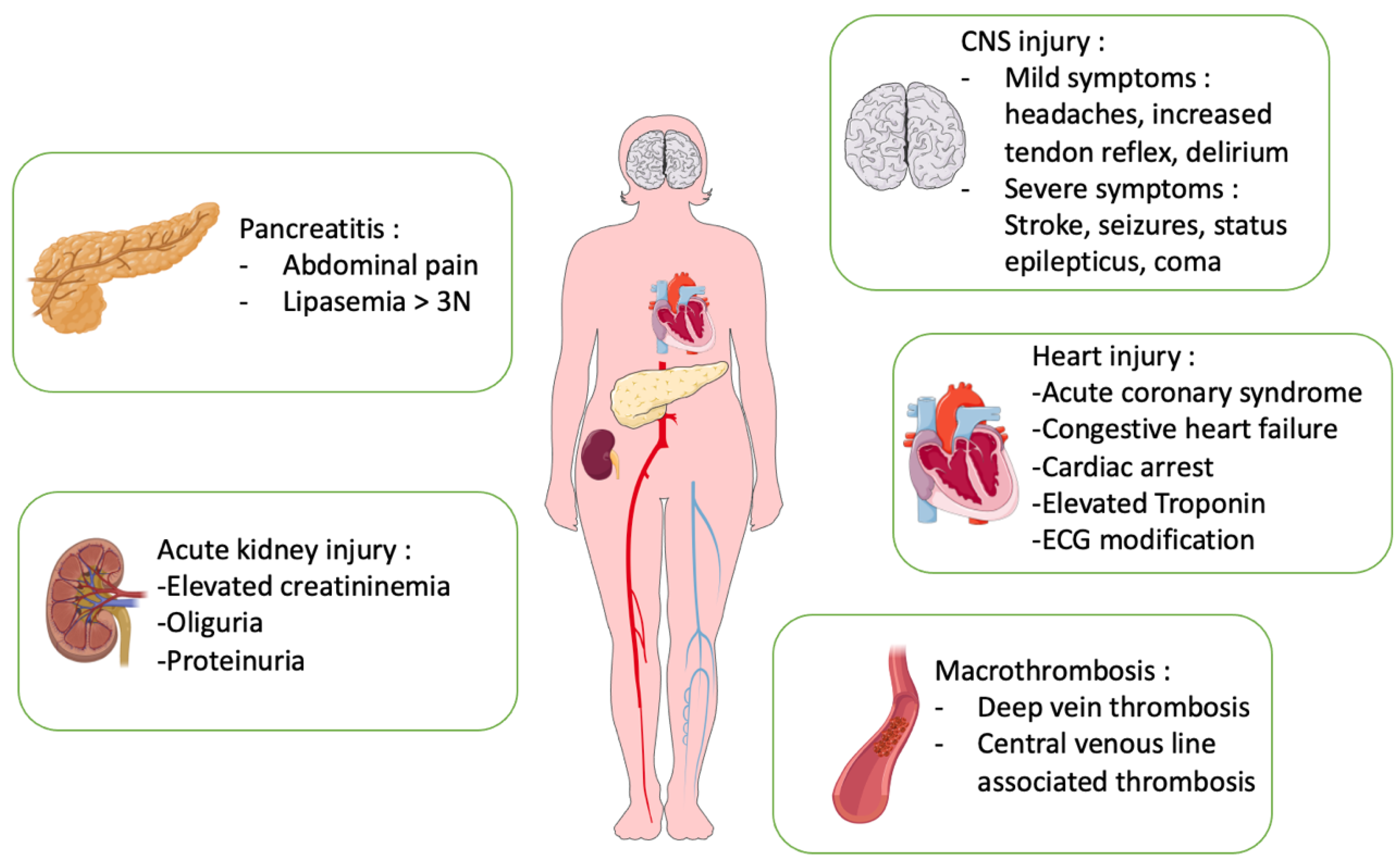
3. Organ Injuries in TTP
3.1. Neurological Manifestations in TTP
3.2. Cardiac Manifestations in TTP
3.3. Acute Kidney Injury (AKI) in TTP
3.4. Pancreatitis and Gastrointestinal Disorder in TTP
3.5. Macrovascular Thrombosis in TTP
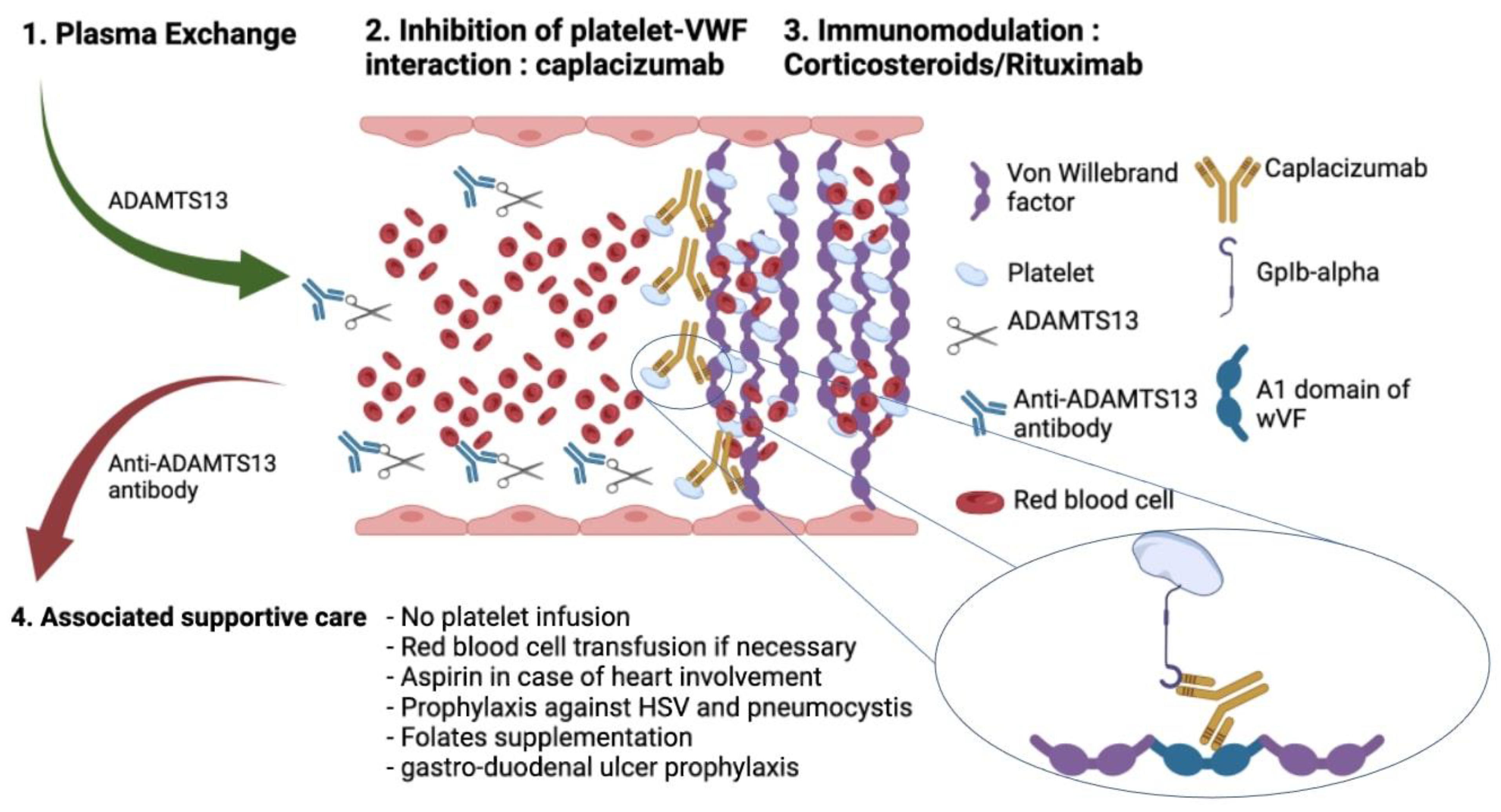
4. ICU Management of TTP
4.1. Initial Management of TTP
4.2. Life-Sustaining Therapies and ICU Supportive Care
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Joly, B.S.; Coppo, P.; Veyradier, A. An Update on Pathogenesis and Diagnosis of Thrombotic Thrombocytopenic Purpura. Expert Rev. Hematol. 2019, 12, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Mariotte, E.; Azoulay, E.; Galicier, L.; Rondeau, E.; Zouiti, F.; Boisseau, P.; Poullin, P.; de Maistre, E.; Provôt, F.; Delmas, Y.; et al. Epidemiology and Pathophysiology of Adulthood-Onset Thrombotic Microangiopathy with Severe ADAMTS13 Deficiency (Thrombotic Thrombocytopenic Purpura): A Cross-Sectional Analysis of the French National Registry for Thrombotic Microangiopathy. Lancet Haematol. 2016, 3, e237–e245. [Google Scholar] [CrossRef]
- Sukumar, S.; Lämmle, B.; Cataland, S.R. Thrombotic Thrombocytopenic Purpura: Pathophysiology, Diagnosis, and Management. J. Clin. Med. 2021, 10, 536. [Google Scholar] [CrossRef]
- Moatti-Cohen, M.; Garrec, C.; Wolf, M.; Boisseau, P.; Galicier, L.; Azoulay, E.; Stepanian, A.; Delmas, Y.; Rondeau, E.; Bezieau, S.; et al. Unexpected Frequency of Upshaw-Schulman Syndrome in Pregnancy-Onset Thrombotic Thrombocytopenic Purpura. Blood 2012, 119, 5888–5897. [Google Scholar] [CrossRef]
- Hussein, E.; Teruya, J. Evaluating the Impact of the ABO Blood Group on the Clinical Outcome of Thrombotic Thrombocytopenic Purpura Associated with Severe ADAMTS13 Deficiency. Vox Sang. 2017, 112, 434–442. [Google Scholar] [CrossRef]
- Zuberi, L.; Yerasuri, D.; Kuriakose, P. Effect of Blood Group on Idiopathic Thrombotic Thrombocytopenic Purpura. J. Clin. Apher. 2009, 24, 131–133. [Google Scholar] [CrossRef] [PubMed]
- Bowen, D.J. An Influence of ABO Blood Group on the Rate of Proteolysis of von Willebrand Factor by ADAMTS13. J. Thromb. Haemost. 2003, 1, 33–40. [Google Scholar] [CrossRef]
- Mariotte, E.; Zafrani, L.; Fadlallah, J.; Galicier, L.; Ghrenassia, E.; Kerhuel, L.; Calvet, L.; Jong, A.D.; Lemiale, V.; Valade, S.; et al. Performance of Diagnostic ScoRes. in Thrombotic Microangiopathy Patients in the Intensive Care Unit: A Monocentric Study. Thromb. Haemost. 2021, 121, 1427–1434. [Google Scholar] [CrossRef]
- Noris, M.; Remuzzi, G. Hemolytic Uremic Syndrome. J. Am. Soc. Nephrol. 2005, 16, 1035–1050. [Google Scholar] [CrossRef] [Green Version]
- Mannucci, P.M. Understanding Organ Dysfunction in Thrombotic Thrombocytopenic Purpura. Intensive Care Med. 2015, 41, 715–718. [Google Scholar] [CrossRef]
