Paraaortic Lymphadenectomy in Gynecologic Oncology—Significance of Vessels Variations
Abstract
1. Introduction
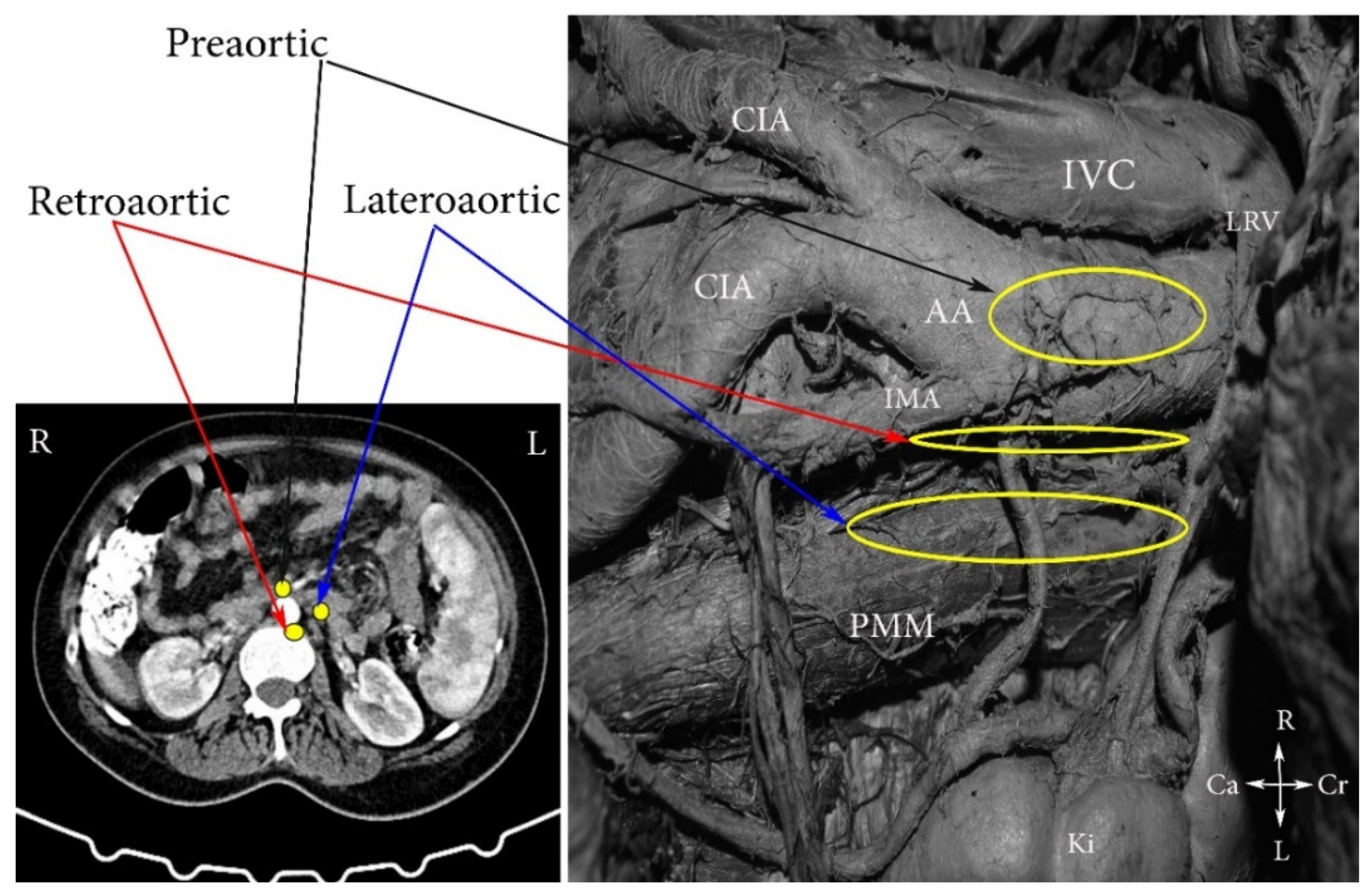
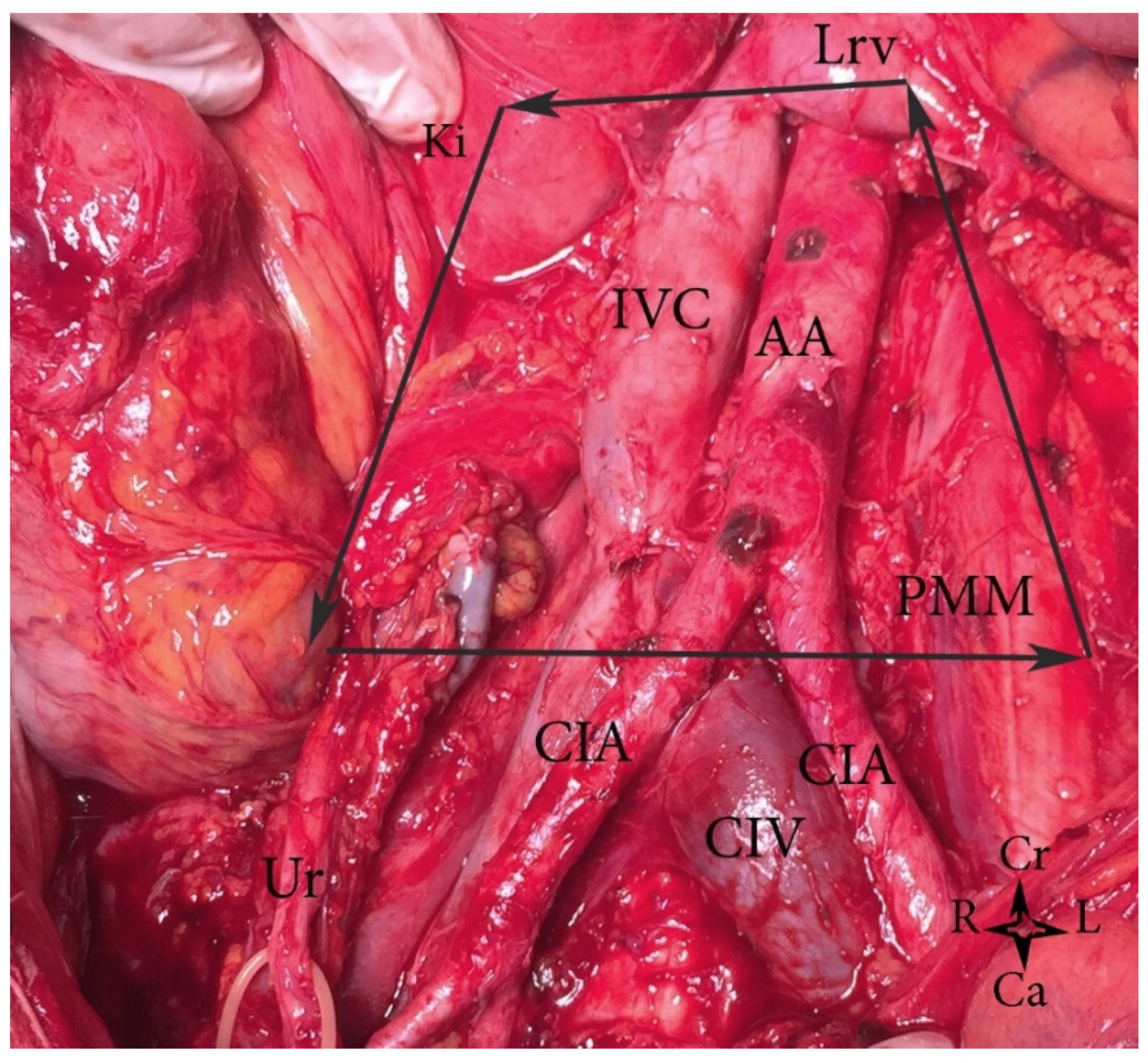
2. Topographical Anatomy in the Paraaortic Regions and Paraaortic (Lumbar) Lymph Nodes’ Anatomical Definition
3. Boundaries of PALND in Gynecological Malignancies
- -
- Right—ureter, Gerota fascia, psoas major muscle, ascending colon;
- -
- Left—ureter, Gerota fascia, psoas major muscle, descending colon;
- -
- Ventrally—left renal vein;
- -
- Dorsally—midpoint of common iliac vessels;
- -
- Caudally—anterior longitudinal ligament.
4. Regions and Their Boundaries during PALND
- (A)
- The high paraaortic (supramesenteric) region is limited: ventrally—LRV; medially—AA; laterally—ureter and Gerota fascia; dorsally—IMA; caudally—psoas major muscle.
