Multiple Aspects of Inappropriate Action of Renin–Angiotensin, Vasopressin, and Oxytocin Systems in Neuropsychiatric and Neurodegenerative Diseases
Abstract
:1. Introduction
2. Renin–Angiotensin System
2.1. A Brief Overview of the RAS
2.2. Systemic RAS and Brain RAS
2.3. RAS in Cardiovascular Disorders
2.4. Inappropriate Function of RAS in Neuropsychiatric/Neurodegenerative Diseases
2.4.1. RAS in Cognitive Disorders
2.4.2. RAS in Stress and Pain
2.4.3. RAS in Depression and Anxiety
2.4.4. RAS in Alzheimer’s Disease
2.4.5. RAS in Parkinson’s Disease and Tardive Dyskinesia
2.4.6. RAS in Schizophrenia and Autism
2.4.7. RAS in Coronavirus Infections
3. Vasopressin and Oxytocin Systems
3.1. Overview of Systemic and Peripheral VPS and OTS
3.2. Vasopressin and Oxytocin in Depression
3.2.1. Vasopressin and Depression
3.2.2. Oxytocin in Depression and Anxiety
3.2.3. Vasopressin and Oxytocin in Alzheimer’s Disease
3.2.4. Vasopressin and Oxytocin in Autism
3.2.5. Vasopressin and Oxytocin in Schizophrenia
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; GBD-NHLBI-JACC Global Burden of Cardiovascular Diseases Writing Group. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K.; Ingelsson, E.; Fornage, M.; Liu, P.; Murphy, A.M.; Newby, L.K.; Newton Cheh, C.; Perez, M.V.; Voora, D.; Woo, D.; et al. Expressed Genome in Cardiovascular Diseases and Stroke: Refinement, Diagnosis, and Prediction: A Scientific Statement From the American Heart Association. Circ. Cardiovasc. Genet. 2017, 10, e000037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correll, C.U.; Solmi, M.; Veronese, N.; Bortolato, B.; Rosson, S.; Santonastaso, P.; Thapa Chhetri, N.; Fornaro, M.; Gallicchio, D.; Collantoni, E.; et al. Prevalence, incidence and mortality from cardiovascular disease in patients with pooled and specific severe mental illness: A large-scale meta-analysis of 3,211,768 patients and 113,383,368 controls. World Psychiatry 2017, 16, 163–180. [Google Scholar] [CrossRef] [Green Version]
- Ardell, J.L.; Andresen, M.C.; Armour, J.A.; Billman, G.E.; Chen, P.S.; Foreman, R.D.; Herring, N.; O’Leary, D.S.; Sabbah, H.N.; Schultz, H.D.; et al. Translational neurocardiology: Preclinical models and cardioneural integrative aspects. J. Physiol. 2016, 594, 3877–3909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimsdale, J.E. Psychological stress and cardiovascular disease. J. Am. Coll. Cardiol. 2008, 51, 1237–1246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lang, U.E.; Borgwardt, S. Molecular mechanisms of depression: Perspectives on new treatment strategies. Cell Physiol. Biochem. 2013, 31, 761–777. [Google Scholar] [CrossRef] [PubMed]
- El Abdellati, K.; De Picker, L.; Morrens, M. Antipsychotic Treatment Failure: A Systematic Review on Risk Factors and Interventions for Treatment Adherence in Psychosis. Front. Neurosci. 2020, 14, 531763. [Google Scholar] [CrossRef]
- Imboden, H.; Patil, J.; Nussberger, J.; Nicoud, F.; Hess, B.; Ahmed, N.; Schaffner, T.; Wellner, M.; Müller, D.; Inagami, T.; et al. Endogenous angiotensinergic system in neurons of rat and human trigeminal ganglia. Regul. Pept. 2009, 154, 23–31. [Google Scholar] [CrossRef] [Green Version]
- Scigliano, G.; Ronchetti, G. Antipsychotic-induced metabolic and cardiovascular side effects in schizophrenia: A novel mechanistic hypothesis. CNS Drugs 2013, 27, 249–257. [Google Scholar] [CrossRef] [Green Version]
- Szczepanska-Sadowska, E.; Cudnoch-Jedrzejewska, A.; Ufnal, M.; Zera, T. Brain and cardiovascular diseases: Common neurogenic background of cardiovascular, metabolic and inflammatory diseases. J. Physiol. Pharmacol. 2010, 61, 509–521. [Google Scholar]
- Yang, T.; Richards, E.M.; Pepine, C.J.; Raizada, M.K. The gut microbiota and the brain-gut-kidney axis in hypertension and chronic kidney disease. Nat. Rev. Nephrol. 2018, 14, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.D. The human cortex responds to an interoceptive challenge. Proc. Natl. Acad. Sci. USA 2004, 101, 6333–6334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ikemoto, S. Brain reward circuitry beyond the mesolimbic dopamine system: A neurobiological theory. Neurosci. Biobehav. Rev. 2010, 35, 129–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKlveen, J.M.; Myers, B.; Herman, J.P. The Medial Prefrontal Cortex: Coordinator of Autonomic, Neuroendocrine, and Behavioral Responses to Stress. J. Neuroendocrinol. 2015, 27, 446–456. [Google Scholar] [CrossRef] [Green Version]
- Rossi, M.A.; Stuber, G.D. Overlapping brain circuits for homeostatic and hedonic feeding. Cell Metab. 2018, 27, 42–56. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Czarzasta, K.; Cudnoch-Jedrzejewska, A. Dysregulation of the renin-angiotensin system and the vasopressinergic system; interactions in cardiovascular disorders. Curr. Hypertens. Rep. 2018, 20, 19. [Google Scholar] [CrossRef] [Green Version]
- Szczepanska-Sadowska, E.; Cudnoch-Jedrzejewska, A.; Sadowski, B. Differential role of specific cardiovascular neuropeptides in pain regulation: Relevance to cardiovascular diseases. Neuropeptides 2020, 81, 102046. [Google Scholar] [CrossRef]
- Correll, C.U.; Joffe, B.I.; Rosen, L.M.; Sullivan, T.B.; Joffe, R.T. Cardiovascular and cerebrovascular risk factors and events associated with second-generation antipsychotic compared to antidepressant use in a non-elderly adult sample: Results from a claims-based inception cohort study. World Psychiatry 2015, 14, 56–63. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.; Goldstein, D.S. Cardiovascular dysautonomia in Parkinson Disease: From pathophysiology to pathogenesis. Neurobiol. Dis. 2012, 46, 572–580. [Google Scholar] [CrossRef] [Green Version]
- Wood, J.D. Enteric nervous system neuropathy: Repair and restoration. Curr. Opin. Gastroenterol. 2011, 27, 106–111. [Google Scholar] [CrossRef]
- Wood, J.D. Enteric neurobiology: Discoveries and directions. Adv. Exp. Med. Biol. 2016, 891, 175–191. [Google Scholar] [CrossRef] [PubMed]
- Pavel, J.; Benicky, J.; Murakami, Y.; Sanchez-Lemus, E.; Saavedra, J.M. Peripherally administered angiotensin II AT1 receptor antagonists are anti-stress compounds in vivo. Ann. N. Y. Acad. Sci. 2008, 1148, 360–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saavedra, J.M.; Benicky, J. Brain and peripheral angiotensin II play a major role in stress. Stress 2007, 10, 185–193. [Google Scholar] [CrossRef]
- Saavedra, J.M.; Sánchez-Lemus, E.; Benicky, J. Blockade of brain angiotensin II AT1 receptors ameliorates stress, anxiety, brain inflammation and ischemia: Therapeutic implications. Psychoneuroendocrinology 2011, 36, 1–18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cudnoch-Jedrzejewska, A.; Szczepanska-Sadowska, E.; Dobruch, J.; Puchalska, L.; Ufnal, M.; Kowalewski, S.; Wsół, A. Differential sensitisation to central cardiovascular effects of angiotensin II in rats with a myocardial infarct: Relevance to stress and interaction with vasopressin. Stress 2008, 11, 290–301. [Google Scholar] [CrossRef] [PubMed]
- Dobruch, J.; Cudnoch-Jedrzejewska, A.; Szczepanska-Sadowska, E. Enhanced involvement of brain vasopressin V1 receptors in cardiovascular responses to stress in rats with myocardial infarction. Stress 2005, 8, 273–284. [Google Scholar] [CrossRef] [PubMed]
- Ufnal, M.; Dudek, M.; Zera, T.; Szczepańska-Sadowska, E. Centrally administered interleukin-1 beta sensitizes to the central pressor action of angiotensin II. Brain Res. 2006, 1100, 64–72. [Google Scholar] [CrossRef]
- Kreyenbuhl, J.; Dickerson, F.B.; Medoff, D.R.; Brown, C.H.; Goldberg, R.W.; Fang, L.; Wohlheiter, K.; Mittal, L.P.; Dixon, L.B. Extent and management of cardiovascular risk factors in patients with type 2 diabetes and serious mental illness. J. Nerv. Ment. Dis. 2006, 194, 404–410. [Google Scholar] [CrossRef] [Green Version]
- Cosarderelioglu, C.; Nidadavolu, L.S.; George, C.J.; Oh, E.S.; Bennett, D.A.; Walston, J.D.; Abadir, P.M. Brain Renin-Angiotensin System at the Intersect of Physical and Cognitive Frailty. Front. Neurosci. 2020, 14, 586314. [Google Scholar] [CrossRef]
- Feng, Y.; Xia, H.; Santos, R.A.; Speth, R.; Lazartigues, E. Angiotensin-converting enzyme 2: A new target for neurogenic hypertension. Exp. Physiol. 2010, 95, 601–606. [Google Scholar] [CrossRef] [Green Version]
- McKinley, M.J.; Albiston, A.L.; Allen, A.M.; Mathai, M.L.; May, C.N.; McAllen, R.M.; Oldfield, B.J.; Mendelsohn, F.A.; Chai, S.Y. The brain renin-angiotensin system: Location and physiological roles. Int. J. Biochem. Cell Biol. 2003, 35, 901–918. [Google Scholar] [CrossRef]
- Paul, M.; Poyan Mehr, A.; Kreutz, R. Physiology of local renin-angiotensin systems. Physiol. Rev. 2006, 86, 747–803. [Google Scholar] [CrossRef] [PubMed]
- Labandeira-Garcia, J.L.; Rodríguez-Perez, A.I.; Garrido-Gil, P.; Rodriguez-Pallares, J.; Lanciego, J.L.; Guerra, M.J. Brain renin-angiotensin System and microglial polarization: Implications for aging and neurodegeneration. Front. Aging Neurosci. 2017, 9, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalba, G.; San José, G.; Moreno, M.U.; Fortuño, M.A.; Fortuño, A.; Beaumont, F.J.; Díez, J. Oxidative stress in arterial hypertension: Role of NAD(P)H oxidase. Hypertension 2001, 38, 1395–1399. [Google Scholar] [CrossRef] [PubMed]
- Horiuchi, M.; Akishita, M.; Dzau, V.J. Recent progress in angiotensin II type 2 receptor research in the cardiovascular system. Hypertension 1999, 33, 613–621. [Google Scholar] [CrossRef] [Green Version]
- Coleman, C.G.; Anrather, J.; Iadecola, C.; Pickel, V.M. Angiotensin II type 2 receptors have a major somatodendritic distribution in vasopressin-containing neurons in the mouse hypothalamic paraventricular nucleus. Neuroscience 2009, 63, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Santisteban, R.; Rodriguez-Perez, A.I.; Muñoz, A.; Reyes-Resina, I.; Labandeira-García, J.L.; Navarro, G.; Franco, R. Angiotensin AT1 and AT2 receptor heteromer expression in the hemilesioned rat model of Parkinson’s disease that increases with levodopa-induced dyskinesia. J. Neuroinflamm. 2020, 17, 243. [Google Scholar] [CrossRef]
- Albiston, A.L.; McDowall, S.G.; Matsacos, D.; Sim, P.; Clune, E.; Mustafa, T.; Lee, J.; Mendelsohn, F.A.; Simpson, R.J.; Connolly, L.M.; et al. Evidence that the angiotensin IV (AT(4)) receptor is the enzyme insulin-regulated aminopeptidase. J. Biol. Chem. 2001, 276, 48623–48626. [Google Scholar] [CrossRef] [Green Version]
- Benoist, C.C.; Kawas, L.H.; Zhu, M.; Tyson, K.A.; Stillmaker, L.; Appleyard, S.M.; Wright, J.W.; Wayman, G.A.; Harding, J.W. The procognitive and synaptogenic effects of angiotensin IV-derived peptides are dependent on activation of the hepatocyte growth factor/c-met system. J. Pharmacol. Exp. Ther. 2014, 351, 390–402. [Google Scholar] [CrossRef] [Green Version]
- Costa-Besada, M.A.; Valenzuela, R.; Garrido-Gil, P.; Villar-Cheda, B.; Parga, J.A.; Lanciego, J.L.; Labandeira-Garcia, J.L. Paracrine and intracrine angiotensin 1-7/Mas receptor axis in the substantia nigra of rodents, monkeys, and humans. Mol. Neurobiol. 2018, 55, 5847–5867. [Google Scholar] [CrossRef]