- Azoulay, E.; Bauer, P.R.; Mariotte, E.; Russell, L.; Knoebl, P.; Martin-Loeches, I.; Pène, F.; Puxty, K.; Povoa, P.; Barratt-Due, A.; et al. Expert Statement on the ICU Management of Patients with Thrombotic Thrombocytopenic Purpura. Intensive Care Med. 2019, 45, 1518–1539. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.L.; Vesely, S.K.; Cataland, S.R.; Coppo, P.; Geldziler, B.; Iorio, A.; Matsumoto, M.; Mustafa, R.A.; Pai, M.; Rock, G.; et al. ISTH Guidelines for Treatment of Thrombotic Thrombocytopenic Purpura. J. Thromb. Haemost. 2020, 18, 2496–2502. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knöbl, P.; Kremer Hovinga, J.A.; Metjian, A.; de la Rubia, J.; Pavenski, K.; Callewaert, F.; et al. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Van de Louw, A.; Mariotte, E.; Darmon, M.; Cohrs, A.; Leslie, D.; Azoulay, E. Outcomes in 1096 Patients with Severe Thrombotic Thrombocytopenic Purpura before the Caplacizumab Era. PLoS ONE 2021, 16, e0256024. [Google Scholar] [CrossRef]
- Ramachandran, P.; Erdinc, B.; Abowali, H.A.; Zahid, U.; Gotlieb, V.; Spitalewitz, S. High Incidence of Thrombotic Thrombocytopenic Purpura Exacerbation Rate among Patients With Morbid Obesity and Drug Abuse. Cureus 2021, 13, e14656. [Google Scholar] [CrossRef] [PubMed]
- Nicol, K.K.; Shelton, B.J.; Knovich, M.A.; Owen, J. Overweight Individuals Are at Increased Risk for Thrombotic Thrombocytopenic Purpura. Am. J. Hematol. 2003, 74, 170–174. [Google Scholar] [CrossRef]
- Benhamou, Y.; Assié, C.; Boelle, P.-Y.; Buffet, M.; Grillberger, R.; Malot, S.; Wynckel, A.; Presne, C.; Choukroun, G.; Poullin, P.; et al. Development and Validation of a Predictive Model for Death in Acquired Severe ADAMTS13 Deficiency-Associated Idiopathic Thrombotic Thrombocytopenic Purpura: The French TMA Reference Center Experience. Haematologica 2012, 97, 1181–1186. [Google Scholar] [CrossRef] [Green Version]
- Kremer Hovinga, J.A.; Vesely, S.K.; Terrell, D.R.; Lämmle, B.; George, J.N. Survival and Relapse in Patients with Thrombotic Thrombocytopenic Purpura. Blood 2010, 115, 1500–1511. [Google Scholar] [CrossRef] [Green Version]
- Goel, R.; King, K.E.; Takemoto, C.M.; Ness, P.M.; Tobian, A.A.R. Prognostic Risk-Stratified Score for Predicting Mortality in Hospitalized Patients with Thrombotic Thrombocytopenic Purpura: Nationally Representative Data from 2007 to 2012. Transfusion 2016, 56, 1451–1458. [Google Scholar] [CrossRef] [Green Version]
- Falter, T.; Herold, S.; Weyer-Elberich, V.; Scheiner, C.; Schmitt, V.; von Auer, C.; Messmer, X.; Wild, P.; Lackner, K.J.; Lämmle, B.; et al. Relapse Rate in Survivors of Acute Autoimmune Thrombotic Thrombocytopenic Purpura Treated with or without Rituximab. Thromb. Haemost. 2018, 118, 1743–1751. [Google Scholar] [CrossRef] [Green Version]
