Heart Transplantation of the Elderly—Old Donors for Old Recipients: Can We Still Achieve Acceptable Results?
Abstract
:1. Introduction
2. Materials and Methods
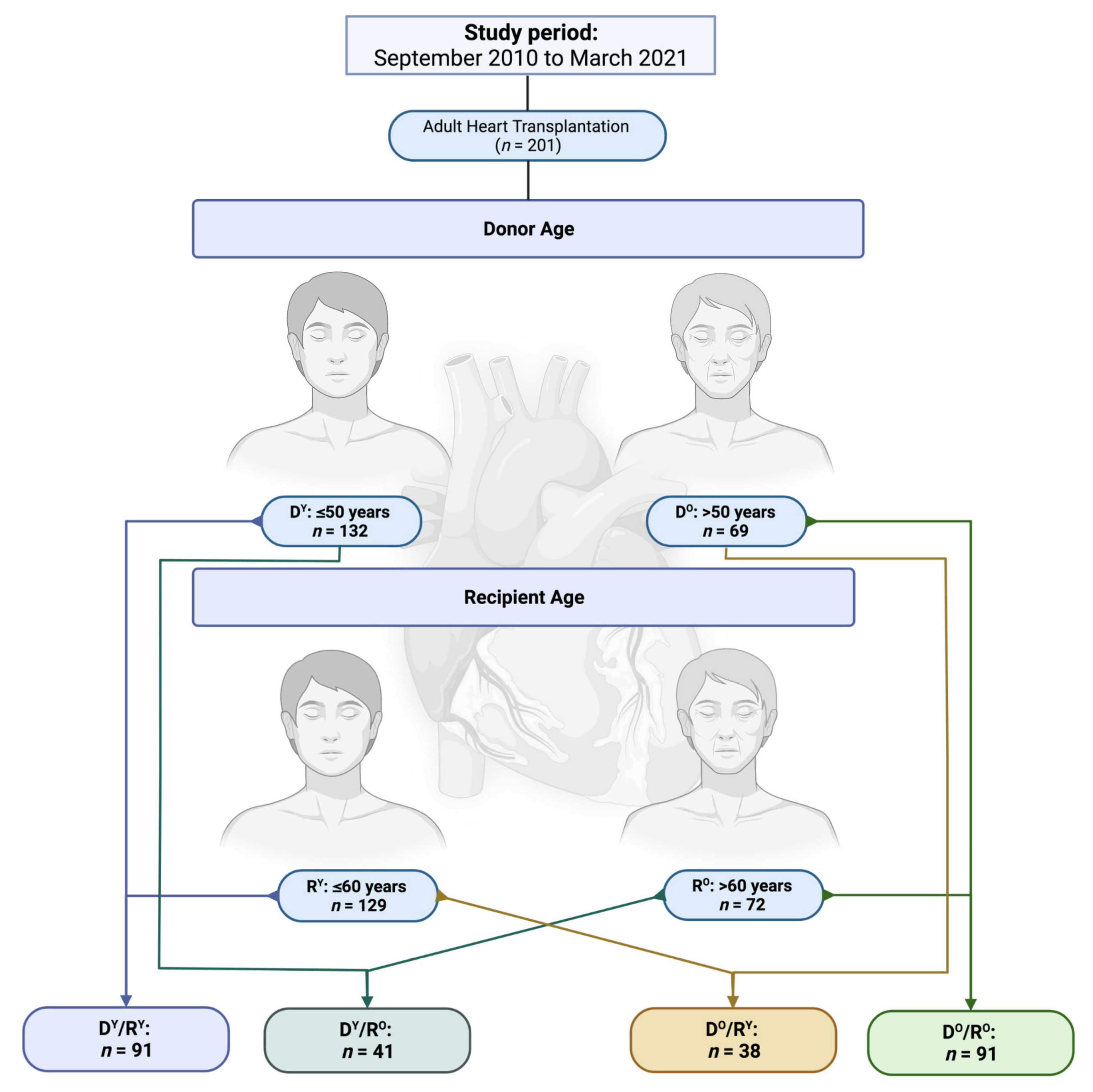
2.1. Patients and Study Design
2.2. Study Objectives and Follow-Up Period
2.3. Surgical Procedure and Perioperative Management
2.4. Statistics
3. Results
3.1. Pre-Transplant Recipient Parameters
3.2. Pre-Transplant Donor Parameters
3.3. Operative Outcome
3.4. Postoperative Survival
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ziaeian, B.; Fonarow, G.C. Epidemiology and aetiology of heart failure. Nat. Rev. Cardiol. 2016, 13, 368–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazurek, J.A.; Jessup, M. Understanding Heart Failure. Heart Fail. Clin. 2017, 13, 1–19. [Google Scholar] [CrossRef]
- Dharmarajan, K.; Rich, M.W. Epidemiology, pathophysiology, and prognosis of heart failure in older adults. Heart Fail. Clin. 2017, 13, 417–426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; Gonzalez-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [CrossRef] [PubMed]
- Yancy, C.W.; Jessup, M.; Bozkurt, B.; Butler, J.; Casey, D.E., Jr.; Drazner, M.H.; Fonarow, G.C.; Geraci, S.A.; Horwich, T.; Januzzi, J.L.; et al. 2013 ACCF/AHA guideline for the management of heart failure: A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013, 62, e147–e239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitale, C.; Jankowska, E.; Hill, L.; Piepoli, M.; Doehner, W.; Anker, S.D.; Lainscak, M.; Jaarsma, T.; Ponikowski, P.; Rosano, G.M.C.; et al. Heart Failure Association/European Society of Cardiology position paper on frailty in patients with heart failure. Eur. J. Heart Fail. 2019, 21, 1299–1305. [Google Scholar] [CrossRef] [PubMed]
- Daneshvar, D.A.; Czer, L.S.; Phan, A.; Trento, A.; Schwarz, E.R. Heart transplantation in the elderly: Why cardiac transplantation does not need to be limited to younger patients but can be safely performed in patients above 65 years of age. Ann. Transplant. 2010, 15, 110–119. [Google Scholar]
- Echterdiek, F.; Schwenger, V.; Döhler, B.; Latus, J.; Kitterer, D.; Heemann, U.; Süsal, C. Kidneys from elderly deceased donors-is 70 the new 60? Front. Immunol. 2019, 10, 2701. [Google Scholar] [CrossRef]
- Schachtner, T.; Otto, N.M.; Reinke, P. Two decades of the Eurotransplant Senior Program: The gender gap in mortality impacts patient survival after kidney transplantation. Clin. Kidney J. 2019, 13, 1091–1100. [Google Scholar] [CrossRef]