- (B)
- The low paraaortic (inframesenteric) region is limited: ventrally by the IMA; medially—by the AA, dorsally—the left common iliac artery (CIA); laterally—the ureter and the Gerota fascia, caudally—the psoas major muscle.
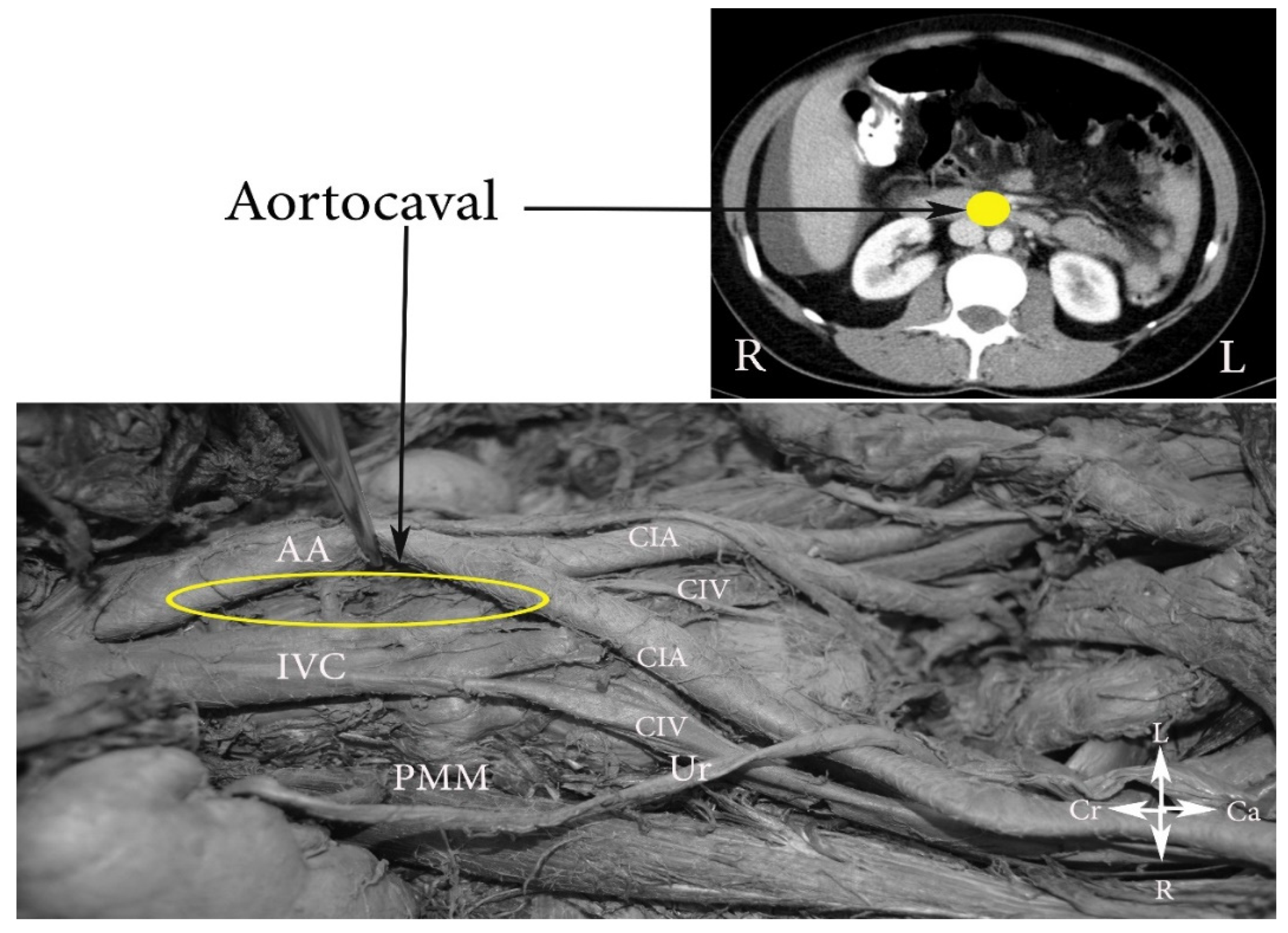
- (C)
- The aortocaval or interaortocaval region (includes preaortic and precaval PALNs) is limited: ventrally—LRV, laterally—left—lateral aspect of the AA, right—lateral aspect of inferior vena cave, dorsally—AA bifurcation, caudally—prevertebral fascia, anterior longitudinal ligament and psoas major muscle.