- Tarasco, E.; Bütikofer, L.; Friedman, K.D.; George, J.N.; Hrachovinova, I.; Knöbl, P.N.; Matsumoto, M.; von Krogh, A.S.; Aebi-Huber, I.; Cermakova, Z.; et al. Annual Incidence and Severity of Acute Episodes in Hereditary Thrombotic Thrombocytopenic Purpura. Blood 2021, 137, 3563–3575. [Google Scholar] [CrossRef] [PubMed]
- Alwan, F.; Vendramin, C.; Vanhoorelbeke, K.; Langley, K.; McDonald, V.; Austin, S.; Clark, A.; Lester, W.; Gooding, R.; Biss, T.; et al. Presenting ADAMTS13 Antibody and Antigen Levels Predict Prognosis in Immune-Mediated Thrombotic Thrombocytopenic Purpura. Blood 2017, 130, 466–471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moschcowitz, E. An acute febrile pleiochromic anemia with hyaline thrombosis of the terminal arterioles and capillaries: An undescribed disease. Arch. Intern. Med. 1925, 36, 89. [Google Scholar] [CrossRef]
- Moschcowitz, E. Hyaline Thrombosis of the Terminal Arterioles and Capillaries. A Hitherto Undescribed Disease. Proc. N. Y. Pathol. Soc. 1924, 24, 21–24. [Google Scholar]
- Hosler, G.A.; Cusumano, A.M.; Hutchins, G.M. Thrombotic Thrombocytopenic Purpura and Hemolytic Uremic Syndrome Are Distinct Pathologic Entities. A Review of 56 Autopsy Cases. Arch. Pathol. Lab. Med. 2003, 127, 834–839. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.-M. Pathophysiology of Thrombotic Thrombocytopenic Purpura. Int. J. Hematol. 2010, 91, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Tsai, H.-M.; Chandler, W.L.; Sarode, R.; Hoffman, R.; Jelacic, S.; Habeeb, R.L.; Watkins, S.L.; Wong, C.S.; Williams, G.D.; Tarr, P.I. Von Willebrand Factor and Von Willebrand Factor-Cleaving Metalloprotease Activity in Escherichia coli O157:H7-Associated Hemolytic Uremic Syndrome. Pediatr. Res. 2001, 49, 653–659. [Google Scholar] [CrossRef] [Green Version]
- Blombery, P.; Kivivali, L.; Pepperell, D.; McQuilten, Z.; Engelbrecht, S.; Polizzotto, M.N.; Phillips, L.E.; Wood, E.; Cohney, S. TTP registry steering committee Diagnosis and Management of Thrombotic Thrombocytopenic Purpura (TTP) in Australia: Findings from the First 5 Years of the Australian TTP/Thrombotic Microangiopathy Registry. Intern. Med. J 2016, 46, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Bugarin-Estrada, E.; Gómez-De León, A.; López-García, Y.K.; Díaz-Chuc, E.A.; Priesca-Marín, J.M.; Ruiz-Argüelles, G.J.; Jaime-Pérez, J.C.; Gómez-Almaguer, D. Clinical Presentation in Thrombotic Thrombocytopenic Purpura: Real-World Data from Two Mexican Institutions. J. Clin. Apher. 2018, 33, 645–653. [Google Scholar] [CrossRef]
- Mirouse, A.; Legriel, S.; Dumas, G.; Labro, G.; Veyradier, A.; Zafrani, L.; Valade, S.; Hourmant, Y.; Boutboul, D.; Darmon, M.; et al. Pattern of Brain Injury in Patients with Thrombotic Thrombocytopenic Purpura in the Precaplacizumab Era. Crit. Care Med. 2021, 49, e931–e940. [Google Scholar] [CrossRef]