- Karamyan, V.T.; Stockmeier, C.A.; Speth, R.C. Human brain contains a novel non-AT1, non-AT2 binding site for active angiotensin peptides. Life Sci. 2008, 83, 421–425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labandeira-Garcia, J.L.; Valenzuela, R.; Costa-Besada, M.A.; Villar-Cheda, B.; Rodriguez-Perez, A.I. The intracellular renin-angiotensin system: Friend or foe. Some light from the dopaminergic neurons. Prog. Neurobiol. 2020, 8, 101919. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.Y.; Liu, Y.; Ng, K.M.; Liong, E.C.; Tipoe, G.L.; Leung, P.S.; Fung, M.L. Upregulation of a local renin-angiotensin system in the rat carotid body during chronic intermittent hypoxia. Exp. Physiol. 2014, 99, 220–231. [Google Scholar] [CrossRef] [PubMed]
- Phillips, M.I.; de Oliveira, E.M. Brain renin angiotensin in disease. J. Mol. Med. 2008, 86, 715–722. [Google Scholar] [CrossRef]
- von Bohlen, O.; Halbach, O.; Albrecht, D. The CNS renin-angiotensin system. Cell Tissue Res. 2006, 326, 599–616. [Google Scholar] [CrossRef]
- Bunnemann, B.; Fuxe, K.; Ganten, D. The renin-angiotensin system in the brain: An update 1993. Regul. Pept. 1993, 46, 487–509. [Google Scholar] [CrossRef]
- Shan, Z.; Shi, P.; Cuadra, A.E.; Dong, Y.; Lamont, G.J.; Li, Q.; Seth, D.M.; Navar, L.G.; Katovich, M.J.; Sumners, C.; et al. Involvement of the brain (pro)renin receptor in cardiovascular homeostasis. Circ. Res. 2010, 107, 934–938. [Google Scholar] [CrossRef]
- Wang, W.-Z.; Gao, L.; Wang, H.-J.; Zucker, I.H.; Wang, W. Interaction between cardiac sympathetic afferent reflex and chemoreflex is mediated by the NTS AT1 receptors in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1216–H1226. [Google Scholar] [CrossRef] [Green Version]
- Rivas-Santisteban, R.; Lillo, J.; Muñoz, A.; Rodríguez-Pérez, A.I.; Labandeira-García, J.L.; Navarro, G.; Franco, R. Novel Interactions Involving the Mas Receptor Show Potential of the Renin-Angiotensin System in the Regulation of Microglia Activation: Altered Expression in Parkinsonism and Dyskinesia. Neurotherapeutics 2021, 18, 998–1016. [Google Scholar] [CrossRef]
- Rodriguez-Perez, A.I.; Sucunza, D.; Pedrosa, M.A.; Garrido-Gil, P.; Kulisevsky, J.; Lanciego, J.L.; Labandeira-Garcia, J.L. Angiotensin type 1 receptor antagonists protect against alpha-synuclein-induced neuroinflammation and dopaminergic neuron death. Neurotherapeutics 2018, 15, 1063–1081. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Perez, A.I.; Garrido-Gil, P.; Pedrosa, M.A.; Garcia-Garrote, M.; Valenzuela, R.; Navarro, G.; Franco, R.; Labandeira-Garcia, J.L. Angiotensin type 2 receptors: Role in aging and neuroinflammation in the substantia nigra. Brain Behav. Immun. 2020, 87, 256–271. [Google Scholar] [CrossRef] [PubMed]
- Ufnal, M.; Zera, T.; Szczepańska-Sadowska, E. Blockade of angiotensin II AT1 receptors inhibits pressor action of centrally administered interleukin-1beta in Sprague Dawley rats. Neuropeptides 2005, 39, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Ufnal, M.; Sikora, M.; Szczepanska-Sadowska, E. Interleukin-1 receptor antagonist reduces the magnitude of the pressor response to acute stress. Neurosci. Lett. 2008, 448, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Zera, T.; Ufnal, M.; Szczepanska-Sadowska, E. Central TNF-alpha elevates blood pressure and sensitizes to central pressor action of angiotensin II in the infarcted rats. J. Physiol. Pharmacol. 2008, 59 (Suppl. 8), 117–121. [Google Scholar] [PubMed]
- Griendling, K.K.; Sorescu, D.; Ushio-Fukai, M. NAD(P)H oxidase: Role in cardiovascular biology and disease. Circ. Res. 2000, 86, 494–501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Morais, S.D.B.; Shanks, J.; Zucker, I.H. Integrative Physiological Aspects of Brain RAS in Hypertension. Curr. Hypertens. Rep. 2018, 20, 10. [Google Scholar] [CrossRef]
- Thomas, W.G.; Sernia, C. Immunocytochemical localization of angiotensinogen in the rat brain. Neuroscience 1988, 25, 319–341. [Google Scholar] [CrossRef]
- Yang, G.; Gray, T.S.; Sigmund, C.D.; Cassell, M.D. The angiotensinogen gene is expressed in both astrocytes and neurons in murine central nervous system. Brain Res. 1999, 817, 123–131. [Google Scholar] [CrossRef]
- Huang, B.S.; Chen, A.; Ahmad, M.; Wang, H.W.; Leenen, F.H. Mineralocorticoid and AT1 receptors in the paraventricular nucleus contribute to sympathetic hyperactivity and cardiac dysfunction in rats post myocardial infarct. J. Physiol. 2014, 592, 3273–3286. [Google Scholar] [CrossRef] [Green Version]
- Kakar, S.S.; Riel, K.K.; Neill, J.D. Differential expression of angiotensin II receptor subtype mRNAs (AT-1A and AT-1B) in the brain. Biochem. Biophys. Res. Commun. 1992, 185, 688–692. [Google Scholar] [CrossRef]
- Lenkei, Z.; Corvol, P.; Llorens-Cortes, C. Comparative expression of vasopressin and angiotensin type-1 receptor mRNA in rat hypothalamic nuclei: A double in situ hybridization study. Brain Res. Mol. Brain Res. 1995, 34, 135–142. [Google Scholar] [CrossRef]
- Von Bohlen und Halbach, O.; Albrecht, D. Mapping of angiotensin AT1 receptors in the rat limbic system. Regul. Pept. 1998, 78, 51–56. [Google Scholar] [CrossRef]
- Gao, L.; Wang, W.; Li, Y.L.; Schultz, H.D.; Liu, D.; Cornish, K.G.; Zucker, I.H. Sympathoexcitation by central ANG II: Roles for AT1 receptor upregulation and NAD(P)H oxidase in RVLM. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H2271–H2279. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Wang, W.Z.; Wang, W.; Zucker, I.H. Imbalance of angiotensin type 1 receptor and angiotensin II type 2 receptor in the rostral ventrolateral medulla: Potential mechanism for sympathetic overactivity in heart failure. Hypertension 2008, 52, 708–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Hara, Y.; Anrather, J.; Speth, R.C.; Iadecola, C.; Pickel, V.M. Angiotensin II subtype 1A (AT1A) receptors in the rat sensory vagal complex: Subcellular localization and association with endogenous angiotensin. Neuroscience 2003, 122, 21–36. [Google Scholar] [CrossRef]
- Milik, E.; Szczepanska-Sadowska, E.; Dobruch, J.; Cudnoch-Jedrzejewska, A.; Maslinski, W. Altered expression of V1a receptors mRNA in the brain and kidney after myocardial infarction and chronic stress. Neuropeptides 2014, 48, 257–266. [Google Scholar] [CrossRef]
- Milik, E.; Cudnoch-Jedrzejewska, A.; Szczepanska-Sadowska, E. Effect of chronic mild stress on AT1 receptor messenger RNA expression in the brain and kidney of rats. Psychosom. Med. 2016, 78, 208–220. [Google Scholar] [CrossRef]
- Porcari, C.Y.; Araujo, I.G.; Urzedo-Rodrigues, L.; De Luca, L.A., Jr.; Menani, J.V.; Caeiro, X.E.; Imboden, H.; Antunes-Rodrigues, J.; Reis, L.C.; Vivas, L.; et al. Whole body sodium depletion modifies AT1 mRNA expression and serotonin content in the dorsal raphe nucleus. J. Neuroendocrinol. 2019, 31, e12703. [Google Scholar] [CrossRef]
- Valenzuela, R.; Costa-Besada, M.A.; Iglesias-Gonzalez, J.; Perez-Costas, E.; Villar-Cheda, B.; Garrido-Gil, P.; Melendez-Ferro, M.; Soto-Otero, R.; Lanciego, J.L.; Henrion, D.; et al. Mitochondrial angiotensin receptors in dopaminergic neurons. Role in cell protection and aging-related vulnerability to neurodegeneration. Cell Death Dis. 2016, 7, e2427. [Google Scholar] [CrossRef] [Green Version]
- Yamazato, M.; Yamazato, Y.; Sun, C.; Diez-Freire, C.; Raizada, M.K. Overexpression of angiotensin-converting enzyme 2 in the rostral ventrolateral medulla causes long-term decrease in blood pressure in the spontaneously hypertensive rats. Hypertension 2007, 49, 926–931. [Google Scholar] [CrossRef] [Green Version]
- Becker, L.K.; Etelvino, G.M.; Walther, T.; Santos, R.A.S.; Campagnole-Santos, M.J. Immunofluorescence localization of the receptor Mas in cardiovascular-related areas of the rat brain. Am. J. Physiol. Circ. Physiol. 2007, 293, H1416–H1424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferguson, A.V. Angiotensinergic regulation of autonomic and neuroendocrine outputs: Critical roles for the subfornical organ and paraventricular nucleus. Neuroendocrinology 2009, 89, 370–376. [Google Scholar] [CrossRef] [PubMed]
- Gaudet, E.A.; Godwin, S.J.; Lukoshkova, E.; Head, G.A. Effect of central endogenous angiotensin II on sympathetic activation induced by hypoxia. Clin. Exp. Hypertens. 1997, 19, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Pitra, S.; Worker, C.J.; Feng, Y.; Stern, J.E. Exacerbated effects of prorenin on hypothalamic magnocellular neuronal activity and vasopressin plasma levels during salt-sensitive hypertension. Am. J. Physiol. Heart Circ. Physiol. 2019, 317, H496–H504. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, Z.; Milligan, C.J.; Paton, J.F.; Deuchars, J. Angiotensin type 1 receptor immunoreactivity in the thoracic spinal cord. Brain Res. 2003, 985, 21–31. [Google Scholar] [CrossRef]
- Geerling, J.C.; Loewy, A.D. Aldosterone in the brain. Am. J. Physiol.-Renal Physiol. 2009, 297, F559–F576. [Google Scholar] [CrossRef]
- Huang, B.S.; White, R.A.; Ahmad, M.; Leenen, F.H. Role of brain corticosterone and aldosterone in central angiotensin II-induced hypertension. Hypertension 2013, 62, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Łoń, S.; Szczepańska-Sadowska, E.; Szczypaczewska, M. Evidence that centrally released arginine vasopressin is involved in central pressor action of angiotensin II. Am. J. Physiol. 1996, 270 (Pt 2), H167–H173. [Google Scholar] [CrossRef]
- Paczwa, P.; Szczepańska-Sadowska, E.; Loń, S.; Ganten, S.L.; Ganten, D. Role of central AT1 and V1 receptors in cardiovascular adaptation to hemorrhage in SD and renin TGR rats. Am. J. Physiol. 1999, 276, H1918–H1926. [Google Scholar] [CrossRef] [Green Version]
- Sztechman, D.; Czarzasta, K.; Cudnoch-Jedrzejewska, A.; Szczepanska-Sadowska, E.; Zera, T. Aldosterone and mineralocorticoid receptors in regulation of the cardiovascular system and pathological remodelling of the heart and arteries. J. Physiol. Pharmacol. 2018, 69, 829–845. [Google Scholar] [CrossRef]
- Ufnal, M.; Sikora, M.; Zera, T.; Szczepanska-Sadowska, E. Simvastatin reduces pressor response to centrally administered angiotensin II. Am. J. Hypertens. 2010, 23, 956–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.H.; Yu, Y.; Kang, Y.M.; Wei, S.G.; Felder, R.B. Aldosterone acts centrally to increase brain renin-angiotensin system activity and oxidative stress in normal rats. Am. J. Physiol. Heart Circ. Physiol. 2008, 294, H1067–H1074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucker, I.H.; Schultz, H.D.; Patel, K.P.; Wang, W.; Gao, L. Regulation of central angiotensin type 1 receptors and sympathetic outflow in heart failure. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1557–H1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Żera, T.; Ufnal, M.; Szczepańska-Sadowska, E. TNF and angiotensin type 1 receptors interact in the brain control of blood pressure in heart failure. Cytokine 2015, 71, 272–277. [Google Scholar] [CrossRef] [PubMed]
- Cudnoch-Jedrzejewska, A.; Dobruch, J.; Puchalska, L.; Szczepańska-Sadowska, E. Interaction of AT1 receptors and V1a receptors-mediated effects in the central cardiovascular control during the post-infarct state. Regul. Pept. 2007, 142, 86–94. [Google Scholar] [CrossRef]
- Cudnoch-Jedrzejewska, A.; Czarzasta, K.; Puchalska, L.; Dobruch, J.; Borowik, O.; Pachucki, J.; Szczepanska-Sadowska, E. Angiotensin converting enzyme inhibition reduces cardiovascular responses to acute stress in myocardially infarcted and chronically stressed rats. Biomed. Res. Int. 2014, 2014, 385082. [Google Scholar] [CrossRef]
- Huang, B.S.; Ahmad, M.; White, R.A.; Marc, Y.; Llorens-Cortes, C.; Leenen, F.H. Inhibition of brain angiotensin III attenuates sympathetic hyperactivity and cardiac dysfunction in rats post-myocardial infarction. Cardiovasc. Res. 2013, 97, 424–431. [Google Scholar] [CrossRef] [Green Version]