- Del Rizzo, D.F.; Menkis, A.H.; Pflugfelder, P.W.; Novick, R.J.; McKenzie, F.N.; Boyd, W.D.; Kostuk, W.J. The role of donor age and ischemic time on survival following orthotopic heart transplantation. J. Heart Lung Transplant. 1999, 18, 310–319. [Google Scholar] [CrossRef]
- Immohr, M.B.; Akhyari, P.; Boettger, C.; Mehdiani, A.; Dalyanoglu, H.; Westenfeld, R.; Tudorache, I.; Aubin, H.; Lichtenberg, A.; Boeken, U. Effects of donor age and ischemia time on outcome after heart transplant: A 10-year single-center experience. Exp. Clin. Transplant. Off. J. Middle East Soc. Organ Transplant. 2021, 19, 351–358. [Google Scholar] [CrossRef]
- Singh, S.S.A.; Dalzell, J.R.; Berry, C.; Al-Attar, N. Primary graft dysfunction after heart transplantation: A thorn amongst the roses. Heart Fail. Rev. 2019, 24, 805–820. [Google Scholar] [CrossRef] [Green Version]
- Kobashigawa, J.; Zuckermann, A.; Macdonald, P.; Leprince, P.; Esmailian, F.; Luu, M.; Mancini, D.; Patel, J.; Razi, R.; Reichenspurner, H.; et al. Report from a consensus conference on primary graft dysfunction after cardiac transplantation. J. Heart Lung Transplant. 2014, 33, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Jawitz, O.K.; Raman, V.; Klapper, J.; Hartwig, M.; Patel, C.B.; Milano, C. Donor and recipient age matching in heart transplantation: Analysis of the UNOS Registry. Transpl. Int. 2019, 32, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Lechiancole, A.; Vendramin, I.; Sponga, S.; Guzzi, G.; Ferrara, V.; Nalli, C.; Di Nora, C.; Bortolotti, U.; Livi, U. Donor-recipient age interaction and the impact on clinical results after heart transplantation. Clin. Transplant. 2020, 34, e14043. [Google Scholar] [CrossRef] [PubMed]
- López-Vilella, R.; González-Vílchez, F.; Crespo-Leiro, M.G.; Segovia-Cubero, J.; Cobo, M.; Delgado-Jiménez, J.; Arizón Del Prado, J.M.; Martínez-Sellés, M.; Sobrino Márquez, J.M.; Mirabet-Pérez, S.; et al. Impact of donor-recipient age on cardiac transplant survival. Subanalysis of the Spanish Heart Transplant Registry. Rev. Esp. Cardiol. 2021, 74, 393–401. [Google Scholar] [CrossRef] [PubMed]
- Ram, E.; Lavee, J.; Kogan, A.; Kassif, Y.; Elian, D.; Freimark, D.; Peled, Y. Does donor-recipient age difference matter in outcome of heart transplantation? Clin. Transplant. 2019, 33, e13593. [Google Scholar] [CrossRef] [PubMed]
- Eurotransplant, I.F. Eurotransplant International Foundation, Annual Report. 2018. Available online: https://www.eurotransplant.org/cms/mediaobject.php?file=ET_Jaarverslag_20181.pdf (accessed on 15 July 2019).