- Berti de Marinis, G.; Novello, S.; Ferrari, S.; Barzon, I.; Cortella, I.; Businaro, M.A.; Fabris, F.; Lombardi, A.M. Correlation between ADAMTS13 Activity and Neurological Impairment in Acute Thrombotic Microangiopathy Patients. J. Thromb. Thrombolysis 2016, 42, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Carcioppolo, D.; Zhang, L.; McCrae, K.R. Management and Outcomes for Patients with TTP: Analysis of 100 Cases at a Single Institution. Am. J. Hematol. 2013, 88, 560–565. [Google Scholar] [CrossRef] [PubMed]
- Burrus, T.M.; Wijdicks, E.F.M.; Rabinstein, A.A. Brain Lesions Are Most Often Reversible in Acute Thrombotic Thrombocytopenic Purpura. Neurology 2009, 73, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Peyvandi, F.; Scully, M.; Kremer Hovinga, J.A.; Cataland, S.; Knöbl, P.; Wu, H.; Artoni, A.; Westwood, J.-P.; Mansouri Taleghani, M.; Jilma, B.; et al. Caplacizumab for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2016, 374, 511–522. [Google Scholar] [CrossRef]
- Jestin, M.; Benhamou, Y.; Schelpe, A.-S.; Roose, E.; Provôt, F.; Galicier, L.; Hié, M.; Presne, C.; Poullin, P.; Wynckel, A.; et al. Preemptive Rituximab Prevents Long-Term Relapses in Immune-Mediated Thrombotic Thrombocytopenic Purpura. Blood 2018, 132, 2143–2153. [Google Scholar] [CrossRef] [Green Version]
- Hie, M.; Gay, J.; Galicier, L.; Provôt, F.; Presne, C.; Poullin, P.; Bonmarchand, G.; Wynckel, A.; Benhamou, Y.; Vanhille, P.; et al. Preemptive Rituximab Infusions after Remission Efficiently Prevent Relapses in Acquired Thrombotic Thrombocytopenic Purpura. Blood 2014, 124, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Cataland, S.R.; Scully, M.A.; Paskavitz, J.; Maruff, P.; Witkoff, L.; Jin, M.; Uva, N.; Gilbert, J.C.; Wu, H.M. Evidence of Persistent Neurologic Injury Following Thrombotic Thrombocytopenic Purpura. Am. J. Hematol. 2011, 86, 87–89. [Google Scholar] [CrossRef]
- Kennedy, A.S.; Lewis, Q.F.; Scott, J.G.; Kremer Hovinga, J.A.; Lämmle, B.; Terrell, D.R.; Vesely, S.K.; George, J.N. Cognitive Deficits after Recovery from Thrombotic Thrombocytopenic Purpura. Transfusion 2009, 49, 1092–1101. [Google Scholar] [CrossRef]
- Han, B.; Page, E.E.; Stewart, L.M.; Deford, C.C.; Scott, J.G.; Schwartz, L.H.; Perdue, J.J.; Terrell, D.R.; Vesely, S.K.; George, J.N. Depression and Cognitive Impairment Following Recovery from Thrombotic Thrombocytopenic Purpura. Am. J. Hematol. 2015, 90, 709–714. [Google Scholar] [CrossRef] [Green Version]
- Deford, C.C.; Reese, J.A.; Schwartz, L.H.; Perdue, J.J.; Kremer Hovinga, J.A.; Lämmle, B.; Terrell, D.R.; Vesely, S.K.; George, J.N. Multiple Major Morbidities and Increased Mortality during Long-Term Follow-up after Recovery from Thrombotic Thrombocytopenic Purpura. Blood 2013, 122, 2023–2029. [Google Scholar] [CrossRef] [Green Version]