- Macova, M.; Pavel, J.; Saavedra, J.M. A peripherally administered, centrally acting angiotensin II AT2 antagonist selectively increases brain AT1 receptors and decreases brain tyrosine hydroxylase transcription, pituitary vasopressin and ACTH. Brain Res. 2009, 1250, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Mitra, A.K.; Gao, L.; Zucker, I.H. Angiotensin II-induced upregulation of AT(1) receptor expression: Sequential activation of NF-kappaB and Elk-1 in neurons. Am. J. Physiol. Cell Physiol. 2010, 299, C561–C569. [Google Scholar] [CrossRef] [Green Version]
- Nunes, F.C.; Braga, V.A. Chronic angiotensin II infusion modulates angiotensin II type I receptor expression in the subfornical organ and the rostral ventrolateral medulla in hypertensive rats. J. Renin-Angiotensin-Aldosterone Syst. 2011, 12, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.G.; Yu, Y.; Zhang, Z.H.; Weiss, R.M.; Felder, R.B. Mitogen-activated protein kinases mediate upregulation of hypothalamic AT1 receptors in heart failure rats. Hypertension 2008, 52, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hiller, H.; Smith, J.A.; de Kloet, A.D.; Krause, E.G. Angiotensin type 1a receptors in the paraventricular nucleus of the hypothalamus control cardiovascular reactivity and anxiety-like behavior in male mice. Physiol. Genomics 2016, 48, 667–676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Jancovski, N.; Bassi, J.K.; Nguyen-Huu, T.P.; Choong, Y.T.; Palma-Rigo, K.; Davern, P.J.; Gurley, S.B.; Thomas, W.G.; Head, G.A.; et al. Angiotensin type 1A receptors in C1 neurons of the rostral ventrolateral medulla modulate the pressor response to aversive stress. J. Neurosci. 2012, 32, 2051–2061. [Google Scholar] [CrossRef] [Green Version]
- Benicky, J.; Sánchez-Lemus, E.; Honda, M.; Pang, T.; Orecna, M.; Wang, J.; Leng, Y.; Chuang, D.M.; Saavedra, J.M. Angiotensin II AT1 Receptor Blockade Ameliorates Brain Inflammation. Neuropsychopharmacology 2011, 36, 857–870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobruch, J.; Paczwa, P.; Łoń, S.; Khosla, M.C.; Szczepańska-Sadowska, E. Hypotensive function of the brain angiotensin-(1-7) in Sprague Dawley and renin transgenic rats. J. Physiol. Pharmacol. 2003, 54, 371–381. [Google Scholar] [PubMed]
- Xia, H.; Suda, S.; Bindom, S.; Feng, Y.; Gurley, S.B.; Seth, D.; Navar, L.G.; Lazartigues, E. ACE2-mediated reduction of oxidative stress in the central nervous system is associated with improvement of autonomic function. PLoS ONE 2011, 6, e22682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, H.; de Queiroz, T.M.; Sriramula, S.; Feng, Y.; Johnson, T.; Mungrue, I.N.; Lazartigues, E. Brain ACE2 overexpression reduces DOCA-salt hypertension independently of endoplasmic reticulum stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2015, 308, R370–R378. [Google Scholar] [CrossRef] [Green Version]
- Sink, K.M.; Leng, X.; Williamson, J.; Kritchevsky, S.B.; Yaffe, K.; Kulle, L.; Yasar, S.; Atkinson, H.; Robbins, M.; Psaty, B.; et al. Angiotensin-converting enzyme inhibitors and cognitive decline in older adults with hypertension: Results from the Cardiovascular Health Study. Arch. Intern. Med. 2009, 169, 1195–1202. [Google Scholar] [CrossRef]
- Yasar, S.; Moored, K.D.; Adam, A.; Zabel, F.; Chuang, Y.F.; Varma, V.R.; Carlson, M.C. Angiotensin II Blood Levels Are Associated with Smaller Hippocampal and Cortical Volumes in Cognitively Normal Older Adults. J. Alzheimers Dis. 2020, 75, 521–529. [Google Scholar] [CrossRef]
- Li, Z.; Cao, Y.; Li, L.; Liang, Y.; Tian, X.; Mo, N.; Liu, Y.; Li, M.; Chui, D.; Guo, X. Prophylactic angiotensin type 1 receptor antagonism confers neuroprotection in an aged rat model of postoperative cognitive dysfunction. Biochem. Biophys. Res. Commun. 2014, 449, 74–80. [Google Scholar] [CrossRef]
- Wilms, H.; Rosenstiel, P.; Unger, T.; Deuschl, G.; Lucius, R. Neuroprotection with angiotensin receptor antagonists: A review of the evidence and potential mechanisms. Am. J. Cardiovasc. Drugs 2005, 5, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Wincewicz, D.; Braszko, J.J. Telmisartan attenuates cognitive impairment caused by chronic stress in rats. Pharmacol. Rep. 2014, 66, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Braszko, J.J.; Walesiuk, A.; Wielgat, P. Cognitive effects attributed to angiotensin II may result from its conversion to angiotensin IV. J. Renin-Angiotensin-Aldosterone Syst. 2006, 7, 168–174. [Google Scholar] [CrossRef] [PubMed]
- Cao, C.; Hasegawa, Y.; Hayashi, K.; Takemoto, Y.; Kim-Mitsuyama, S. Chronic Angiotensin 1-7 Infusion Prevents Angiotensin-II-Induced Cognitive Dysfunction and Skeletal Muscle Injury in a Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2019, 69, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Hellner, K.; Walther, T.; Schubert, M.; Albrecht, D. Angiotensin-(1-7) enhances LTP in the hippocampus through the G-protein-coupled receptor Mas. Mol. Cell. Neurosci. 2005, 29, 427–435. [Google Scholar] [CrossRef]
- Barnes, J.M.; Barnes, N.M.; Costall, B.; Horovitz, Z.P.; Ironside, J.W.; Naylor, R.J.; Williams, T.J. Angiotensin II inhibits cortical cholinergic function: Implications for cognition. J. Cardiovasc. Pharmacol. 1990, 16, 234–238. [Google Scholar] [CrossRef]
- Barnes, N.M.; Cheng, C.H.; Costall, B.; Naylor, R.J.; Williams, T.J.; Wischik, C.M. Angiotensin converting enzyme density is increased in temporal cortex from patients with Alzheimer’s disease. Eur. J. Pharmacol. 1991, 200, 289–292. [Google Scholar] [CrossRef]
- Savaskan, E.; Hock, C.; Olivieri, G.; Bruttel, S.; Rosenberg, C.; Hulette, C.; Müller-Spahn, F. Cortical alterations of angiotensin converting enzyme, angiotensin II and AT1 receptor in Alzheimer’s dementia. Neurobiol. Aging 2001, 22, 541–546. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Swomley, A.M.; Sultana, R. Amyloid β-peptide (1-42)-induced oxidative stress in Alzheimer disease: Importance in disease pathogenesis and progression. Antioxid. Redox Signal. 2013, 19, 823–835. [Google Scholar] [CrossRef] [Green Version]
- Guimond, M.O.; Gallo-Payet, N. How does angiotensin AT(2) receptor activation help neuronal differentiation and improve neuronal pathological situations? Front. Endocrinol. 2012, 3, 164. [Google Scholar] [CrossRef] [Green Version]
- Masters, C.L.; Simms, G.; Weinman, N.A.; Multhaup, G.; McDonald, B.L.; Beyreuther, K. Amyloid plaque core protein in Alzheimer disease and Down syndrome. Proc. Natl. Acad. Sci. USA 1985, 82, 4245–4249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quitterer, U.; AbdAlla, S. Improvements of symptoms of Alzheimer’s disease by inhibition of the angiotensin system. Pharmacol. Res. 2020, 154, 104230. [Google Scholar] [CrossRef] [PubMed]
- Takeda, S.; Sato, N.; Takeuchi, D.; Kurinami, H.; Shinohara, M.; Niisato, K.; Kano, M.; Ogihara, T.; Rakugi, H.; Morishita, R. Angiotensin receptor blocker prevented beta amyloid-induced cognitive impairment associated with recovery of neurovascular coupling. Hypertension 2009, 54, 1345–1352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, T.; Zhang, Y.-D.; Zhou, J.-S.; Zhu, X.-C.; Tian, Y.-Y.; Zhao, H.D.; Lu, H.; Gao, Q.; Tan, L.; Yu, J.T. Angiotensin-(1-7) is Reduced and Inversely Correlates with Tau Hyperphosphorylation in Animal Models of Alzheimer’s Disease. Mol. Neurobiol. 2016, 53, 2489. [Google Scholar] [CrossRef]
- Aguilera, G.; Kiss, A.; Luo, X. Increased expression of type 1 angiotensin II receptors in the hypothalamic paraventricular nucleus following stress and glucocorticoid administration. J. Neuroendocrinol. 1995, 7, 775–783. [Google Scholar] [CrossRef]
- Brasil, T.F.S.; Fassini, A.; Corrêa, F.M. AT1 and AT2 receptors in the prelimbic cortex modulate the cardiovascular response evoked by acute exposure to restraint stress in rats. Cell. Mol. Neurobiol. 2018, 38, 305–316. [Google Scholar] [CrossRef]
- Costa, A.C.O.; Becker, L.K.; Moraes, É.R.; Romero, T.R.L.; Guzzo, L.; Santos, R.A.S.; Duarte, I.D.G. Angiotensin-(1–7) induces peripheral antinociception through Mas receptor activation in an opioid-independent pathway. Pharmacology 2012, 89, 137–144. [Google Scholar] [CrossRef]
- Zhao, Y.; Qin, Y.; Liu, T.; Hao, D. Chronic nerve injury-induced Mas receptor expression in dorsal root ganglion neurons alleviates neuropathic pain. Exp. Ther. Med. 2015, 10, 2384–2388. [Google Scholar] [CrossRef] [Green Version]
- Saab, Y.B.; Gard, P.R.; Yeoman, M.S.; Mfarrej, B.; El-Moalem, H.; Ingram, M.J. Renin-angiotensin-system gene polymorphisms and depression. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 1113–1118. [Google Scholar] [CrossRef]
- Kangussu, L.M.; Almeida-Santos, A.F.; Bader, M.; Alenina, N.; Fontes, M.A.P.; Santos, R.A.S.; Aguiar, D.C.; Campagnole-Santos, M.J. Angiotensin-(1-7) attenuates the anxiety and depression-like behaviors in transgenic rats with low brain angiotensinogen. Behav. Brain Res. 2013, 257, 25–30. [Google Scholar] [CrossRef]
- Hajjar, I.; Kritchevsky, S.; Newman, A.B.; Li, R.; Yaffe, K.; Simonsick, E.M.; Lipsitz, L.A.; Health, Aging and Body Composition Study. Renin angiotensin system gene polymorphisms modify angiotensin-converting enzyme inhibitors’ effect on cognitive function: The health, aging and body composition study. J. Am. Geriatr. Soc. 2010, 58, 1035–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, K.M.; Kraal, A.Z.; Flowers, S.A.; Ellingrod, V.L. Cardiovascular Pharmacogenomics and Cognitive Function in Patients with Schizophrenia. Pharmacotherapy 2017, 37, 1122–1130. [Google Scholar] [CrossRef] [PubMed]
- Gadelha, A.; Vendramini, A.M.; Yonamine, C.M.; Nering, M.; Berberian, A.; Suiama, M.A.; Oliveira, V.; Lima-Landman, M.T.; Breen, G.; Bressan, R.A.; et al. Convergent evidences from human and animal studies implicate angiotensin I-converting enzyme activity in cognitive performance in schizophrenia. Transl. Psychiatry 2015, 5, e691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, X.; Song, X.; Zhao, M.; Jarskog, L.F.; Natarajan, R.; Shukair, N.; Freudenreich, O.; Henderson, D.C.; Goff, D.C. The effect of adjunctive telmisartan treatment on psychopathology and cognition in patients with schizophrenia. Acta Psychiatr. Scand. 2017, 136, 465–472. [Google Scholar] [CrossRef]
- Dominguez-Meijide, A.; Villar-Cheda, B.; Garrido-Gil, P.; Sierrra-Paredes, G.; Guerra, M.J.; Labandeira-Garcia, J.L. Effect of chronic treatment with angiotensin type 1 receptor antagonists on striatal dopamine levels in normal rats and in a rat model of Parkinson’s disease treated with L-DOPA. Neuropharmacology 2014, 76, 156–168. [Google Scholar] [CrossRef]
- Joglar, B.; Rodriguez-Pallares, J.; Rodriguez-Perez, A.I.; Rey, P.; Guerra, M.J.; Labandeira-Garcia, J.L. The inflammatory response in the MPTP model of Parkinson’s disease is mediated by brain angiotensin: Relevance to progression of the disease. J. Neurochem. 2009, 109, 656–669. [Google Scholar] [CrossRef]
- Sonsalla, P.K.; Coleman, C.; Wong, L.Y.; Harris, S.L.; Richardson, J.R.; Gadad, B.S.; Li, W.; German, D.C. The angiotensin converting enzyme inhibitor captopril protects nigrostriatal dopamine neurons in animal models of parkinsonism. Exp. Neurol. 2013, 250, 376–383. [Google Scholar] [CrossRef] [Green Version]