- Fuchs, M.; Schibilsky, D.; Zeh, W.; Berchtold-Herz, M.; Beyersdorf, F.; Siepe, M. Does the heart transplant have a future? Eur. J. Cardiothorac. Surg. 2019, 55, i38–i48. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.C.; Sareyyupoglu, B.; Pham, S.M. Left ventricular assist devices in the elderly: Marching forward with cautions. J. Card. Surg. 2020, 35, 3409–3411. [Google Scholar] [CrossRef]
- Lindvall, C.; Udelsman, B.; Malhotra, D.; Brovman, E.Y.; Urman, R.D.; D’Alessandro, D.A.; Tulsky, J.A. In-hospital mortality in older patients after ventricular assist device implantation: A national cohort study. J. Thorac. Cardiovasc. Surg. 2018, 158, 466–475.e464. [Google Scholar] [CrossRef]
- Gazda, A.J.; Kwak, M.J.; Akkanti, B.; Nathan, S.; Kumar, S.; de Armas, I.S.; Baer, P.; Patel, B.; Kar, B.; Gregoric, I.D. Complications of LVAD utilization in older adults. Heart Lung 2020, 50, 75–79. [Google Scholar] [CrossRef] [PubMed]
- Crespo-Leiro, M.G.; Anker, S.D.; Maggioni, A.P.; Coats, A.J.; Filippatos, G.; Ruschitzka, F.; Ferrari, R.; Piepoli, M.F.; Delgado Jimenez, J.F.; Metra, M.; et al. European Society of Cardiology Heart Failure Long-Term Registry (ESC-HF-LT): 1-year follow-up outcomes and differences across regions. Eur. J. Heart Fail. 2016, 18, 613–625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, N.R.; Roalfe, A.K.; Adoki, I.; Hobbs, F.D.R.; Taylor, C.J. Survival of patients with chronic heart failure in the community: A systematic review and meta-analysis. Eur. J. Heart Fail. 2019, 21, 1306–1325. [Google Scholar] [CrossRef] [PubMed]
- Claes, S.; Berchtold-Herz, M.; Zhou, Q.; Trummer, G.; Bock, M.; Zirlik, A.; Beyersdorf, F.; Bode, C.; Grundmann, S. Towards a cardiac allocation score: A retrospective calculation for 73 patients from a German transplant center. J. Cardiothorac. Surg. 2017, 12, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| DY/RY | DO/RY | DY/RO | DO/RO | p-Value | |
|---|---|---|---|---|---|
| Recipient Variables | (n = 91) | (n = 38) | (n = 41) | (n = 31) | |
| Age, y (SD) | 48 (11) | 52 (8) | 64 (3) | 65 (3) | <0.01 |
| Female gender, n (%) | 26 (28.6) | 12 (31.6) | 8 (19.5) | 7 (22.6) | 0.59 |
| Height, cm (SD) | 175 (8) | 173 (11) | 176 (7) | 174 (7) | 0.57 |
| Weight, kg (SD) | 78 (16) | 75 (16) | 79 (16) | 79 (13) | 0.52 |
| Body mass index, kg/m2 (SD) | 25.7 (4.9) | 25.2 (5.0) | 25.5 (4.0) | 26.2 (3.8) | 0.64 |
| Panel-reactive antibodies, % (SD) | 3.1 (14.5) | 1.3 (6.6) | 3.1 (19.1) | 0.2 (0.9) | 0.66 |
| High urgency wait list status, n (%) | 52 (57.1) | 19 (50.0) | 19 (46.3) | 7 (22.6) | 0.01 |
| Aetiology | |||||