- Lewis, Q.F.; Lanneau, M.S.; Mathias, S.D.; Terrell, D.R.; Vesely, S.K.; George, J.N. Long-Term Deficits in Health-Related Quality of Life after Recovery from Thrombotic Thrombocytopenic Purpura. Transfusion 2009, 49, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, S.; Abbas, H.; McCrae, K.R. Increased Morbidity during Long-Term Follow-up of Survivors of Thrombotic Thrombocytopenic Purpura. Am. J. Hematol. 2015, 90, E208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ridolfi, R.L.; Hutchins, G.M.; Bell, W.R. The Heart and Cardiac Conduction System in Thrombotic Thrombocytopenic Purpura. A Clinicopathologic Study of 17 Autopsied Patients. Ann. Intern. Med. 1979, 91, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.M.; Abu-Fadel, M.; Vesely, S.K.; George, J.N. Clinical Cardiac Involvement in Thrombotic Thrombocytopenic Purpura: A Systematic Review. Transfusion 2008, 48, 382–392. [Google Scholar] [CrossRef]
- Hughes, C.; McEwan, J.R.; Longair, I.; Hughes, S.; Cohen, H.; Machin, S.; Scully, M. Cardiac Involvement in Acute Thrombotic Thrombocytopenic Purpura: Association with Troponin T and IgG Antibodies to ADAMTS 13. J. Thromb. Haemost. 2009, 7, 529–536. [Google Scholar] [CrossRef]
- Benhamou, Y.; Boelle, P.-Y.; Baudin, B.; Ederhy, S.; Gras, J.; Galicier, L.; Azoulay, E.; Provôt, F.; Maury, E.; Pène, F.; et al. Cardiac Troponin-I on Diagnosis Predicts Early Death and Refractoriness in Acquired Thrombotic Thrombocytopenic Purpura. Experience of the French Thrombotic Microangiopathies Reference Center. J. Thromb. Haemost. 2015, 13, 293–302. [Google Scholar] [CrossRef]
- Mariotte, E.; Blet, A.; Galicier, L.; Darmon, M.; Parquet, N.; Lengline, E.; Boutboul, D.; Canet, E.; Traineau, R.; Schlemmer, B.; et al. Unresponsive Thrombotic Thrombocytopenic Purpura in Critically Ill Adults. Intensive Care Med. 2013, 39, 1272–1281. [Google Scholar] [CrossRef]
- Fourmont, A.-M.; Zafrani, L.; Mariotte, E.; Galicier, L.; Joly, B.; Merceron, S.; Bertinchamp, R.; Lemiale, V.; de Jong, A.; Valade, S.; et al. The Clinical FeatuRes. of Cardiac Involvement in Patients with Severe Thrombotic Thrombocytopenic Purpura. Intensive Care Med. 2018, 44, 963–965. [Google Scholar] [CrossRef]
- Bobbio-Pallavicini, E.; Gugliotta, L.; Centurioni, R.; Porta, C.; Vianelli, N.; Billio, A.; Tacconi, F.; Ascari, E. Antiplatelet Agents in Thrombotic Thrombocytopenic Purpura (TTP). Results of a Randomized Multicenter Trial by the Italian Cooperative Group for TTP. Haematologica 1997, 82, 429–435. [Google Scholar]