- Thakur, K.S.; Prakash, A.; Bisht, R.; Bansal, P.K. Beneficial effect of candesartan and lisinopril against haloperidol-induced tardive dyskinesia in rat. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 917–929. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Xu, J.; Hou, Y.; Leverenz, J.B.; Kallianpur, A.; Mehra, R.; Liu, Y.; Yu, H.; Pieper, A.A.; Jehi, L.; et al. Network medicine links SARS-CoV-2/COVID-19 infection to brain microvascular injury and neuroinflammation in dementia-like cognitive impairment. Alzheimer’s Res. Ther. 2021, 13, 110. [Google Scholar] [CrossRef]
- Purba, J.S.; Hoogendijk, W.J.; Hofman, M.A.; Swaab, D.F. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch. Gen. Psychiatry 1996, 53, 137–143. [Google Scholar] [CrossRef]
- Van West, D.; Del-Favero, J.; Aulchenko, Y.; Oswald, P.; Souery, D.; Forsgren, T.; Sluijs, S.; Bel-Kacem, S.; Adolfsson, R.; Mendlewicz, J.; et al. A major SNP haplotype of the arginine vasopressin 1B receptor protects against recurrent major depression. Mol. Psychiatry 2004, 9, 287–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zai, C.C.; Muir, K.E.; Nowrouzi, B.; Shaikh, S.A.; Choi, E.; Berall, L.; Trépanier, M.O.; Beitchman, J.H.; Kennedy, J.L. Possible genetic association between vasopressin receptor 1B and child aggression. Psychiatry Res. 2012, 200, 784–788. [Google Scholar] [CrossRef] [PubMed]
- Ben-Efraim, Y.J.; Wasserman, D.; Wasserman, J.; Sokolowski, M.V. Family-based study of AVPR1B association and interaction with stressful life events on depression and anxiety in suicide attempts. Neuropsychopharmacology 2013, 38, 1504–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamiya, M.; Sabia, H.D.; Marella, J.; Fava, M.; Nemeroff, C.B.; Umeuchi, H.; Iijima, M.; Chaki, S.; Nishino, I. Efficacy and safety of TS-121, a novel vasopressin V(1B) receptor antagonist, as adjunctive treatment for patients with major depressive disorder: A randomized, double-blind, placebo-controlled study. J. Psychiatr. Res. 2020, 128, 43–51. [Google Scholar] [CrossRef]
- Katz, D.A.; Locke, C.; Greco, N.; Liu, W.; Tracy, K.A. Hypothalamic-pituitary-adrenal axis and depression symptom effects of an arginine vasopressin type 1B receptor antagonist in a one-week randomized Phase 1b trial. Brain Behav. 2017, 7, e00628. [Google Scholar] [CrossRef] [Green Version]
- Agorastos, A.; Sommer, A.; Heinig, A.; Wiedemann, K.; Demiralay, C. Vasopressin Surrogate Marker Copeptin as a Potential Novel Endocrine Biomarker for Antidepressant Treatment Response in Major Depression: A Pilot Study. Front. Psychiatry 2020, 11, 453. [Google Scholar] [CrossRef]
- Griebel, G.; Simiand, J.; Serradeil-Le Gal, C.; Wagnon, J.; Pascal, M.; Scatton, B.; Maffrand, J.P.; Soubrie, P. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc. Natl. Acad. Sci. USA 2002, 99, 6370–6375. [Google Scholar] [CrossRef] [Green Version]
- Iijima, M.; Chaki, S. An arginine vasopressin V1b antagonist, SSR149415 elicits antidepressant-like effects in an olfactory bulbectomy model. Prog. Neuropsychopharmacol. Biol. Psychiatry 2007, 31, 622–627. [Google Scholar] [CrossRef]
- Iijima, M.; Yoshimizu, T.; Shimazaki, T.; Tokugawa, K.; Fukumoto, K.; Kurosu, S.; Kuwada, T.; Sekiguchi, Y.; Chaki, S. Antidepressant and anxiolytic profiles of newly synthesized arginine vasopressin V1B receptor antagonists: TASP0233278 and TASP0390325. Br. J. Pharmacol. 2014, 171, 3511–3525. [Google Scholar] [CrossRef] [Green Version]
- Cudnoch-Jedrzejewska, A.; Puchalska, L.; Szczepanska-Sadowska, E.; Wsol, A.; Kowalewski, S.; Czarzasta, K. The effect of blockade of the central V1 vasopressin receptors on anhedonia in chronically stressed infarcted and non-infarcted rats. Physiol. Behav. 2014, 135, 208–214. [Google Scholar] [CrossRef]
- Cudnoch-Jedrzejewska, A.; Szczepanska-Sadowska, E.; Dobruch, J.; Gomolka, R.; Puchalska, L. Brain vasopressin V(1) receptors contribute to enhanced cardiovascular responses to acute stress in chronically stressed rats and rats with myocardial infarction. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2010, 298, R672–R680. [Google Scholar] [CrossRef] [PubMed]
- Cilz, N.I.; Cymerblit-Sabba, A.; Young, W.S. Oxytocin and vasopressin in the rodent hippocampus. Genes Brain Behav. 2019, 18, e12535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wsol, A.; Cudnoch-Jedrzejewska, A.; Szczepanska-Sadowska, E.; Kowalewski, S.; Dobruch, J. Central oxytocin modulation of acute stress-induced cardiovascular responses after myocardial infarction in the rat. Stress 2009, 12, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Wsol, A.; Szczepanska-Sadowska, E.; Kowalewski, S.; Puchalska, L.; Cudnoch-Jedrzejewska, A. Oxytocin differently regulates pressor responses to stress in WKY and SHR rats: The role of central oxytocin and V1a receptors. Stress 2014, 17, 117–125. [Google Scholar] [CrossRef]
- Wsol, A.; Wojno, O.; Puchalska, L.; Wrzesien, R.; Szczepanska-Sadowska, E.; Cudnoch-Jedrzejewska, A. Impaired hypotensive effects of centrally acting oxytocin in SHR and WKY rats exposed to chronic mild stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R160–R172. [Google Scholar] [CrossRef]
- Breuer, M.E.; van Gaalen, M.M.; Wernet, W.; Claessens, S.E.; Oosting, R.S.; Behl, B.; Korte, S.M.; Schoemaker, H.; Gross, G.; Olivier, B.; et al. SSR149415, a non-peptide vasopressin V1b receptor antagonist, has long-lasting antidepressant effects in the olfactory bulbectomy-induced hyperactivity depression model. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2009, 379, 101–106. [Google Scholar] [CrossRef]
- Serradeil-Le Gal, C.; Derick, S.; Brossard, G.; Manning, M.; Simiand, J.; Gaillard, R.; Griebel, G.; Guillon, G. Functional and pharmacological characterization of the first specific agonist and antagonist for the V1b receptor in mammals. Stress 2003, 6, 199–206. [Google Scholar] [CrossRef]
- Raskind, M.A.; Peskind, E.R.; Lampe, T.H.; Risse, S.C.; Taborsky, G.J., Jr.; Dorsa, D. Cerebrospinal fluid vasopressin, oxytocin, somatostatin, and beta-endorphin in Alzheimer’s disease. Arch. Gen Psychiatry 1986, 43, 382–388. [Google Scholar] [CrossRef]
- Mazurek, M.F.; Beal, M.F.; Bird, E.D.; Martin, J.B. Vasopressin in Alzheimer’s disease: A study of postmortem brain concentrations. Ann. Neurol. 1986, 20, 665–670. [Google Scholar] [CrossRef]
- Goudsmit, E.; Filers, E.; Swaab, D.F. Changes in vasopressin neurons and fibers in aging and Alzheimer’s disease: Reversibility in the rat. Prog. Clin. Biol. Res. 1989, 317, 1193–1208. [Google Scholar]
- Meynen, G.; Unmehopa, U.A.; Hofman, M.A.; Swaab, D.F.; Hoogendijk, W.J. Hypothalamic vasopressin and oxytocin mRNA expression in relation to depressive state in Alzheimer’s disease: A difference with major depressive disorder. J. Neuroendocrinol. 2009, 21, 722–729. [Google Scholar] [CrossRef] [PubMed]
- Van Zwieten, E.J.; Ravid, R.; Hoogendijk, W.J.; Swaab, D.F. Stable vasopressin innervation in the degenerating human locus coeruleus in Alzheimer’s disease. Brain Res. 1994, 649, 329–333. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Zhao, F.; Wang, C.; Zhang, J.; Bai, Y.; Zhou, F.; Wang, Z.; Guo, J.; Qi, J. AVP(4-8) Improves Cognitive Behaviors and Hippocampal Synaptic Plasticity in the APP/PS1 Mouse Model of Alzheimer’s Disease. Neurosci. Bull. 2020, 36, 254–262. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.S.; Williams Avram, S.K.; Cymerblit-Sabba, A.; Song, J.; Young, W.S. Targeted activation of the hippocampal CA2 area strongly enhances social memory. Mol. Psychiatry 2016, 21, 1137–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frederiksen, S.O.; Ekman, R.; Gottfries, C.G.; Widerlöv, E.; Jonsson, S. Reduced concentrations of galanin, arginine vasopressin, neuropeptide Y and peptide YY in the temporal cortex but not in the hypothalamus of brains from schizophrenics. Acta Psychiatr. Scand. 1991, 83, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Busch, J.R.; Jacobsen, C.; Lynnerup, N.; Banner, J.; Møller, M. Expression of vasopressin mRNA in the hypothalamus of individuals with a diagnosis of schizophrenia. Brain Behav. 2019, 9, e01355. [Google Scholar] [CrossRef] [Green Version]
- Guzel, D.; Yazici, A.B.; Pek, T.M.; Doganay, S.; Simsek, A.B.S.; Saglam, K.; Turan, C.; Yazici, E. Atrial natriuretic peptide and posterior pituitary neurohormone changes in patients with acute schizophrenia. Neuropsychiatr. Dis. Treat. 2018, 14, 1855–1860. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Tao, H.; Yang, X.; Huang, K.; Zhang, X.; Li, C. Decreased Serum Oxytocin and Increased Homocysteine in First-Episode Schizophrenia Patients. Front. Psychiatry 2019, 10, 217. [Google Scholar] [CrossRef] [Green Version]
- Rubin, L.H.; Carter, C.S.; Bishop, J.R.; Pournajafi-Nazarloo, H.; Harris, M.S.; Hill, S.K.; Reilly, J.L.; Sweeney, J.A. Peripheral vasopressin but not oxytocin relates to severity of acute psychosis in women with acutely-ill untreated first-episode psychosis. Schizophr. Res. 2013, 146, 138–143. [Google Scholar] [CrossRef] [Green Version]
- Goldman, M.B. The mechanism of life-threatening water imbalance in schizophrenia and its relationship to the underlying psychiatric illness. Brain Res. Rev. 2009, 61, 210–220. [Google Scholar] [CrossRef] [Green Version]
- Goldman, M.B.; Robertson, G.L.; Luchins, D.J.; Hedeker, D.; Pandey, G.N. Psychotic exacerbations and enhanced vasopressin secretion in schizophrenic patients with hyponatremia and polydipsia. Arch. Gen. Psychiatry 1997, 54, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Ohsawa, H.; Kishimoto, T.; Shimayoshi, N.; Matsumura, K.; Tahara, K.; Kitera, K.; Higashiura, N.; Noriyama, Y.; Matsumoto, H.; Hirai, M.; et al. Atrial natriuretic peptide and arginine vasopressin secretion in schizophrenic patients. Acta Psychiatr. Scand. 1993, 88, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, S.M.; Farokhnia, M.; Rezaei, F.; Gougol, A.; Yekehtaz, H.; Iranpour, N.; Salehi, B.; Tabrizi, M.; Tajdini, M.; Ghaleiha, A.; et al. Intranasal desmopressin as an adjunct to risperidone for negative symptoms of schizophrenia: A randomized, double-blind, placebo-controlled, clinical trial. Eur. Neuropsychopharmacol. 2014, 24, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Teltsh, O.; Kanyas-Sarner, K.; Rigbi, A.; Greenbaum, L.; Lerer, B.; Kohn, Y. Oxytocin and vasopressin genes are significantly associated with schizophrenia in a large Arab-Israeli pedigree. Int. J. Neuropsychopharmacol. 2012, 15, 309–319. [Google Scholar] [CrossRef] [Green Version]
- Cilia, J.; Gartlon, J.E.; Shilliam, C.; Dawson, L.A.; Moore, S.H.; Jones, D.N.C. Further neurochemical and behavioural investigation of Brattleboro rats as a putative model of schizophrenia. J. Psychopharmacol. 2010, 24, 407–419. [Google Scholar] [CrossRef]
- Demeter, K.; Török, B.; Fodor, A.; Varga, J.; Ferenczi, S.; Kovács, K.J.; Eszik, I.; Szegedi, V.; Zelena, D. Possible contribution of epigenetic changes in the development of schizophrenia-like behavior in vasopressin-deficient Brattleboro rats. Behav. Brain Res. 2016, 300, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Egashira, N.; Tanoue, A.; Matsuda, T.; Koushi, E.; Harada, S.; Takano, Y.; Tsujimoto, G.; Mishima, K.; Iwasaki, K.; Fujiwara, M. Impaired social interaction and reduced anxiety-related behavior in vasopressin V1a receptor knockout mice. Behav. Brain Res. 2007, 178, 123–127. [Google Scholar] [CrossRef]
- Potasiewicz, A.; Holuj, M.; Litwa, E.; Gzielo, K.; Socha, L.; Popik, P.; Nikiforuk, A. Social dysfunction in the neurodevelopmental model of schizophrenia in male and female rats: Behavioural and biochemical studies. Neuropharmacology 2020, 170, 108040. [Google Scholar] [CrossRef]