| Ischemic cardiomyopathy, n (%) | 24 (26.4) | 18 (47.4) | 23 (56.1) | 16 (51.6) | 0.20 |
| Dilated cardiomyopathy, n (%) | 55 (60.4) | 19 (50.0) | 16 (39.0) | 13 (41.9) | |
| Other, n (%) | 12 (13.2) | 1 (2.6) | 2 (4.8) | 2 (6.4) | |
| Ventricular assist device, n (%) | 50 (54.9) | 17 (44.7) | 23 (56.1) | 18 (58.1) | 0.66 |
| Extracorporeal life support, n (%) | 6 (6.7) | 2 (5.3) | 1 (2.4) | 0 (0.0) | 0.57 |
| Concomitant diseases | |||||
| Diabetes mellitus, n (%) | 17 (8.7) | 9 (23.7) | 9 (22.0) | 6 (19.4) | 0.58 |
| Haemodialyis, n (%) | 7 (7.8) | 1 (2.6) | 1 (2.6) | 1 (3.2) | 0.65 |
| Smoking, n (%) | 21 (23.1) | 7 (18.4) | 8 (19.5) | 8 (25.8) | 0.74 |
| Arterial hypertension, n (%) | 50 (54.9) | 25 (65.8) | 26 (63.4) | 17 (54.8) | 0.72 |
| Pulmonary hypertension, n (%) | 8 (8.8) | 5 (13.2) | 2 (4.9) | 4 (12.9) | 0.52 |
| COPD, n (%) | 7 (7.7) | 2 (5.3) | 2 (4.9) | 4 (12.9) | 0.60 |
| Cardiopulmonary resuscitation, n (%) | 13 (14.3) | 5 (13.2) | 5 (12.2) | 0 (0.0) | 0.17 |
| Mechanical ventilation, n (%) | 8 (8.8) | 5 (13.2) | 1 (2.4) | 0 (0.0) | 0.01 |
| Blood transfusion, n (%) | 8 (8.8) | 1 (2.6) | 2 (4.9) | 1 (3.2) | 0.58 |
| Laboratory values | |||||
| Hemoglobin, g/dL (SD) | 11.6 (2.4) | 11.5 (2.3) | 12.4 (1.9) | 12.6 (2.4) | 0.05 |
| Bilirubin, mg/dL (SD) | 1.0 (1.2) | 0.8 (0.9) | 0.8 (0.6) | 1.4 (0.4) | 0.83 |
| Creatinine, mg/dL (SD) | 1.4 (1.3) | 1.3 (0.5) | 1.5 (0.7) | 1.4 (0.4) | 0.19 |
| AST, U/L (SD) | 49 (87) | 41 (34) | 29 (15) | 30 (12) | 0.46 |
| Lactate dehydrogenase, U/L (SD) | 413 (460) | 288 (142) | 279 (108) | 285 (86) | 0.87 |
| DY/RY | DO/RY | DY/RO | DO/RO | p-Value | |
|---|---|---|---|---|---|
| Donor Variables | (n = 91) | (n = 38) | (n = 41) | (n = 31) | |
| Age, y (SD) | 35 (10) | 56 (4) | 38 (10) | 58 (10) | <0.01 |
| Female gender, n (%) | 38 (41.8) | 23 (60.5) | 12 (29.3) | 15 (48.4) | 0.04 |
| Height, cm (SD) | 176 (9) | 172 (8) | 177 (6) | 173 (8) | 0.05 |
| Weight, kg (SD) | 80 (15) | 79 (11) | 79 (17) | 81 (15) | 0.81 |
| Body mass index, kg/m2 (SD) | 25.6 (4.1) | 26.7 (3.1) | 25.2 (5.0) | 27.9 (7.0) | 0.01 |
| Predicted Heart Mass Ratio, % (SD) | 13.8 (10.4) | 14.7 (13.4) | 12.6 (9.7) | 11.6 (7.9) | 0.87 |
| Cardiopulmonary resuscitation, n (%) | 24 (26.4) | 3 (7.9) | 19 (46.3) | 6 (19.4) | <0.01 |
| Duration, min (SD) | 18 (13) | 13 (3) | 17 (13) | 22 (17) | 0.92 |
| Norepinephrine, µg/kg/min (SD) | 0.12 (0.16) | 0.14 (0.33) | 0.14 (0.21) | 0.10 (0.09) | 0.68 |
| Ejection fraction, % (SD) | 61 (9) | 62 (10) | 57 (10) | 62 (7) | 0.28 |
| Concomitant diseases | |||||
| Arterial hypertension, n (%) | 14/41 (34.1) | 18/25 (72.0) | 10/22 (45.5) | 16/20 (22.2) | <0.01 |