- Patschan, D.; Witzke, O.; Dührsen, U.; Erbel, R.; Philipp, T.; Herget-Rosenthal, S. Acute Myocardial Infarction in Thrombotic Microangiopathies--Clinical Characteristics, Risk Factors and Outcome. Nephrol. Dial. Transplant. 2006, 21, 1549–1554. [Google Scholar] [CrossRef] [Green Version]
- Atreya, A.R.; Arora, S.; Sivalingam, S.K.; Giugliano, G.R. ST Segment Elevation Myocardial Infarction as a Presenting Feature of Thrombotic Thrombocytopenic Purpura. J. Cardiovasc. Dis. Res. 2012, 3, 167–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, H.H.; Minutello, R.; Juliano, N.; Wong, S.C. A Rare Cause of Acute Myocardial Infarction: Thrombotic Thrombocytopenic Purpura. Int. J. Cardiol. 2009, 133, e1–e2. [Google Scholar] [CrossRef]
- Oshima, T.; Ikutomi, M.; Shinohara, H.; Ishiwata, J.; Fukino, K.; Amaki, T.; Nakamura, F. Acute Myocardial Infarction Caused by Thrombotic Microangiopathy Complicated With Myelodysplastic Syndrome. Int. Heart J. 2016, 57, 634–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doll, J.A.; Kelly, J.P. ST-Segment Elevation Myocardial Infarction Treated with Thrombolytic Therapy in a Patient with Thrombotic Thrombocytopenic Purpura. J. Thromb. Thrombolysis 2014, 38, 124–126. [Google Scholar] [CrossRef] [PubMed]
- Veyradier, A.; Obert, B.; Houllier, A.; Meyer, D.; Girma, J.P. Specific von Willebrand Factor-Cleaving Protease in Thrombotic Microangiopathies: A Study of 111 Cases. Blood 2001, 98, 1765–1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khwaja, A. KDIGO Clinical Practice Guidelines for Acute Kidney Injury. Nephron. Clin. Pract. 2012, 120, c179–c184. [Google Scholar] [CrossRef]
- Zafrani, L.; Mariotte, E.; Darmon, M.; Canet, E.; Merceron, S.; Boutboul, D.; Veyradier, A.; Galicier, L.; Azoulay, E. Acute Renal Failure Is Prevalent in Patients with Thrombotic Thrombocytopenic Purpura Associated with Low Plasma ADAMTS13 Activity. J. Thromb. Haemost. 2015, 13, 380–389. [Google Scholar] [CrossRef]
- Coppo, P.; Bengoufa, D.; Veyradier, A.; Wolf, M.; Bussel, A.; Millot, G.A.; Malot, S.; Heshmati, F.; Mira, J.-P.; Boulanger, E.; et al. Severe ADAMTS13 Deficiency in Adult Idiopathic Thrombotic Microangiopathies Defines a Subset of Patients Characterized by Various Autoimmune Manifestations, Lower Platelet Count, and Mild Renal Involvement. Medicine 2004, 83, 233–244. [Google Scholar] [CrossRef]
- Tsai, H.-M. The Kidney in Thrombotic Thrombocytopenic Purpura. Minerva Med. 2007, 98, 731–747. [Google Scholar]
- George, J.N.; Chen, Q.; Deford, C.C.; Al-Nouri, Z. Ten Patient Stories Illustrating the Extraordinarily Diverse Clinical FeatuRes. of Patients with Thrombotic Thrombocytopenic Purpura and Severe ADAMTS13 Deficiency. J. Clin. Apher. 2012, 27, 302–311. [Google Scholar] [CrossRef]
- Chiasakul, T.; Cuker, A. Clinical and Laboratory Diagnosis of TTP: An Integrated Approach. Hematol. Am. Soc. Hematol. Educ. Program 2018, 2018, 530–538. [Google Scholar] [CrossRef] [Green Version]