- Oztan, O.; Garner, J.P.; Partap, S.; Sherr, E.H.; Hardan, A.Y.; Farmer, C.; Thurm, A.; Swedo, S.E.; Parker, K.J. Cerebrospinal fluid vasopressin and symptom severity in children with autism. Ann. Neurol. 2018, 84, 611–615. [Google Scholar] [CrossRef]
- Oztan, O.; Garner, J.P.; Constantino, J.N.; Parker, K.J. Neonatal CSF vasopressin concentration predicts later medical record diagnoses of autism spectrum disorder. Proc. Natl. Acad. Sci. USA 2020, 117, 10609–10613. [Google Scholar] [CrossRef]
- Oztan, O.; Jackson, L.P.; Libove, R.A.; Sumiyoshi, R.D.; Phillips, J.M.; Garner, J.P.; Hardan, A.Y.; Parker, K.J. Biomarker discovery for disease status and symptom severity in children with autism. Psychoneuroendocrinology 2018, 89, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Francis, S.M.; Kim, S.J.; Kistner-Griffin, E.; Guter, S.; Cook, E.H.; Jacob, S. ASD and Genetic Associations with Receptors for Oxytocin and Vasopressin-AVPR1A, AVPR1B, and OXTR. Front. Neurosci. 2016, 10, 516. [Google Scholar] [CrossRef] [PubMed]
- Cataldo, I.; Azhari, A.; Esposito, G. A Review of Oxytocin and Arginine-Vasopressin Receptors and Their Modulation of Autism Spectrum Disorder. Front. Mol. Neurosci. 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Y.; Cho, S.C.; Yoo, H.J.; Cho, I.H.; Park, M.; Kim, B.N.; Kim, J.W.; Shin, M.S.; Park, T.W.; Son, J.W.; et al. Association study between single nucleotide polymorphisms in promoter region of AVPR1A and Korean autism spectrum disorders. Neurosci. Lett. 2010, 479, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.Y.; Cho, S.C.; Yoo, H.J.; Cho, I.H.; Park, M.; Yoe, J.; Kim, S.A. Family-based association study of microsatellites in the 5′ flanking region of AVPR1A with autism spectrum disorder in the Korean population. Psychiatry Res. 2010, 178, 199–201. [Google Scholar] [CrossRef]
- Parker, K.J.; Oztan, O.; Libove, R.A.; Sumiyoshi, R.D.; Jackson, L.P.; Karhson, D.S.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. Intranasal oxytocin treatment for social deficits and biomarkers of response in children with autism. Proc. Natl. Acad. Sci. USA 2017, 114, 8119–8124. [Google Scholar] [CrossRef] [Green Version]
- Parker, K.J.; Oztan, O.; Libove, R.A.; Mohsin, N.; Karhson, D.S.; Sumiyoshi, R.D.; Summers, J.E.; Hinman, K.E.; Motonaga, K.S.; Phillips, J.M.; et al. A randomized placebo-controlled pilot trial shows that intranasal vasopressin improves social deficits in children with autism. Sci. Transl. Med. 2019, 11, eaau7356. [Google Scholar] [CrossRef]
- Brambilla, M.; Manenti, R.; de Girolamo, G.; Adenzato, M.; Bocchio-Chiavetto, L.; Cotelli, M. Effects of Intranasal Oxytocin on Long-Term Memory in Healthy Humans: A Systematic Review. Drug Dev. Res. 2016, 77, 479–488. [Google Scholar] [CrossRef] [Green Version]
- Lucassen, P.J.; Tilders, F.J.; Salehi, A.; Swaab, D.F. Neuropeptides vasopressin (AVP), oxytocin (OXT) and corticotropin-releasing hormone (CRH) in the human hypothalamus: Activity changes in aging, Alzheimer’s disease and depression. Aging 1997, 9 (Suppl. 4), 48–50. [Google Scholar] [CrossRef]
- Mazurek, M.F.; Beal, M.F.; Bird, E.D.; Martin, J.B. Oxytocin in Alzheimer’s disease: Postmortem brain levels. Neurology 1987, 37, 1001–1003. [Google Scholar] [CrossRef]
- Van Zwieten, E.J.; Ravid, R.; Swaab, D.F. Differential vasopressin and oxytocin innervation of the human parabrachial nucleus: No changes in Alzheimer’s disease. Brain Res. 1996, 711, 146–152. [Google Scholar] [CrossRef] [Green Version]
- Wierda, M.; Goudsmit, E.; van der Woude, P.F.; Purba, J.S.; Hofman, M.A.; Bogte, H.; Swaab, D.F. Oxytocin cell number in the human paraventricular nucleus remains constant with aging and in Alzheimer’s disease. Neurobiol. Aging 1991, 12, 511–516. [Google Scholar] [CrossRef] [Green Version]
- Petekkaya, E.; Burakgazi, G.; Kuş, B.; Melek, İ.M.; Arpacı, A. Comparative study of the volume of the temporal lobe sections and neuropeptide effect in Alzheimer’s patients and healthy persons. Int. J. Neurosci. 2021, 131, 725–734. [Google Scholar] [CrossRef]
- Anderberg, U.M.; Uvnäs-Moberg, K. Plasma oxytocin levels in female fibromyalgia syndrome patients. Z. Rheumatol. 2000, 59, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Dai, D.; Li, Q.C.; Zhu, Q.B.; Hu, S.H.; Balesar, R.; Swaab, D.; Bao, A.M. Direct Involvement of Androgen Receptor in Oxytocin Gene Expression: Possible Relevance for Mood Disorders. Neuropsychopharmacology 2017, 42, 2064–2071. [Google Scholar] [CrossRef] [PubMed]
- Meynen, G.; Unmehopa, U.A.; Hofman, M.A.; Swaab, D.F.; Hoogendijk, W.J. Hypothalamic oxytocin mRNA expression and melancholic depression. Mol. Psychiatry 2007, 12, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Ozsoy, S.; Esel, E.; Kula, M. Serum oxytocin levels in patients with depression and the effects of gender and antidepressant treatment. Psychiatry Res. 2009, 169, 249–252. [Google Scholar] [CrossRef]
- Scantamburlo, G.; Hansenne, M.; Fuchs, S.; Pitchot, W.; Maréchal, P.; Pequeux, C.; Ansseau, M.; Legros, J.J. Plasma oxytocin levels and anxiety in patients with major depression. Psychoneuroendocrinology 2007, 32, 407–410. [Google Scholar] [CrossRef] [Green Version]
- Tsai, T.Y.; Tseng, H.H.; Chi, M.H.; Chang, H.H.; Wu, C.K.; Yang, Y.K.; Chen, P.S. The Interaction of Oxytocin and Social Support, Loneliness, and Cortisol Level in Major Depression. Clin. Psychopharmacol. Neurosci. 2019, 17, 487–494. [Google Scholar] [CrossRef] [Green Version]
- Holt-Lunstad, J.; Birmingham, W.; Light, K.C. The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology 2011, 36, 1249–1256. [Google Scholar] [CrossRef]
- Parker, K.J.; Kenna, H.A.; Zeitzer, J.M.; Keller, J.; Blasey, C.M.; Amico, J.A.; Schatzberg, A.F. Preliminary evidence that plasma oxytocin levels are elevated in major depression. Psychiatry Res. 2010, 178, 359–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrener, C.D.; Valentin, E.M.; Gallin, C.; Richey, L.; Ross, D.B.; Hood, C.J.; Lori, A.; Cubells, J.; Rauch, S.A.M.; Rilling, J.K. The role of oxytocin signaling in depression and suicidality in returning war veterans. Psychoneuroendocrinology 2021, 126, 105085. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Larkin, T. Plasma cortisol and oxytocin levels predict help-seeking intentions for depressive symptoms. Psychoneuroendocrinology 2018, 87, 159–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jobst, A.; Sabaß, L.; Hall, D.; Brücklmeier, B.; Buchheim, A.; Hall, J.; Sarubin, N.; Zill, P.; Falkai, P.; Brakemeier, E.L.; et al. Oxytocin plasma levels predict the outcome of psychotherapy: A pilot study in chronic depression. J. Affect. Disord. 2018, 227, 206–213. [Google Scholar] [CrossRef]
- Costa, B.; Pini, S.; Martini, C.; Abelli, M.; Gabelloni, P.; Ciampi, O.; Muti, M.; Gesi, C.; Lari, L.; Cardini, A.; et al. Mutation analysis of oxytocin gene in individuals with adult separation anxiety. Psychiatry Res. 2009, 168, 87–93. [Google Scholar] [CrossRef]
- Costa, B.; Pini, S.; Gabelloni, P.; Abelli, M.; Lari, L.; Cardini, A.; Muti, M.; Gesi, C.; Landi, S.; Galderisi, S.; et al. Oxytocin receptor polymorphisms and adult attachment style in patients with depression. Psychoneuroendocrinology 2009, 34, 1506–1514. [Google Scholar] [CrossRef]
- Thompson, R.J.; Parker, K.J.; Hallmayer, J.F.; Waugh, C.E.; Gotlib, I.H. Oxytocin receptor gene polymorphism (rs2254298) interacts with familial risk for psychopathology to predict symptoms of depression and anxiety in adolescent girls. Psychoneuroendocrinology 2011, 36, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Costa, B.; Pini, S.; Baldwin, D.S.; Silove, D.; Manicavasagar, V.; Abelli, M.; Coppedè, F.; Martini, C. Oxytocin receptor and G-protein polymorphisms in patients with depression and separation anxiety. J. Affect. Disord. 2017, 218, 365–373. [Google Scholar] [CrossRef] [Green Version]
- Choi, D.; Tsuchiya, K.J.; Takei, N. Interaction effect of oxytocin receptor (OXTR) rs53576 genotype and maternal postpartum depression on child behavioural problems. Sci. Rep. 2019, 9, 7685. [Google Scholar] [CrossRef]
- Parris, M.S.; Grunebaum, M.F.; Galfalvy, H.C.; Andronikashvili, A.; Burke, A.K.; Yin, H.; Min, E.; Huang, Y.Y.; Mann, J.J. Attempted suicide and oxytocin-related gene polymorphisms. J. Affect. Disord. 2018, 238, 62–68. [Google Scholar] [CrossRef]
- Neumann, I.D.; Torner, L.; Wigger, A. Brain oxytocin: Differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience 2000, 95, 567–575. [Google Scholar] [CrossRef]
- Stuebe, A.M.; Grewen, K.; Meltzer-Brody, S. Association between maternal mood and oxytocin response to breastfeeding. J. Women’s Health 2013, 22, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Jobst, A.; Dehning, S.; Ruf, S.; Notz, T.; Buchheim, A.; Henning-Fast, K.; Meißner, D.; Meyer, S.; Bondy, B.; Müller, N.; et al. Oxytocin and vasopressin levels are decreased in the plasma of male schizophrenia patients. Acta Neuropsychiatr. 2014, 26, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halverson, T.; Jarskog, L.F.; Pedersen, C.; Penn, D. Effects of oxytocin on empathy, introspective accuracy, and social symptoms in schizophrenia: A 12-week twice-daily randomized controlled trial. Schizophr. Res. 2019, 204, 178–182. [Google Scholar] [CrossRef]
- Woolley, J.D.; Chuang, B.; Lam, O.; Lai, W.; O’Donovan, A.; Rankin, K.P.; Mathalon, D.H.; Vinogradov, S. Oxytocin administration enhances controlled social cognition in patients with schizophrenia. Psychoneuroendocrinology 2014, 47, 116–125. [Google Scholar] [CrossRef] [Green Version]
- Dagani, J.; Sisti, D.; Abelli, M.; Di Paolo, L.; Pini, S.; Raimondi, S.; Rocchi, M.B.; Saviotti, F.M.; Scocco, P.; Totaro, S.; et al. Do we need oxytocin to treat schizophrenia? A randomized clinical trial. Schizophr. Res. 2016, 172, 158–164. [Google Scholar] [CrossRef]
- Jarskog, L.F.; Pedersen, C.A.; Johnson, J.L.; Hamer, R.M.; Rau, S.W.; Elliott, T.; Penn, D.L. A 12-week randomized controlled trial of twice-daily intranasal oxytocin for social cognitive deficits in people with schizophrenia. Schizophr. Res. 2017, 185, 88–95. [Google Scholar] [CrossRef]
- Oya, K.; Matsuda, Y.; Matsunaga, S.; Kishi, T.; Iwata, N. Efficacy and safety of oxytocin augmentation therapy for schizophrenia: An updated systematic review and meta-analysis of randomized, placebo-controlled trials. Eur. Arch. Psychiatry Clin. Neurosci. 2016, 266, 439–450. [Google Scholar] [CrossRef]
- Williams, D.R.; Bürkner, P.C. Effects of intranasal oxytocin on symptoms of schizophrenia: A multivariate Bayesian meta-analysis. Psychoneuroendocrinology 2017, 75, 141–151. [Google Scholar] [CrossRef]
- Ylisaukko-oja, T.; Alarcón, M.; Cantor, R.M.; Auranen, M.; Vanhala, R.; Kempas, E.; von Wendt, L.; Järvelä, I.; Geschwind, D.H.; Peltonen, L. Search for autism loci by combined analysis of Autism Genetic Resource Exchange and Finnish families. Ann. Neurol. 2006, 59, 145–155. [Google Scholar] [CrossRef]