- (D)
- The paracaval region (includes laterocaval and retrocaval PALNs) is limited: ventrally—right renal vein (RRV); dorsally—midpoint of the lateral aspect of right CIA, laterally—right ureter and right psoas major muscle, caudally—the psoas major muscle.
5. Pathways of Lymphatic Spread to the Paraaortic Region in Gynecological Pelvic Cancers
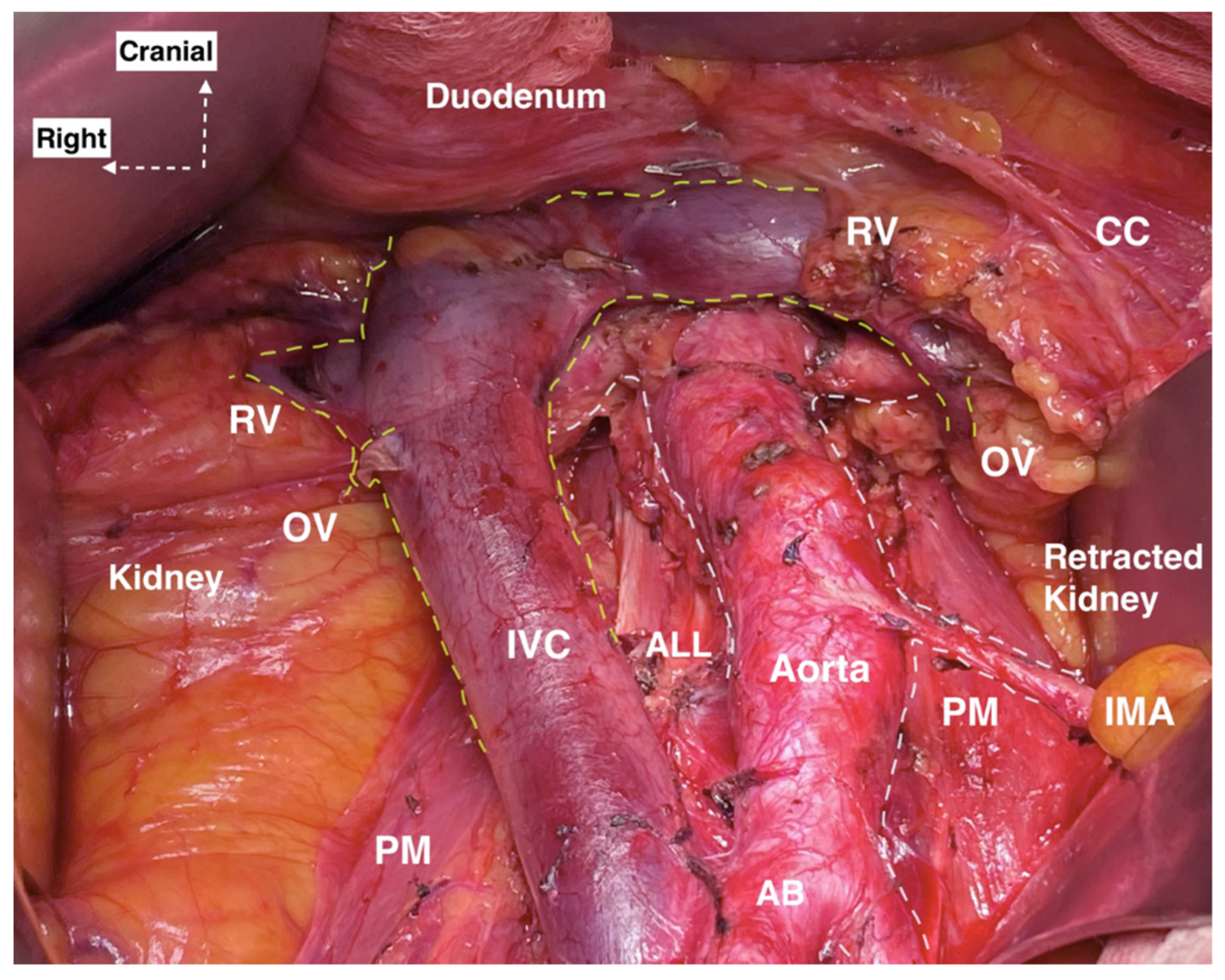
6. Systematic Paraaortic Lymphadenectomy Technique
- A xypho-pubic skin incision is performed for better exposure of the AA and IVC from their bifurcation up to the renal veins.