- Fujino, Y.; Inoue, Y.; Onodera, M.; Kikuchi, S.; Sato, M.; Kojika, M.; Sato, H.; Suzuki, K.; Matsumoto, M. Acute Pancreatitis-Induced Thrombotic Thrombocytopenic Purpura with Recurrent Acute Pancreatitis. Clin. J. Gastroenterol. 2016, 9, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, I.P.; Kremer Hovinga, J.A.; Lämmle, B.; Peter, H.J.; Schiemann, U. Acute Pancreatitis and Thrombotic Thrombocytopenic Purpura. Eur. J. Med. Res. 2008, 13, 481–482. [Google Scholar] [PubMed]
- Gotlieb, V.K.; Erma, V.; Jacob, R.; Reddy, P.; Taha, H.; Ullah, A. Thrombotic Thrombocytopenic Purpura INDUCED PANCREATITIS– A Rare Complication. Blood 2010, 116, 4683. [Google Scholar] [CrossRef]
- Thachil, J. Lessons from Acute Pancreatitis-Induced Thrombotic Thrombocytopenic Purpura. Eur. J. Intern. Med. 2009, 20, 739–743. [Google Scholar] [CrossRef] [PubMed]
- Swisher, K.K.; Doan, J.T.; Vesely, S.K.; Kwaan, H.C.; Kim, B.; Lämmle, B.; Hovinga, J.A.K.; George, J.N. Pancreatitis Preceding Acute Episodes of Thrombotic Thrombocytopenic Purpura-Hemolytic Uremic Syndrome: Report of Five Patients with a Systematic Review of Published Reports. Haematologica 2007, 92, 936–943. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camous, L.; Veyradier, A.; Darmon, M.; Galicier, L.; Mariotte, E.; Canet, E.; Parquet, N.; Azoulay, É. Macrovascular Thrombosis in Critically Ill Patients with Thrombotic Micro-Angiopathies. Intern. Emerg. Med. 2014, 9, 267–272. [Google Scholar] [CrossRef]
- Joly, B.S.; Coppo, P.; Veyradier, A. Thrombotic Thrombocytopenic Purpura. Blood 2017, 129, 2836–2846. [Google Scholar] [CrossRef] [Green Version]
- Coppo, P. French Reference Center for Thrombotic Microangiopathies Treatment of Autoimmune Thrombotic Thrombocytopenic Purpura in the More Severe Forms. Transfus. Apher. Sci. 2017, 56, 52–56. [Google Scholar] [CrossRef]
- Coppo, P.; Froissart, A. French Reference Center for Thrombotic Microangiopathies Treatment of Thrombotic Thrombocytopenic Purpura beyond Therapeutic Plasma Exchange. Hematol. Am. Soc. Hematol. Educ. Program 2015, 2015, 637–643. [Google Scholar] [CrossRef] [Green Version]
- Balduini, C.L.; Gugliotta, L.; Luppi, M.; Laurenti, L.; Klersy, C.; Pieresca, C.; Quintini, G.; Iuliano, F.; Re, R.; Spedini, P.; et al. High versus Standard Dose Methylprednisolone in the Acute Phase of Idiopathic Thrombotic Thrombocytopenic Purpura: A Randomized Study. Ann. Hematol. 2010, 89, 591–596. [Google Scholar] [CrossRef] [Green Version]