- Wu, S.; Jia, M.; Ruan, Y.; Liu, J.; Guo, Y.; Shuang, M.; Gong, X.; Zhang, Y.; Yang, X.; Zhang, D. Positive association of the oxytocin receptor gene (OXTR) with autism in the Chinese Han population. Biol. Psychiatry 2005, 58, 74–77. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Brune, C.W.; Carter, C.S.; Leventhal, B.L.; Lord, C.; Cook, E.H., Jr. Association of the oxytocin receptor gene (OXTR) in Caucasian children and adolescents with autism. Neurosci. Lett. 2007, 417, 6–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Kawamura, Y.; Shimada, T.; Otowa, T.; Koishi, S.; Sugiyama, T.; Nishida, H.; Hashimoto, O.; Nakagami, R.; Tochigi, M.; et al. Association of the oxytocin receptor (OXTR) gene polymorphisms with autism spectrum disorder (ASD) in the Japanese population. J. Hum. Genet. 2010, 55, 137–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skuse, D.H.; Lori, A.; Cubells, J.F.; Lee, I.; Conneely, K.N.; Puura, K.; Lehtimäki, T.; Binder, E.B.; Young, L.J. Common polymorphism in the oxytocin receptor gene (OXTR) is associated with human social recognition skills. Proc. Natl. Acad. Sci. USA 2014, 111, 1987–1992. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, D.B.; Datta, D.; Jones, S.T.; Batey Lee, E.; Sutcliffe, J.S.; Hammock, E.A.; Levitt, P. Association of oxytocin receptor (OXTR) gene variants with multiple phenotype domains of autism spectrum disorder. J. Neurodev. Disord. 2011, 3, 101–112. [Google Scholar] [CrossRef] [Green Version]
- LoParo, D.; Waldman, I.D. The oxytocin receptor gene (OXTR) is associated with autism spectrum disorder: A meta-analysis. Mol. Psychiatry 2015, 20, 640–646. [Google Scholar] [CrossRef] [PubMed]
- Kranz, T.M.; Kopp, M.; Waltes, R.; Sachse, M.; Duketis, E.; Jarczok, T.A.; Degenhardt, F.; Görgen, K.; Meyer, J.; Freitag, C.M.; et al. Meta-analysis and association of two common polymorphisms of the human oxytocin receptor gene in autism spectrum disorder. Autism Res. 2016, 9, 1036–1045. [Google Scholar] [CrossRef]
- Green, L.; Fein, D.; Modahl, C.; Feinstein, C.; Waterhouse, L.; Morris, M. Oxytocin and autistic disorder: Alterations in peptide forms. Biol. Psychiatry 2001, 50, 609–613. [Google Scholar] [CrossRef]
- Husarova, V.M.; Lakatosova, S.; Pivovarciova, A.; Babinska, K.; Bakos, J.; Durdiakova, J.; Kubranska, A.; Ondrejka, I.; Ostatnikova, D. Plasma Oxytocin in Children with Autism and Its Correlations with Behavioral Parameters in Children and Parents. Psychiatry Investig. 2016, 13, 174–183. [Google Scholar] [CrossRef]
- John, S.; Jaeggi, A.V. Oxytocin levels tend to be lower in autistic children: A meta-analysis of 31 studies. Autism 2021, 25, 2152–2161. [Google Scholar] [CrossRef]
- Modahl, C.; Green, L.; Fein, D.; Morris, M.; Waterhouse, L.; Feinstein, C.; Levin, H. Plasma oxytocin levels in autistic children. Biol. Psychiatry 1998, 43, 270–277. [Google Scholar] [CrossRef]
- Hollander, E.; Novotny, S.; Hanratty, M.; Yaffe, R.; DeCaria, C.M.; Aronowitz, B.R.; Mosovich, S. Oxytocin infusion reduces repetitive behaviors in adults with autistic and Asperger’s disorders. Neuropsychopharmacology 2003, 28, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Yamasue, H.; Okada, T.; Munesue, T.; Kuroda, M.; Fujioka, T.; Uno, Y.; Matsumoto, K.; Kuwabara, H.; Mori, D.; Okamoto, Y.; et al. Effect of intranasal oxytocin on the core social symptoms of autism spectrum disorder: A randomized clinical trial. Mol. Psychiatry 2020, 25, 1849–1858. [Google Scholar] [CrossRef] [PubMed]
- Kruppa, J.A.; Gossen, A.; Weiß, E.O.; Kohls, G.; Großheinrich, N.; Cholemkery, H.; Freitag, C.M.; Karges, W.; Wölfle, E.; Sinzig, J.; et al. Neural modulation of social reinforcement learning by intranasal oxytocin in male adults with high-functioning autism spectrum disorder: A randomized trial. Neuropsychopharmacology 2019, 44, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Bernaerts, S.; Boets, B.; Bosmans, G.; Steyaert, J.; Alaerts, K. Behavioral effects of multiple-dose oxytocin treatment in autism: A randomized, placebo-controlled trial with long-term follow-up. Mol. Autism 2020, 11, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadds, M.R.; MacDonald, E.; Cauchi, A.; Williams, K.; Levy, F.; Brennan, J. Nasal oxytocin for social deficits in childhood autism: A randomized controlled trial. J. Autism Dev. Disord. 2014, 44, 521–531. [Google Scholar] [CrossRef]
- Henneberry, E.; Lamy, M.; Dominick, K.C.; Erickson, C.A. Decades of Progress in the Psychopharmacology of Autism Spectrum Disorder. J. Autism Dev. Disord. 2021, 51, 4370–4394. [Google Scholar] [CrossRef]
- Cai, Q.; Feng, L.; Yap, K.Z. Systematic review and meta-analysis of reported adverse events of long-term intranasal oxytocin treatment for autism spectrum disorder. Psychiatry Clin. Neurosci. 2018, 72, 140–151. [Google Scholar] [CrossRef]
- Wincewicz, D.; Braszko, J.J. Validation of Brain Angiotensin System Blockade as a Novel Drug Target in Pharmacological Treatment of Neuropsychiatric Disorders. Pharmacopsychiatry 2017, 50, 233–247. [Google Scholar] [CrossRef]
- Royea, J.; Hamel, E. Brain angiotensin II and angiotensin IV receptors as potential Alzheimer’s disease therapeutic targets. Geroscience 2020, 42, 1237–1256. [Google Scholar] [CrossRef]
- Thomas, W.G.; Mendelsohn, F.A. Angiotensin receptors: Form and function and distribution. Int. J. Biochem. Cell Biol. 2003, 35, 774–779. [Google Scholar] [CrossRef]
- Armando, I.; Carranza, A.; Nishimura, Y.; Barontini, M.; Ito, T.; Saavedra, J.M. Candesartan decreases the sympatho-adrenal and hormonal response to isolation stress. J. Renin-Angiotensin-Aldosterone Syst. 2001, 2 (Suppl. 1), S130–S135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armando, I.; Carranza, A.; Nishimura, Y.; Hoe, K.L.; Barontini, M.; Terrón, J.A.; Falcón-Neri, A.; Ito, T.; Juorio, A.V.; Saavedra, J.M. Peripheral administration of an angiotensin II AT(1) receptor antagonist decreases the hypothalamic-pituitary-adrenal response to isolation stress. Endocrinology 2001, 142, 3880–3889. [Google Scholar] [CrossRef] [PubMed]
- Costa-Ferreira, W.; Vieira, J.O.; Almeida, J.; Gomes-de-Souza, L.; Crestani, C.C. Involvement of Type 1 Angiontensin II Receptor (AT1) in Cardiovascular Changes Induced by Chronic Emotional Stress: Comparison between Homotypic and Heterotypic Stressors. Front. Pharmacol. 2016, 7, 262. [Google Scholar] [CrossRef] [Green Version]
- Pelegrini-da-Silva, A.; Martins, A.R.; Prado, W.A. A new role for the renin-angiotensin system in the rat periaqueductal gray matter: Angiotensin receptor-mediated modulation of nociception. Neuroscience 2005, 132, 453–463. [Google Scholar] [CrossRef]
- Raghavendra, V.; Chopra, K.; Kulkarni, S.K. Brain renin angiotensin system (RAS) in stress-induced analgesia and impaired retention. Peptides 1999, 20, 335–342. [Google Scholar] [CrossRef]
- Kumar, A.; Bilker, W.; Lavretsky, H.; Gottlieb, G. Volumetric asymmetries in late-onset mood disorders: An attenuation of frontal asymmetry with depression severity. Psychiatry Res. 2000, 100, 41–47. [Google Scholar] [CrossRef]
- Pandya, M.; Altinay, M.; Malone, D.A., Jr.; Anand, A. Where in the brain is depression? Curr. Psychiatry Rep. 2012, 14, 634–642. [Google Scholar] [CrossRef] [Green Version]
- Drevets, W.C.; Price, J.L.; Simpson, J.R., Jr.; Todd, R.D.; Reich, T.; Vannier, M.; Raichle, M.E. Subgenual prefrontal cortex abnormalities in mood disorders. Nature 1997, 386, 824–827. [Google Scholar] [CrossRef]
- Mayberg, H.S.; Liotti, M.; Brannan, S.K.; McGinnis, S.; Mahurin, R.K.; Jerabek, P.A.; Silva, J.A.; Tekell, J.L.; Martin, C.C.; Lancaster, J.L.; et al. Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am. J. Psychiatry 1999, 156, 675–682. [Google Scholar] [CrossRef]
- Vogt, B.A. Midcingulate cortex: Structure, connections, homologies, functions and diseases. J. Chem. Neuroanat. 2016, 74, 28–46. [Google Scholar] [CrossRef] [PubMed]
- Coffey, C.E.; Wilkinson, W.E.; Weiner, R.D.; Parashos, I.A.; Djang, W.T.; Webb, M.C.; Figiel, G.S.; Spritzer, C.E. Quantitative cerebral anatomy in depression. A controlled magnetic resonance imaging study. Arch. Gen. Psychiatry 1993, 50, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Schweitzer, I.; Tuckwell, V.; Ames, D.; O’Brien, J. Structural neuroimaging studies in late-life depression: A review. World J. Biol. Psychiatry 2001, 2, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Sprengelmeyer, R.; Steele, J.D.; Mwangi, B.; Kumar, P.; Christmas, D.; Milders, M.; Matthews, K. The insular cortex and the neuroanatomy of major depression. J. Affect. Disord. 2011, 133, 120–127. [Google Scholar] [CrossRef]
- Gard, P.R. Implications of the angiotensin converting enzyme gene insertion/deletion polymorphism in health and disease: A snapshot review. Int. J. Mol. Epidemiol. Genet. 2010, 1, 145–157. [Google Scholar]
- Zannas, A.S.; McQuoid, D.R.; Payne, M.E.; MacFall, J.R.; Ashley-Koch, A.; Steffens, D.C.; Potter, G.G.; Taylor, W.D. Association of gene variants of the renin-angiotensin system with accelerated hippocampal volume loss and cognitive decline in old age. Am. J. Psychiatry 2014, 171, 1214–1221. [Google Scholar] [CrossRef] [Green Version]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Mucke, L. Alzheimer mechanisms and therapeutic strategies. Cell 2012, 148, 1204–1222. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Ho, L.; Chen, L.; Zhao, Z.; Zhao, W.; Qian, X.; Humala, N.; Seror, I.; Bartholomew, S.; Rosendorff, C.; et al. Valsartan lowers brain beta-amyloid protein levels and improves spatial learning in a mouse model of Alzheimer disease. J. Clin. Investig. 2007, 117, 3393–3402. [Google Scholar] [CrossRef]
- AbdAlla, S.; Langer, A.; Fu, X.; Quitterer, U. ACE inhibition with captopril retards the development of signs of neurodegeneration in an animal model of Alzheimer’s disease. Int. J. Mol. Sci. 2013, 14, 16917–16942. [Google Scholar] [CrossRef]
- Anderson, C.; Teo, K.; Gao, P.; Arima, H.; Dans, A.; Unger, T.; Commerford, P.; Dyal, L.; Schumacher, H.; Pogue, J.; et al. Renin-angiotensin system blockade and cognitive function in patients at high risk of cardiovascular disease: Analysis of data from the ONTARGET and TRANSCEND studies. Lancet Neurol. 2011, 10, 43–53. [Google Scholar] [CrossRef]
- Chang-Quan, H.; Hui, W.; Chao-Min, W.; Zheng-Rong, W.; Jun-Wen, G.; Yong-Hong, L.; Yan-You, L.; Qing-Xiu, L. The association of antihypertensive medication use with risk of cognitive decline and dementia: A meta-analysis of longitudinal studies. Int. J. Clin. Pract. 2011, 65, 1295–1305. [Google Scholar] [CrossRef] [PubMed]
- Ohrui, T.; Tomita, N.; Sato-Nakagawa, T.; Matsui, T.; Maruyama, M.; Niwa, K.; Arai, H.; Sasaki, H. Effects of brain-penetrating ACE inhibitors on Alzheimer disease progression. Neurology 2004, 63, 1324–1325. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, M.A.; Ratti, G.; Di Salvo, G.; Natale, F. Does the angiotensin II receptor antagonist losartan improve cognitive function? Drugs Aging 2002, 19, 723–732. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, S.; Wang, H.F.; Wang, X.; Li, J.; Xing, C.M. The association of renin-angiotensin system blockade use with the risks of cognitive impairment of aging and Alzheimer’s disease: A meta-analysis. J. Clin. Neurosci. 2016, 33, 32–38. [Google Scholar] [CrossRef]
- Barthold, D.; Joyce, G.; Diaz Brinton, R.; Wharton, W.; Kehoe, P.G.; Zissimopoulos, J. Association of combination statin and antihypertensive therapy with reduced Alzheimer’s disease and related dementia risk. PLoS ONE 2020, 15, e0229541. [Google Scholar] [CrossRef] [Green Version]
- Valappil, R.A.; Black, J.E.; Broderick, M.J.; Carrillo, O.; Frenette, E.; Sullivan, S.S.; Goldman, S.M.; Tanner, C.M.; Langston, J.W. Exploring the electrocardiogram as a potential tool to screen for premotor Parkinson’s disease. Mov. Disord. 2010, 25, 2296–2303. [Google Scholar] [CrossRef]