- Small bowels, omentum, colon transversum are exteriorized cranio-laterally and the sigmoid colon is retracted caudo-laterally.
- Two separate incisions of the posterior parietal peritoneum are performed: the first along the right paracolic gutter to the level of the hepatocolic ligament and the second incision along the ileocolic junction to the level of the ligament of Treitz.
- Entering the avascular plane between Gerota’s and Toldt’s fascia.
- Identification of the right ovarian pedicle and separation from the right ureter.
- The transversal part of duodenum is dissected superiorly.
- The areolar tissue is dissected between the left CIA and sigmoid mesentery. Identification of the IMA, left ovarian pedicle and left ureter is performed.
- Mobilization and lateralization of the ureters is performed. Ureteric vessels should be preserved in order to prevent fistulas.
- Ligation of ovarian vessels at their insertion.
- The dissection of PALNs is performed in the caudal to the cranial direction.
- Laterocaval, retrocaval and precaval lymph nodes are dissected. The presence of the lympho-vascular anastomosis draining into the IVC anteriorly should be considered.
- Preaortic lymph node dissection. Attention during dissection for the lumbar vessels. Ligation of the aortocaval lymphatic is performed.
7. Complications of PALND
8. Risks and Benefits of Systematic PALND
9. Vessels Variations in the Paraaortic Region
9.1. Renal Arteries Anatomy
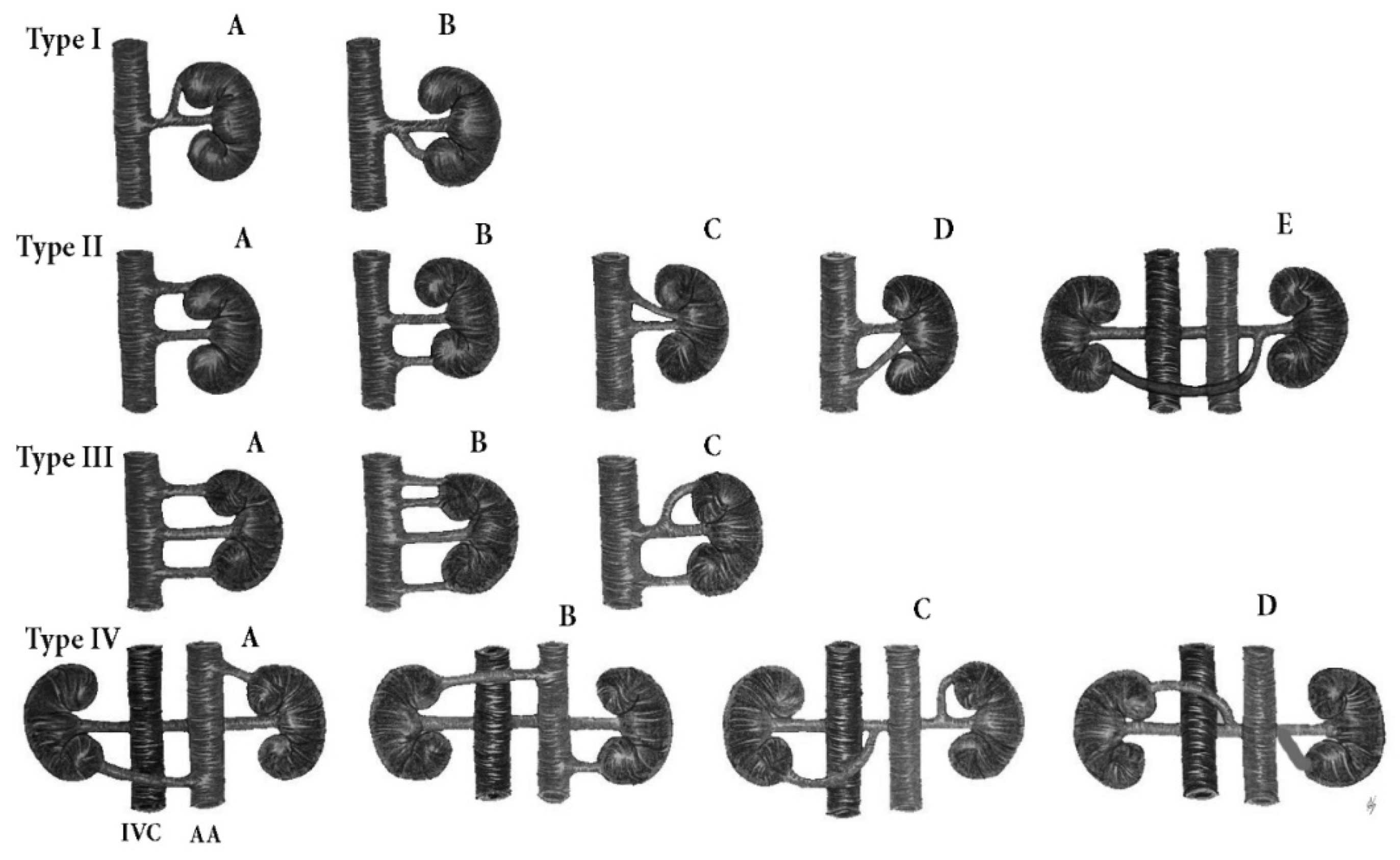
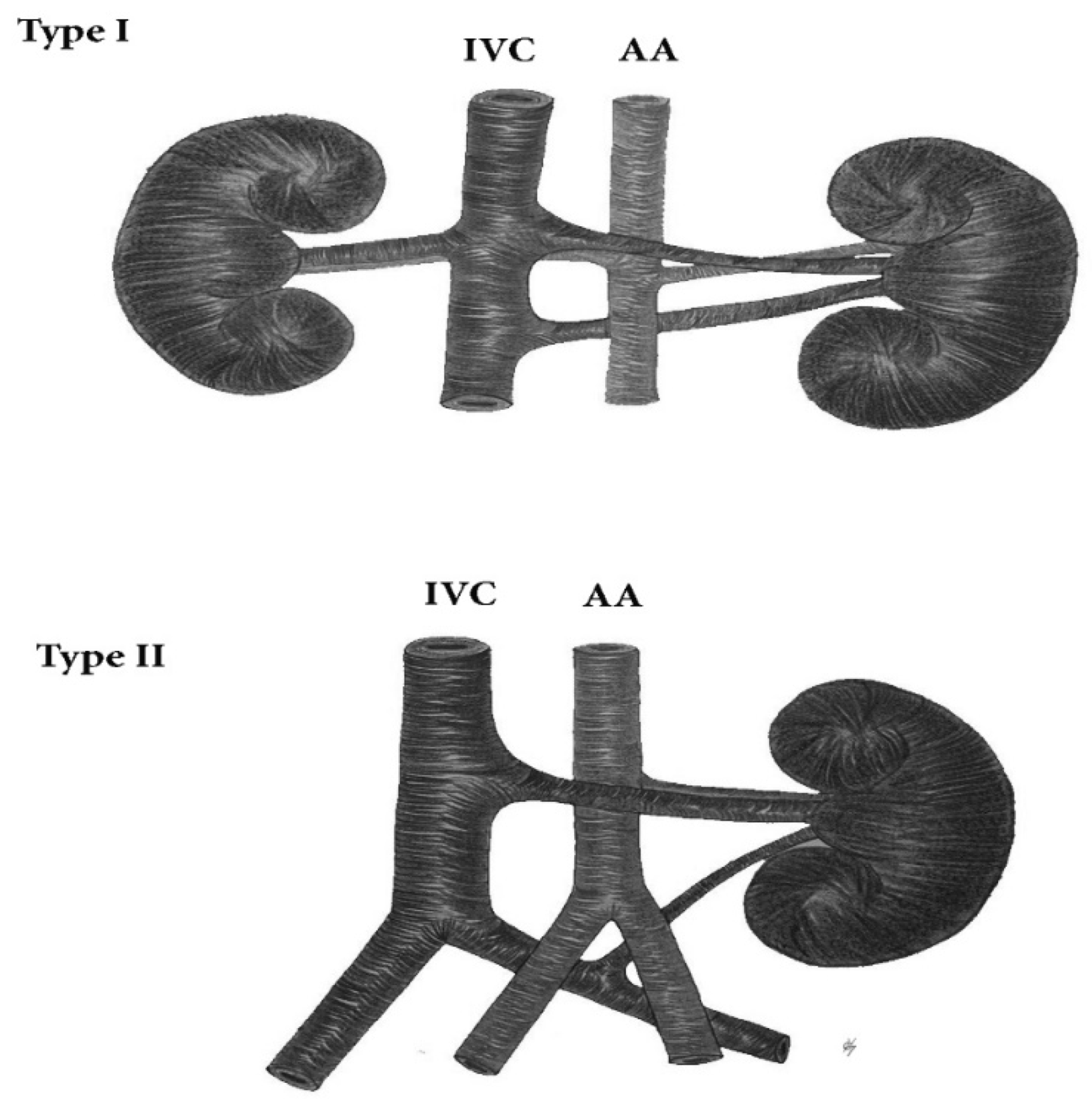
9.1.1. Renal Arteries Anatomical Variations
Type IA—Anatomical Variations of the Origin of Renal Arteries, Which Arise from the Abdominal Aorta
Type IB—Anatomical Variations of the Origin of Renal Arteries, Which Arise from the Abdominal Aorta Branches