| Diabetes mellitus, n (%) | 6/37 (16.2) | 2/11 (18.2) | 0/15 (0.0) | 5/10 (50.0) | 0.02 |
| Smoking, n (%) | 49/76 (64.5) | 16/30 (53.3) | 21/39 (53.8) | 14/26 (53.8) | 0.56 |
| Drug abuse, n (%) | 8/75 (10.7) | 1/31 (3.2) | 8/34 (23.5) | 0/24 (0.0) | 0.02 |
| Laboratory values | |||||
| Hemoglobin, g/dL (SD) | 10.1 (2.8) | 9.9 (1.9) | 10.3 (2.9) | 10.3 (2.4) | 0.90 |
| White blood cells, 1 × 109/L (SD) | 15.1 (5.8) | 14.9 (5.8) | 14.3 (4.4) | 21.0 (39.2) | 0.89 |
| Lactate dehydrogenase, U/L (SD) | 510 (681) | 352 (257) | 525 (414) | 347 (191) | 0.04 |
| Creatinine kinase, U/L (SD) | 2029 (8139) | 438 (643) | 1068 (2326) | 682 (1350) | 0.11 |
| C-reactive protein, mg/L (SD) | 163 (232) | 234 (416) | 157 (110) | 151 (96) | 0.55 |
| DY/RY | DO/RY | DY/RO | DO/RO | p-Value | |
|---|---|---|---|---|---|
| Outcome Variables | (n = 91) | (n = 38) | (n = 41) | (n = 31) | |
| Total graft ischemic time, min (SD) | 228 (55) | 208 (45) | 218 (48) | 199 (37) | 0.02 |
| Transport time, min (SD) | 162 (55) | 142 (42) | 151 (46) | 134 (42) | 0.02 |
| Warm ischemia, min (SD) | 66 (15) | 66 (11) | 67 (13) | 65 (16) | 0.71 |
| Primary graft dysfunction | |||||
| Peak catecholamine | |||||
| Dobutamine, µg/kg/min (SD) | 4.81 (2.16) | 5.45 (2.84) | 4.40 (2.29) | 3.27 (2.43) | 0.15 |
| Epinephrine, µg/kg/min (SD) | 0.21 (0.18) | 0.27 (0.22) | 0.20 (0.17) | 0.32 (0.23) | 0.05 |
| Norepinephrine, µg/kg/min (SD) | 0.35 (0.25) | 0.37 (0.26) | 0.35 (0.34) | 0.37 (0.31) | 0.94 |
| va-ECMO, n (%) | 27 (29.7) | 10 (26.3) | 16 (39.0) | 9 (29.0) | 0.64 |
| Support duration, d (SD) | 9.4 (9.3) | 5.7 (5.1) | 6.7 (3.6) | 9.9 (4.9) | 0.34 |
| Deceased on support, n (%) | 6/26 (23.1) | 4/10 (40.0) | 2/16 (12.5) | 2/8 (25.0) | 0.46 |
| Postoperative morbidity | |||||
| Infective complications, n (%) | 19/88 (21.6) | 10/36 (27.8) | 8/40 (20.0) | 12/30 (40.0) | 0.21 |
| Acute graft rejection, n (%) | 7/87 (8.0) | 2/36 (5.6) | 3/40 (7.5) | 2/30 (6.7) | 1.00 |
| Hemodialysis on ICU, n (%) | 43/89 (48.3) | 23/37 (62.2) | 25/40 (62.5) | 19/30 (63.3) | 0.27 |
| Neurological complications, n (%) | 17/88 (19.3) | 5/36 (13.9) | 7/40 (17.5) | 8/30 (26.7) | 0.63 |
| Re-thoracotomy, n (%) | 25/88 (28.4) | 12/37 (32.4) | 13/40 (32.5) | 9/31 (29.0) | 0.93 |
| Postoperative hospital stay, d (SD) | 42 (28) | 41 (24) | 51 (39) | 54 (52) | 0.68 |
| Postoperative ICU/IMC stay, d (SD) | 23 (27) | 20 (20) | 27 (31) | 30 (31) | 0.20 |
| Mechanical ventilation, h (SD) | 145 (197) | 109 (141) | 197 (210) | 183 (232) | 0.29 |
| Blood transfusion | |||||
| Packed red blood cells, mL (SD) | 3716 (5321) | 3085 (3186) | 3309 (2704) | 4646 (5572) | 0.70 |