- Froissart, A.; Buffet, M.; Veyradier, A.; Poullin, P.; Provôt, F.; Malot, S.; Schwarzinger, M.; Galicier, L.; Vanhille, P.; Vernant, J.-P.; et al. Efficacy and Safety of First-Line Rituximab in Severe, Acquired Thrombotic Thrombocytopenic Purpura with a Suboptimal Response to Plasma Exchange. Experience of the French Thrombotic Microangiopathies Reference Center. Crit. Care Med. 2012, 40, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Scully, M.; McDonald, V.; Cavenagh, J.; Hunt, B.J.; Longair, I.; Cohen, H.; Machin, S.J. A Phase 2 Study of the Safety and Efficacy of Rituximab with Plasma Exchange in Acute Acquired Thrombotic Thrombocytopenic Purpura. Blood 2011, 118, 1746–1753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owattanapanich, W.; Wongprasert, C.; Rotchanapanya, W.; Owattanapanich, N.; Ruchutrakool, T. Comparison of the Long-Term Remission of Rituximab and Conventional Treatment for Acquired Thrombotic Thrombocytopenic Purpura: A Systematic Review and Meta-Analysis. Clin. Appl. Thromb. Hemost. 2019, 25, 1076029618825309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scully, M.; Hunt, B.J.; Benjamin, S.; Liesner, R.; Rose, P.; Peyvandi, F.; Cheung, B.; Machin, S.J. British Committee for Standards in Haematology Guidelines on the Diagnosis and Management of Thrombotic Thrombocytopenic Purpura and Other Thrombotic Microangiopathies. Br. J. Haematol. 2012, 158, 323–335. [Google Scholar] [CrossRef] [PubMed]
- Riviere, E.; Saint-Léger, M.; James, C.; Delmas, Y.; Clouzeau, B.; Bui, N.; Vital, A.; Coppo, P.; Gruson, D.; Boyer, A. Platelet Transfusion and Catheter Insertion for Plasma Exchange in Patients with Thrombotic Thrombocytopenic Purpura and a Low Platelet Count. Transfusion 2015, 55, 1798–1802. [Google Scholar] [CrossRef] [PubMed]
- Benhamou, Y.; Baudel, J.-L.; Wynckel, A.; Galicier, L.; Azoulay, E.; Provôt, F.; Pène, F.; Mira, J.-P.; Presne, C.; Poullin, P.; et al. Are Platelet Transfusions Harmful in Acquired Thrombotic Thrombocytopenic Purpura at the Acute Phase? Experience of the French Thrombotic Microangiopathies Reference Center. Am. J. Hematol. 2015, 90, E127–E129. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fodil, S.; Zafrani, L. Severe Thrombotic Thrombocytopenic Purpura (TTP) with Organ Failure in Critically Ill Patients. J. Clin. Med. 2022, 11, 1103. https://doi.org/10.3390/jcm11041103
Fodil S, Zafrani L. Severe Thrombotic Thrombocytopenic Purpura (TTP) with Organ Failure in Critically Ill Patients. Journal of Clinical Medicine. 2022; 11(4):1103. https://doi.org/10.3390/jcm11041103
Chicago/Turabian StyleFodil, Sofiane, and Lara Zafrani. 2022. "Severe Thrombotic Thrombocytopenic Purpura (TTP) with Organ Failure in Critically Ill Patients" Journal of Clinical Medicine 11, no. 4: 1103. https://doi.org/10.3390/jcm11041103
APA StyleFodil, S., & Zafrani, L. (2022). Severe Thrombotic Thrombocytopenic Purpura (TTP) with Organ Failure in Critically Ill Patients. Journal of Clinical Medicine, 11(4), 1103. https://doi.org/10.3390/jcm11041103