- Wright, J.W.; Harding, J.W. Importance of the brain Angiotensin system in Parkinson’s disease. Parkinson’s Dis. 2012, 2012, 860923. [Google Scholar] [CrossRef] [Green Version]
- Su, G.; Dou, H.; Zhao, L.; Wang, H.; Liu, G.; Huang, B.; Peng, B. The angiotensin-converting enzyme (ACE) I/D polymorphism in Parkinson’s disease. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 428–433. [Google Scholar] [CrossRef] [Green Version]
- Attar, R.; Wester, A.; Koul, S.; Eggert, S.; Polcwiartek, C.; Jernberg, T.; Erlinge, D.; Andell, P. Higher risk of major adverse cardiac events after acute myocardial infarction in patients with schizophrenia. Open Heart 2020, 7, e001286. [Google Scholar] [CrossRef]
- Castillejos, M.C.; Martín-Pérez, C.; García-Ruiz, A.; Mayoral-Cleries, F.; Moreno-Küstner, B. Recording of cardiovascular risk factors by general practitioners in patients with schizophrenia. Ann. Gen. Psychiatry 2020, 19, 34. [Google Scholar] [CrossRef] [PubMed]
- Daumit, G.L.; Goff, D.C.; Meyer, J.M.; Davis, V.G.; Nasrallah, H.A.; McEvoy, J.P.; Rosenheck, R.; Davis, S.M.; Hsiao, J.K.; Stroup, T.S.; et al. Antipsychotic effects on estimated 10-year coronary heart disease risk in the CATIE schizophrenia study. Schizophr. Res. 2008, 105, 175–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, S.M.; Sullivan, K.M.; Liu, F.; DiPaula, B.A.; Jose, P.A.; Kitchen, C.A.; Feldman, S.M.; Kelly, D.L. Blood Pressure and Heart Rate Changes during Clozapine Treatment. Psychiatr. Q. 2017, 88, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Fan, X. The Possible Role of the Angiotensin System in the Pathophysiology of Schizophrenia: Implications for Pharmacotherapy. CNS Drugs 2019, 33, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Kugathasan, P.; Horsdal, H.T.; Aagaard, J.; Jensen, S.E.; Laursen, T.M.; Nielsen, R.E. Association of Secondary Preventive Cardiovascular Treatment after Myocardial Infarction with Mortality among Patients with Schizophrenia. JAMA Psychiatry 2018, 75, 1234–1240. [Google Scholar] [CrossRef] [Green Version]
- Song, G.G.; Lee, Y.H. The insertion/deletion polymorphism in the angiotensin-converting enzyme and susceptibility to schizophrenia or Parkinson’s disease: A meta-analysis. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 434–442. [Google Scholar] [CrossRef]
- Kruse, D.; Pantelis, C.; Rudd, R.; Quek, J.; Herbert, P.; McKinley, M. Treatment of psychogenic polydipsia: Comparison of risperidone and olanzapine, and the effects of an adjunctive angiotensin-II receptor blocking drug (irbesartan). Aust. N. Z. J. Psychiatry 2001, 35, 65–68. [Google Scholar] [CrossRef]
- Firouzabadi, N.; Ghazanfari, N.; Alavi Shoushtari, A.; Erfani, N.; Fathi, F.; Bazrafkan, M.; Bahramali, E. Genetic Variants of Angiotensin-Converting Enzyme Are Linked to Autism: A Case-Control Study. PLoS ONE 2016, 11, e0153667. [Google Scholar] [CrossRef] [Green Version]
- Welcome, M.O.; Mastorakis, N.E. Neuropathophysiology of coronavirus disease 2019: Neuroinflammation and blood brain barrier disruption are critical pathophysiological processes that contribute to the clinical symptoms of SARS-CoV-2 infection. Inflammopharmacology 2021, 29, 939–963. [Google Scholar] [CrossRef]
- Magalhaes, G.S.; Rodrigues-Machado, M.D.G.; Motta-Santos, D.; Campagnole-Santos, M.J.; Santos, R.A.S. Activation of Ang-(1-7)/Mas Receptor Is a Possible Strategy to Treat Coronavirus (SARS-CoV-2) Infection. Front. Physiol. 2020, 11, 730. [Google Scholar] [CrossRef]
- Zamai, L. The Yin and Yang of ACE/ACE2 pathways: The rationale for the use of renin-angiotensin system inhibitors in COVID-19 patients. Cells 2000, 9, 1704. [Google Scholar] [CrossRef] [PubMed]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doobay, M.F.; Talman, L.S.; Obr, T.D.; Tian, X.; Davisson, R.L.; Lazartigues, E. Differential expression of neuronal ACE2 in transgenic mice with overexpression of the brain renin-angiotensin system. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 292, R373–R381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, P.E.; Chappell, M.C.; Ferrario, C.M.; Tallant, E.A. Distinct roles for ANG II and ANG-(1-7) in the regulation of angiotensin-converting enzyme 2 in rat astrocytes. Am. J. Physiol. Cell. Physiol. 2006, 290, C420–C426. [Google Scholar] [CrossRef] [PubMed]
- Sakima, A.; Averill, D.B.; Gallagher, P.E.; Kasper, S.O.; Tommasi, E.N.; Ferrario, C.M.; Diz, D.I. Impaired heart rate baroreflex in older rats: Role of endogenous angiotensin-(1-7) at the nucleus tractus solitarii. Hypertension 2005, 46, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Buzhdygan, T.P.; DeOre, B.J.; Baldwin-Leclair, A.; Bullock, T.A.; McGary, H.M.; Khan, J.A.; Razmpour, R.; Hale, J.F.; Galie, P.A.; Potula, R.; et al. The SARS-CoV-2 spike protein alters barrier function in 2D static and 3D microfluidic in-vitro models of the human blood-brain barrier. Neurobiol. Dis. 2020, 146, 105131. [Google Scholar] [CrossRef]
- Kehoe, P.G.; Wong, S.; Al Mulhim, N.; Palmer, L.E.; Miners, J.S. Angiotensin-converting enzyme 2 is reduced in Alzheimer’s disease in association with increasing amyloid-β and tau pathology. Alzheimer’s Res. Ther. 2016, 8, 50. [Google Scholar] [CrossRef] [Green Version]
- Chen, R.; Wang, K.; Yu, J.; Howard, D.; French, L.; Chen, Z.; Wen, C.; Xu, Z. The Spatial and Cell-Type Distribution of SARS-CoV-2 Receptor ACE2 in the Human and Mouse Brains. Front. Neurol. 2021, 11, 573095. [Google Scholar] [CrossRef]
- McMahon, C.L.; Staples, H.; Gazi, M.; Carrion, R.; Hsieh, J. SARS-CoV-2 targets glial cells in human cortical organoids. Stem Cell Rep. 2021, 16, 1156–1164. [Google Scholar] [CrossRef]
- Netland, J.; Meyerholz, D.K.; Moore, S.; Cassell, M.; Perlman, S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J. Virol. 2008, 82, 7264–7275. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; He, L.; Zhang, Q.; Huang, Z.; Che, X.; Hou, J.; Wang, H.; Shen, H.; Qiu, L.; Li, Z.; et al. Organ distribution of severe acute respiratory syndrome (SARS) associated coronavirus (SARS-CoV) in SARS patients: Implications for pathogenesis and virus transmission pathways. J. Pathol. 2004, 203, 622–630. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhong, S.; Liu, J.; Li, L.; Li, Y.; Wu, X.; Li, Z.; Deng, P.; Zhang, J.; Zhong, N.; et al. Detection of severe acute respiratory syndrome coronavirus in the brain: Potential role of the chemokine mig in pathogenesis. Clin. Infect. Dis. 2005, 41, 1089–1096. [Google Scholar] [CrossRef] [Green Version]
- Song, E.; Zhang, C.; Israelow, B.; Lu-Culligan, A.; Prado, A.V.; Skriabine, S.; Lu, P.; Weizman, O.E.; Liu, F.; Dai, Y.; et al. Neuroinvasion of SARS-CoV-2 in human and mouse brain. J. Exp. Med. 2021, 218, e20202135. [Google Scholar] [CrossRef] [PubMed]
- Brognara, F.; Felippe, I.S.A.; Salgado, H.C.; Paton, J.F.R. Autonomic innervation of the carotid body as a determinant of its sensitivity: Implications for cardiovascular physiology and pathology. Cardiovasc. Res. 2021, 117, 1015–1032. [Google Scholar] [CrossRef] [PubMed]
- Machado, B.H.; Paton, J.F.R. Relevance of carotid bodies in COVID-19: A hypothetical viewpoint. Auton Neurosci. 2021, 233, 102810. [Google Scholar] [CrossRef] [PubMed]
- Porzionato, A.; Emmi, A.; Stocco, E.; Barbon, S.; Boscolo-Berto, R.; Macchi, V.; De Caro, R. The potential role of the carotid body in COVID-19. Am. J. Physiol. Lung Cell. Mol. Physiol. 2020, 319, L620–L626. [Google Scholar] [CrossRef] [PubMed]
- Villadiego, J.; Ramírez-Lorca, R.; Cala, F.; Labandeira-García, J.L.; Esteban, M.; Toledo-Aral, J.J.; López-Barneo, J. Is Carotid Body Infection Responsible for Silent Hypoxemia in COVID-19 Patients? Function 2020, 2, zqaa032. [Google Scholar] [CrossRef]
- Lambermont, B.; Davenne, E.; Maclot, F.; Delvenne, P. SARS-CoV-2 in carotid body. Intensive Care Med. 2021, 47, 342–343. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Wsol, A.; Cudnoch-Jedrzejewska, A.; Żera, T. Complementary role of oxytocin and vasopressin in cardiovascular regulation. Int. J. Mol. Sci. 2021, 22, 11465. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Zera, T.; Sosnowski, P.; Cudnoch-Jedrzejewska, A.; Puszko, A.; Misicka, A. Vasopressin and related peptides; potential value in diagnosis, prognosis and treatment of clinical disorders. Curr. Drug Metab. 2017, 18, 306–345. [Google Scholar] [CrossRef]
- Gimpl, G.; Fahrenholz, F. The oxytocin receptor system: Structure, function, and regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Góźdź, A.; Szczepańska-Sadowska, E.; Maśliński, W.; Kumosa, M.; Szczepańska, K.; Dobruch, J. Differential expression of vasopressin V1a and V1b receptors mRNA in the brain of renin transgenic TGR (mRen2) 27 and Sprague–Dawley rats. Brain Res. Bull. 2003, 59, 399–3403. [Google Scholar] [CrossRef]
- Hallbeck, M.; Blomqvist, A. Spinal cord-projecting vasopressinergic neurons in the rat paraventricular hypothalamus. J. Comp. Neurol. 1999, 411, 201–211. [Google Scholar] [CrossRef]
- Hallbeck, M.; Hermanson, O.; Blomqvist, A. Distribution of preprovasopressin mRNA in the rat central nervous system. J. Comp. Neurol. 1999, 411, 181–200. [Google Scholar] [CrossRef]
- Phillips, P.A.; Abrahams, J.M.; Kelly, J.; Paxinos, G.; Grzonka, Z.; Mendelsohn, F.A.; Johnston, C.I. Localization of vasopressin binding sites in rat brain by in vitro autoradiography using a radioiodinated V1 receptor antagonist. Neuroscience 1988, 27, 749–761. [Google Scholar] [CrossRef]
- Young, L.; Toloczko, D.; Insel, R. Localization of vasopressin (V1a) receptor binding and mRNA in the rhesus monkey brain. J. Neuroendocrinol. 1999, 11, 291–298. [Google Scholar] [CrossRef]
- Czarzasta, K.; Wojno, O.; Zera, T.; Puchalska, L.; Dobruch, J.; Cudnoch-Jedrzejewska, A. The influence of post-infarct heart failure and high fat diet on the expression of apelin APJ and vasopressin V1a and V1b receptors. Neuropeptides 2019, 78, 101975. [Google Scholar] [CrossRef]
- Mathai, M.L.; Evered, M.D.; McKinley, M.J. Central losartan blocks natriuretic, vasopressin, and pressor responses to central hypertonic NaCl in sheep. Am. J. Physiol. 1998, 275, R548–R554. [Google Scholar] [CrossRef]
- Schrier, R.W. Interactions between angiotensin II and arginine vasopressin in water homeostasis. Kidney Int. 2009, 76, 137–139. [Google Scholar] [CrossRef] [Green Version]
- Caldwell, H.K. Oxytocin and Vasopressin: Powerful Regulators of Social Behavior. Neuroscientist 2017, 23, 517–528. [Google Scholar] [CrossRef]
- Caldwell, H.K.; Aulino, E.A.; Rodriguez, K.M.; Witchey, S.K.; Yaw, A.M. Social Context, Stress, Neuropsychiatric Disorders, and the Vasopressin 1b Receptor. Front. Neurosci. 2017, 11, 567. [Google Scholar] [CrossRef] [PubMed]
- De Wied, D. Neuropeptides as psychotropic drugs. Acta Neuropsychiatr. 1992, 4, 1–7. [Google Scholar] [CrossRef] [PubMed]
- De Wied, D.; Sigling, H.O. Neuropeptides involved in the pathophysiology of schizophrenia and major depression. Neurotox. Res. 2002, 4, 453–468. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, J.B.; Aftab, A.; Radhakrishnan, R.; Widge, A.; Rodriguez, C.I.; Carpenter, L.L.; Nemeroff, C.B.; McDonald, W.M.; Kalin, N.H.; APA Council of Research Task Force on Novel Biomarkers and Treatments. Hormonal Treatments for Major Depressive Disorder: State of the Art. Am. J. Psychiatry 2020, 177, 686–705. [Google Scholar] [CrossRef]
- Frank, E.; Landgraf, R. The vasopressin system-from antidiuresis to psychopathology. Eur. J. Pharmacol. 2008, 583, 226–242. [Google Scholar] [CrossRef]