| Fresh frozen plasma, mL (SD) | 5646 (8252) | 3909 (3179) | 6679 (5497) | 8802 (8972) | 0.09 |
| Platelets, ml (SD) | 1012 (2588) | 833 (1198) | 1106 (1308) | 1775 (2719) | 0.06 |
| 30-day survival, n (%) | 85/90 (94.4) | 32/38 (84.2) | 38/40 (95.0) | 25/31 (80.6) | 0.05 |
| Cause of death within 30 days | 0.48 | ||||
| Graft failure | 1 (20.0) | 1 (16.7) | 0 (0.0) | 2 (33.3) | |
| Sepsis/MODS | 2 (40.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) | |
| Coagulopathy | 1 (20.0) | 2 (33.3) | 1 (50.0) | 1 (16.7) | |
| Cerebral injury | 1 (20.0.) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Visceral ischemia | 0 (0.0) | 0 (0.0) | 1 (50.0) | 0 (0.0) | |
| Other/unknown | 0 (0.0) | 3 (50.0) | 0 (0.0) | 1 (16.7) | |
| 1-year survival, n (%) | 62/72 (86.1) | 23/31 (74.2) | 26/33 (78.8) | 14/25 (56.0) | 0.02 |
| Cause of death between 30 days and 1 year | 0.52 | ||||
| Graft failure | 0 (0.0) | 0 (0.0) | 2 (40.0) | 0 (0.0) | |
| Sepsis/MODS | 2 (40.0) | 1 (50.0) | 1 (20.0) | 3 (60.0) | |
| Coagulopathy | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Cerebral injury | 0 (0.0) | 0 (0.0) | 1 (20.0) | 0 (0.0) | |
| Visceral ischemia | 1 (20.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | |
| Other/unknown | 1 (20.0) | 1 (50.0) | 1 (20.0) | 2 (40.0) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Immohr, M.B.; Aubin, H.; Westenfeld, R.; Erbel-Khurtsidze, S.; Tudorache, I.; Akhyari, P.; Lichtenberg, A.; Boeken, U. Heart Transplantation of the Elderly—Old Donors for Old Recipients: Can We Still Achieve Acceptable Results? J. Clin. Med. 2022, 11, 929. https://doi.org/10.3390/jcm11040929
Immohr MB, Aubin H, Westenfeld R, Erbel-Khurtsidze S, Tudorache I, Akhyari P, Lichtenberg A, Boeken U. Heart Transplantation of the Elderly—Old Donors for Old Recipients: Can We Still Achieve Acceptable Results? Journal of Clinical Medicine. 2022; 11(4):929. https://doi.org/10.3390/jcm11040929
Chicago/Turabian StyleImmohr, Moritz Benjamin, Hug Aubin, Ralf Westenfeld, Sophiko Erbel-Khurtsidze, Igor Tudorache, Payam Akhyari, Artur Lichtenberg, and Udo Boeken. 2022. "Heart Transplantation of the Elderly—Old Donors for Old Recipients: Can We Still Achieve Acceptable Results?" Journal of Clinical Medicine 11, no. 4: 929. https://doi.org/10.3390/jcm11040929
APA StyleImmohr, M. B., Aubin, H., Westenfeld, R., Erbel-Khurtsidze, S., Tudorache, I., Akhyari, P., Lichtenberg, A., & Boeken, U. (2022). Heart Transplantation of the Elderly—Old Donors for Old Recipients: Can We Still Achieve Acceptable Results? Journal of Clinical Medicine, 11(4), 929. https://doi.org/10.3390/jcm11040929