- Iovino, M.; Messana, T.; De Pergola, G.; Iovino, E.; Dicuonzo, F.; Guastamacchia, E.; Giagulli, V.A.; Triggiani, V. The Role of Neurohypophyseal Hormones Vasopressin and Oxytocin in Neuropsychiatric Disorders. Endocr. Metab. Immune Disord. Drug Targets Actions 2018, 18, 341–347. [Google Scholar] [CrossRef] [PubMed]
- Kunitake, Y.; Imamura, Y.; Mizoguchi, Y.; Matsushima, J.; Tateishi, H.; Murakawa-Hirachi, T.; Nabeta, H.; Kawashima, T.; Kojima, N.; Yamada, S.; et al. Serum Oxytocin Levels and Logical Memory in Older People in Rural Japan. J. Geriatr. Psychiatry Neurol. 2021, 34, 156–161. [Google Scholar] [CrossRef]
- De Winter, R.F.; van Hemert, A.M.; De Rijk, R.H.; Zwinderman, K.H.; Frankhuijzen-Sierevogel, A.C.; Wiegant, V.M.; Goekoop, J.G. Anxious-retarded depression: Relation with plasma vasopressin and cortisol. Neuropsychopharmacology 2003, 28, 140–147. [Google Scholar] [CrossRef] [Green Version]
- Gjerris, A.; Hammer, M.; Vendsborg, P.; Christensen, N.J.; Rafaelsen, O.J. Cerebrospinal fluid vasopressin-changes in depression. Br. J. Psychiatry 1985, 147, 696–701. [Google Scholar] [CrossRef]
- Rubin, L.H.; Carter, C.S.; Bishop, J.R.; Pournajafi-Nazarloo, H.; Drogos, L.L.; Hill, S.K.; Ruocco, A.C.; Keedy, S.K.; Reilly, J.L.; Keshavan, M.S.; et al. Reduced levels of vasopressin and reduced behavioral modulation of oxytocin in psychotic disorders. Schizophr. Bull. 2014, 40, 1374–1384. [Google Scholar] [CrossRef] [Green Version]
- Surget, A.; Belzung, C. Involvement of vasopressin in affective disorders. Eur. J. Pharmacol. 2008, 583, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Van Londen, L.; Goekoop, J.G.; van Kempen, G.M.; Frankhuijzen-Sierevogel, A.C.; Wiegant, V.M.; van der Velde, E.A.; De Wied, D. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology 1997, 17, 284–292. [Google Scholar] [CrossRef] [Green Version]
- Rutigliano, G.; Rocchetti, M.; Paloyelis, Y.; Gilleen, J.; Sardella, A.; Cappucciati, M.; Palombini, E.; Dell’Osso, L.; Caverzasi, E.; Politi, P.; et al. Peripheral oxytocin and vasopressin: Biomarkers of psychiatric disorders? A comprehensive systematic review and preliminary meta-analysis. Psychiatry Res. 2016, 241, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Barhale, C.; Raju, M.S.V.K.; Pawar, A.V.; Kadam, K.; De Sousa, A.; Andrade, C. Serum Oxytocin Concentration in Patients Receiving Electroconvulsive Therapy: An Exploratory Study and Review of Literature. J. ECT 2017, 33, 122–125. [Google Scholar] [CrossRef] [PubMed]
- Demitrack, M.A.; Gold, P.W. Oxytocin: Neurobiologic considerations and their implications for affective illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 1988, 12 (Suppl. 1), S23–S51. [Google Scholar] [CrossRef]
- Sasayama, D.; Hattori, K.; Teraishi, T.; Hori, H.; Ota, M.; Yoshida, S.; Arima, K.; Higuchi, T.; Amano, N.; Kunugi, H. Negative correlation between cerebrospinal fluid oxytocin levels and negative symptoms of male patients with schizophrenia. Schizophr. Res. 2012, 139, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Pitts, A.F.; Samuelson, S.D.; Meller, W.H.; Bissette, G.; Nemeroff, C.B.; Kathol, R.G. Cerebrospinal fluid corticotropin-releasing hormone, vasopressin, and oxytocin concentrations in treated patients with major depression and controls. Biol. Psychiatry 1995, 38, 330–335. [Google Scholar] [CrossRef]
- Lee, M.R.; Sheskier, M.B.; Farokhnia, M.; Feng, N.; Marenco, S.; Lipska, B.K.; Leggio, L. Oxytocin receptor mRNA expression in dorsolateral prefrontal cortex in major psychiatric disorders: A human post-mortem study. Psychoneuroendocrinology 2018, 96, 143–147. [Google Scholar] [CrossRef]
- Landgraf, R.; Neumann, I.D. Vasopressin and oxytocin release within the brain: A dynamic concept of multiple and variable modes of neuropeptide communication. Front. Neuroendocrinol. 2004, 25, 150–176. [Google Scholar] [CrossRef]
- Heinrichs, M.; Meinlschmidt, G.; Neumann, I.; Wagner, S.; Kirschbaum, C.; Ehlert, U.; Hellhammer, D.H. Effects of suckling on hypothalamic-pituitary-adrenal axis responses to psychosocial stress in postpartum lactating women. J. Clin. Endocrinol. Metab. 2001, 86, 4798–4804. [Google Scholar] [CrossRef]
- Skrundz, M.; Bolten, M.; Nast, I.; Hellhammer, D.H.; Meinlschmidt, G. Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology 2011, 36, 1886–1893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Cagna, F.; Fusar-Poli, L.; Damiani, S.; Rocchetti, M.; Giovanna, G.; Mori, A.; Politi, P.; Brondino, N. The Role of Intranasal Oxytocin in Anxiety and Depressive Disorders: A Systematic Review of Randomized Controlled Trials. Clin. Psychopharmacol. Neurosci. 2019, 17, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Engel, S.; Laufer, S.; Knaevelsrud, C.; Schumacher, S. The endogenous oxytocin system in depressive disorders: A systematic review and meta-analysis. Psychoneuroendocrinology 2019, 101, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Soeken, T.A.; Cromer, S.J.; Martinez, S.R.; Hardy, L.R.; Strathearn, L. Oxytocin and postpartum depression: Delivering on what’s known and what’s not. Brain Res. 2014, 1580, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Moura, D.; Canavarro, M.C.; Figueiredo-Braga, M. Oxytocin and depression in the perinatal period-a systematic review. Arch. Women’s Ment. Health 2016, 19, 561–570. [Google Scholar] [CrossRef]
- Scantamburlo, G.; Hansenne, M.; Geenen, V.; Legros, J.J.; Ansseau, M. Additional intranasal oxytocin to escitalopram improves depressive symptoms in resistant depression: An open trial. Eur. Psychiatry 2015, 30, 65–68. [Google Scholar] [CrossRef]
- Pincus, D.; Kose, S.; Arana, A.; Johnson, K.; Morgan, P.S.; Borckardt, J.; Herbsman, T.; Hardaway, F.; George, M.S.; Panksepp, J.; et al. Inverse effects of oxytocin on attributing mental activity to others in depressed and healthy subjects: A double-blind placebo controlled FMRI study. Front. Psychiatry 2010, 1, 134. [Google Scholar] [CrossRef] [Green Version]
- Pitman, R.K.; Orr, S.P.; Lasko, N.B. Effects of intranasal vasopressin and oxytocin on physiologic responding during personal combat imagery in Vietnam veterans with posttraumatic stress disorder. Psychiatry Res. 1993, 48, 107–117. [Google Scholar] [CrossRef]
- Lardenoije, R.; Roubroeks, J.A.Y.; Pishva, E.; Leber, M.; Wagner, H.; Iatrou, A.; Smith, A.R.; Smith, R.G.; Eijssen, L.M.T.; Kleineidam, L.; et al. Alzheimer’s disease-associated (hydroxy)methylomic changes in the brain and blood. Clin. Epigenetics 2019, 11, 164. [Google Scholar] [CrossRef]
- Green, J.J.; Hollander, E. Autism and oxytocin: New developments in translational approaches to therapeutics. Neurotherapeutics 2010, 7, 250–257. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.F.; Dai, Y.C.; Wu, J.; Jia, N.X.; Zhang, J.S.; Shou, X.J.; Han, S.P.; Zhang, R.; Han, J.S. Plasma oxytocin and arginine-vasopressin levels in childre with autism spectrum disorders in China: Association with symptoms. Neurosci. Bull. 2016, 32, 423–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendaus, M.; Jomha, F.A.; Alhammadi, A.H. Vasopressin in the Amelioration of Social Functioning in Autism Spectrum Disorder. J. Clin. Med. 2019, 8, 1061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yatawara, C.J.; Einfeld, S.L.; Hickie, I.B.; Davenport, T.A.; Guastella, A.J. The effect of oxytocin nasal spray on social interaction deficits observed in young children with autism: A randomized clinical crossover trial. Mol. Psychiatry 2016, 21, 1225–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rich, M.E.; Caldwell, H.K. A Role for Oxytocin in the Etiology and Treatment of Schizophrenia. Front. Endocrinol. 2015, 6, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
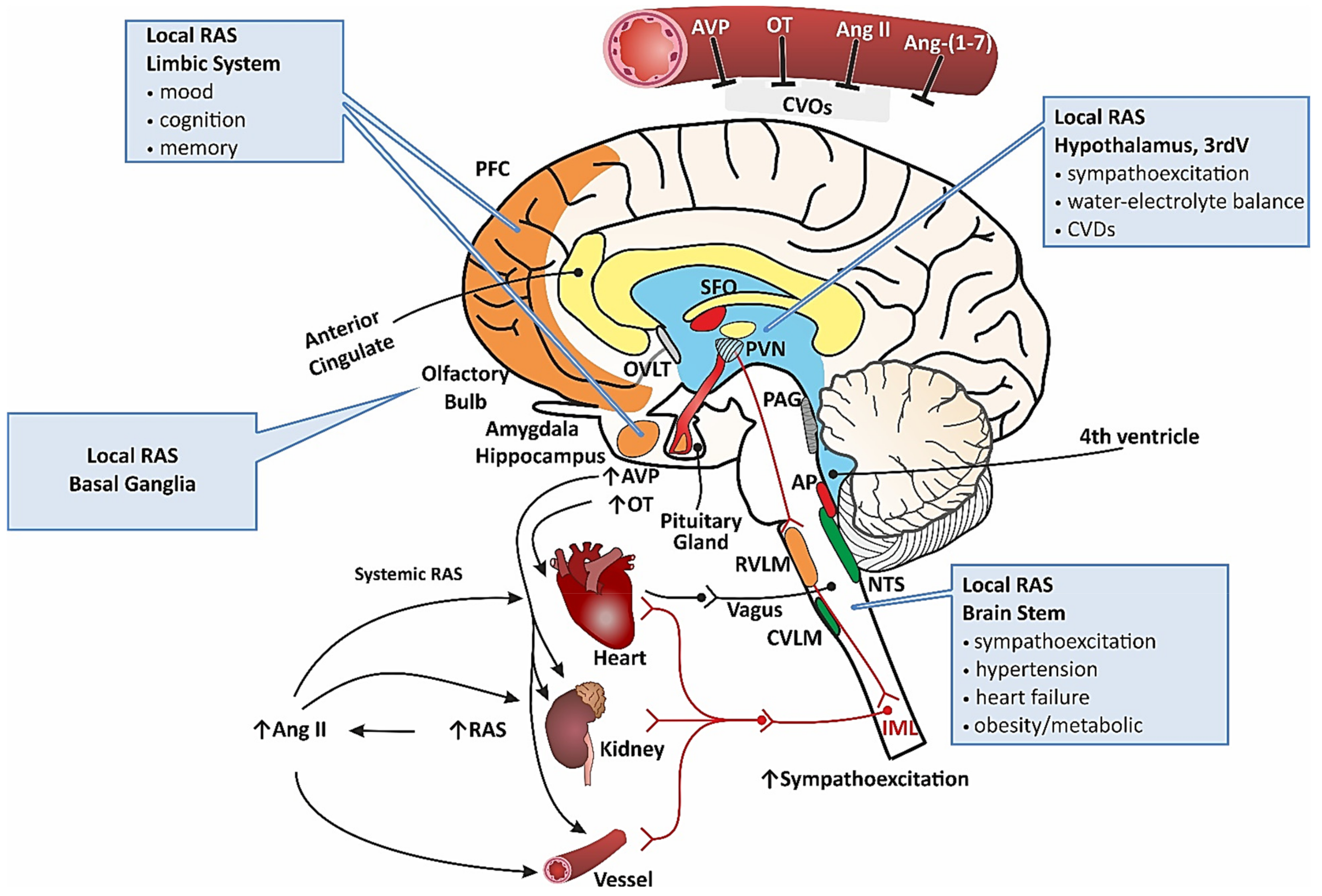
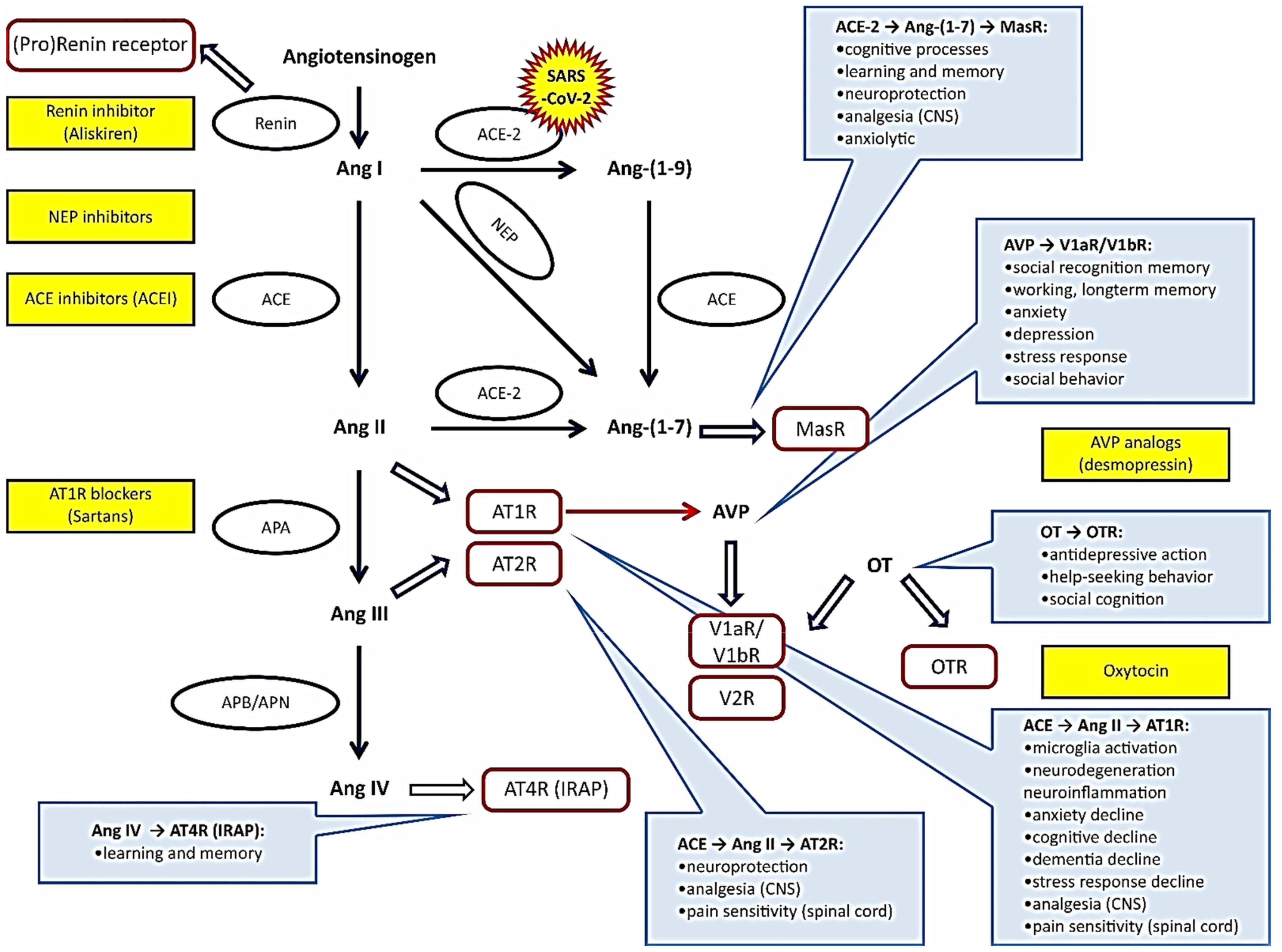
| Neuropsychiatric/Neurodegenrative Disorder | Functional Action | References |
|---|---|---|
| Renin–angiotensin system (RAS) | ||
| Cognitive disorders | Human and rodent studies: | |
| [98,99,100,101,102] | |
| Rodent studies: | ||
| [103,104,105] | |
| Alzheimer’s disease | Human studies: | |
| [106,107,108] | |
| Human and rodent studies: | ||
| [109,110,111,112,113] | |
| Rodent studies: | ||
| [114] | |
| Stress and pain | Human and rodent studies: | |
| [16,23,25,66,67,85,87,115] | |
| Rodent studies: | ||
| [116] | |
| [117,118] | |
| Affective disorders | Human studies: | |
| [119] | |
| [120] | |
| Schizophrenia | Human studies: | |
| [121,122] | |
| [123] | |
| [124] | |
| Parkinson’s disease | Rodent studies: | |
| [51] | |
| [125,126,127] | |
| [37,125] | |
| Tardive dyskinesia | Rodent studies: | |
| [128] | |
| Psychiatric symptoms in COVID 19 | Human studies: | |
| [129] | |
| ||
| Vasopressin system (VPS) | ||
| Affective disorders | Human studies: | |
| [130] | |
| [131] | |
| [132] | |
| [133] | |
| [134,135,136] | |
| [134,135] | |
| Rodent studies: | ||
| [137,138,139] | |
| [140] | |
| [26,140,141,142,143,144,145] | |
| [146,147] | |
| Alzheimer’s disease | Human studies: | |
| [148] | |
| [149] | |
| [150] | |
| [151,152] | |
| Rodent studies: | ||
| [153] | |
| [154] | |
| Schizophrenia | Human studies: | |
| [155] | |
| [156] | |
| [157,158,159] | |
| [159] | |
| [160,161,162] | |
| [163] | |
| [164] | |
| Rodent studies: | ||
| [165,166,167] | |
| [168] | |
| Autism spectrum disorder | Human studies: | |
| [169,170,171] | |
| [170] | |
| [172,173,174,175] | |
| [176,177] | |
| Oxytocin system (OTS) | ||
| Alzheimer’s disease | Human studies: | |
| [151,178,179,180,181,182] | |
| [183] | |
| Affective disorders | Human studies: | |
| [130,184,185,186] | |
| [184,187,188,189,190,191,192] | |
| [184,193,194] | |
| [195,196,197,198,199,200] | |
| [201] | |
| [202] | |
| Rodent studies: | ||
| [201] | |
| Schizophrenia |
| |
| [164] | |
| [158,203] | |
| [157] | |
| [204,205] | |
| [206,207,208,209] | |
| Rodent studies: | ||
| [168] | |
| Autism spectrum disorder | Human studies: | |
| [210,211,212,213,214,215,216,217] | |
| [218,219,220,221] | |
| [222,223] | |
| [176,224] | |
| [225,226,227] | |
| [228] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szczepanska-Sadowska, E.; Wsol, A.; Cudnoch-Jedrzejewska, A.; Czarzasta, K.; Żera, T. Multiple Aspects of Inappropriate Action of Renin–Angiotensin, Vasopressin, and Oxytocin Systems in Neuropsychiatric and Neurodegenerative Diseases. J. Clin. Med. 2022, 11, 908. https://doi.org/10.3390/jcm11040908
Szczepanska-Sadowska E, Wsol A, Cudnoch-Jedrzejewska A, Czarzasta K, Żera T. Multiple Aspects of Inappropriate Action of Renin–Angiotensin, Vasopressin, and Oxytocin Systems in Neuropsychiatric and Neurodegenerative Diseases. Journal of Clinical Medicine. 2022; 11(4):908. https://doi.org/10.3390/jcm11040908
Chicago/Turabian StyleSzczepanska-Sadowska, Ewa, Agnieszka Wsol, Agnieszka Cudnoch-Jedrzejewska, Katarzyna Czarzasta, and Tymoteusz Żera. 2022. "Multiple Aspects of Inappropriate Action of Renin–Angiotensin, Vasopressin, and Oxytocin Systems in Neuropsychiatric and Neurodegenerative Diseases" Journal of Clinical Medicine 11, no. 4: 908. https://doi.org/10.3390/jcm11040908
APA StyleSzczepanska-Sadowska, E., Wsol, A., Cudnoch-Jedrzejewska, A., Czarzasta, K., & Żera, T. (2022). Multiple Aspects of Inappropriate Action of Renin–Angiotensin, Vasopressin, and Oxytocin Systems in Neuropsychiatric and Neurodegenerative Diseases. Journal of Clinical Medicine, 11(4), 908. https://doi.org/10.3390/jcm11040908