Surgical Considerations during PALND
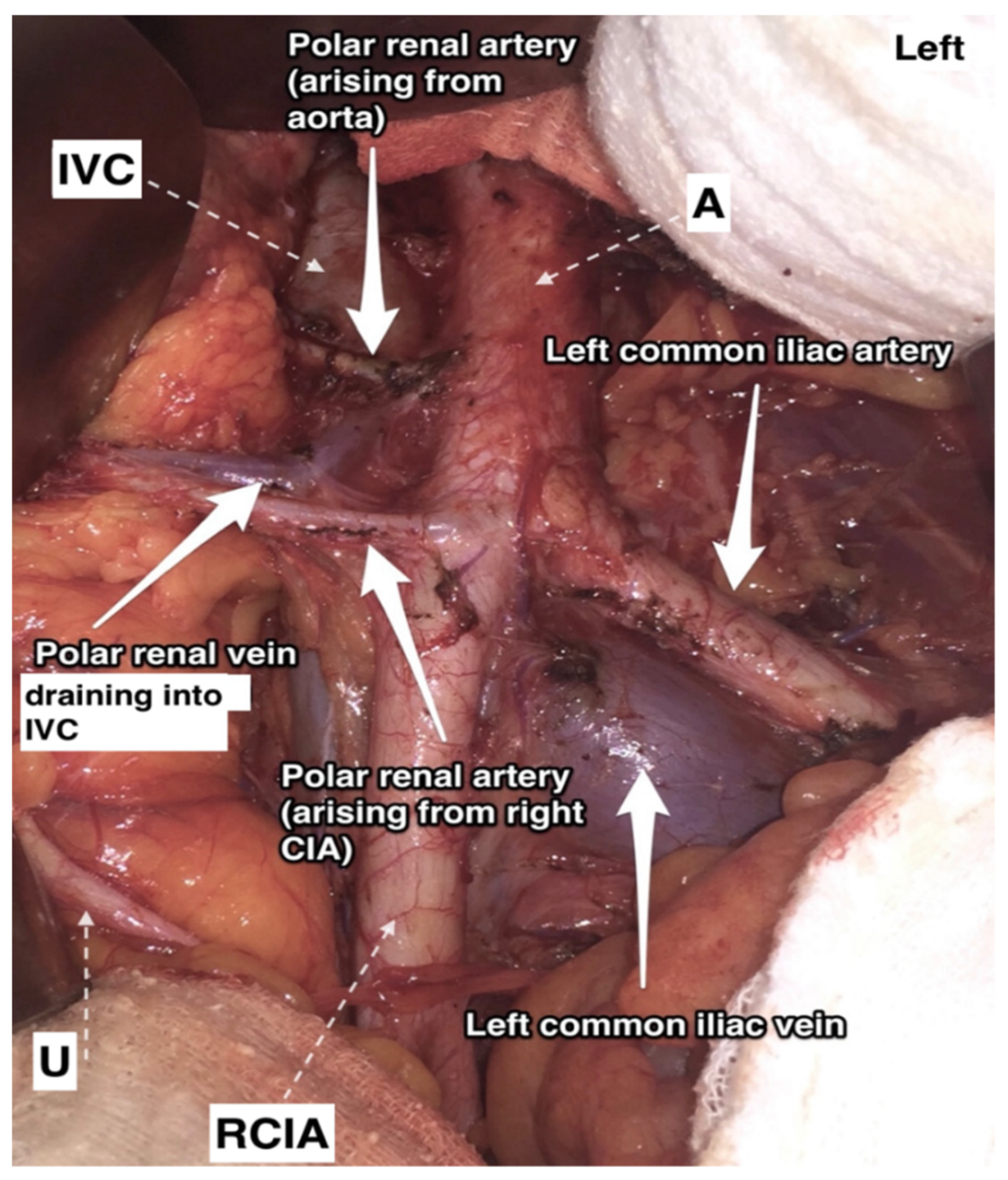
9.1.2. Additional and Accessory Renal Arteries
Additional Renal Arteries
Surgical Considerations
Accessory Renal Arteries
Surgical Considerations
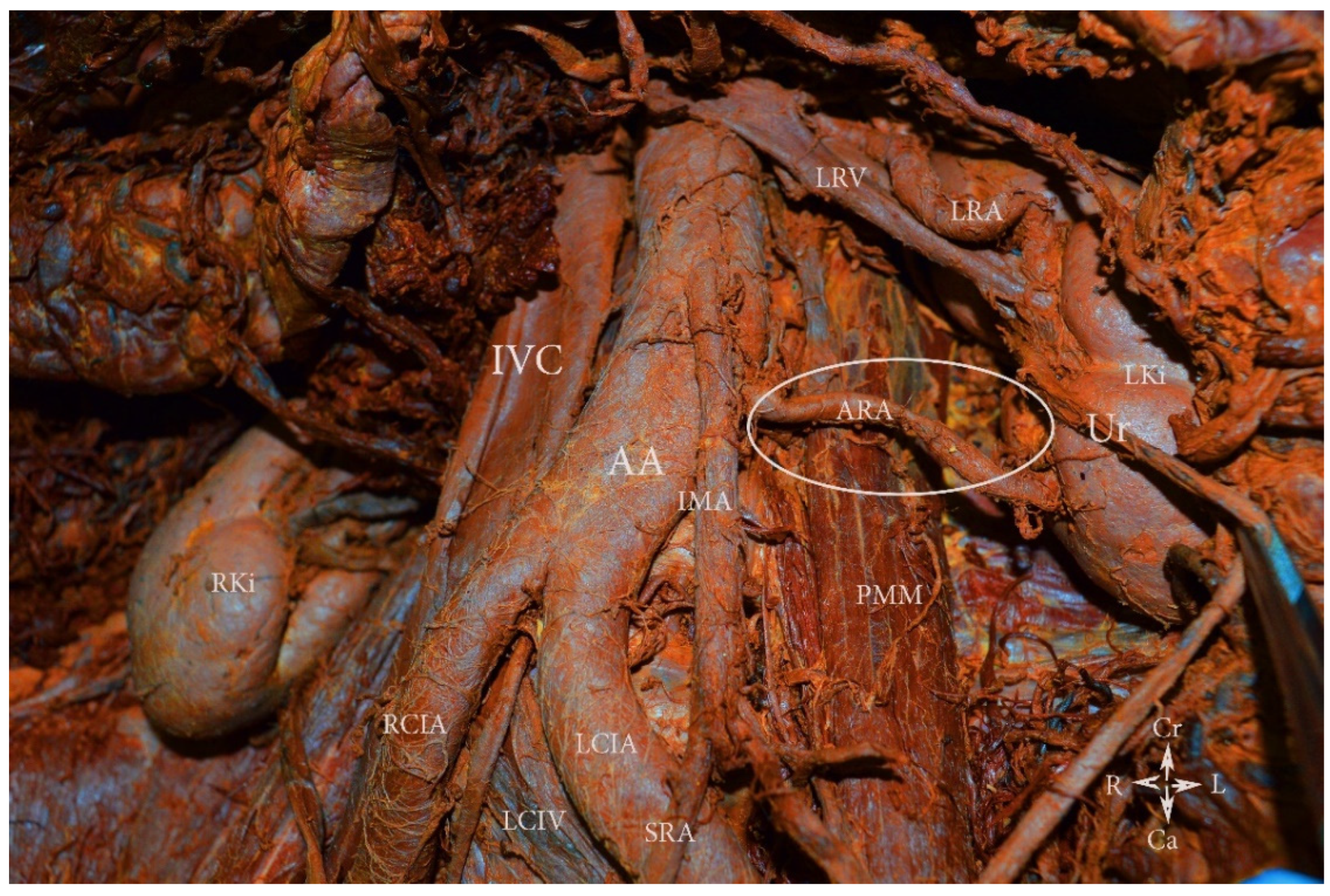
9.1.3. Precaval Right Renal Artery
Surgical Considerations
9.2. Renal Veins Anatomy
9.2.1. Renal Veins Variations
9.2.2. Variations of the Draining Pattern of the Left Renal Vein
Surgical Considerations
9.2.3. Additional Renal Veins
Surgical Considerations
9.2.4. Circumaortic Left Renal Vein
Surgical Considerations
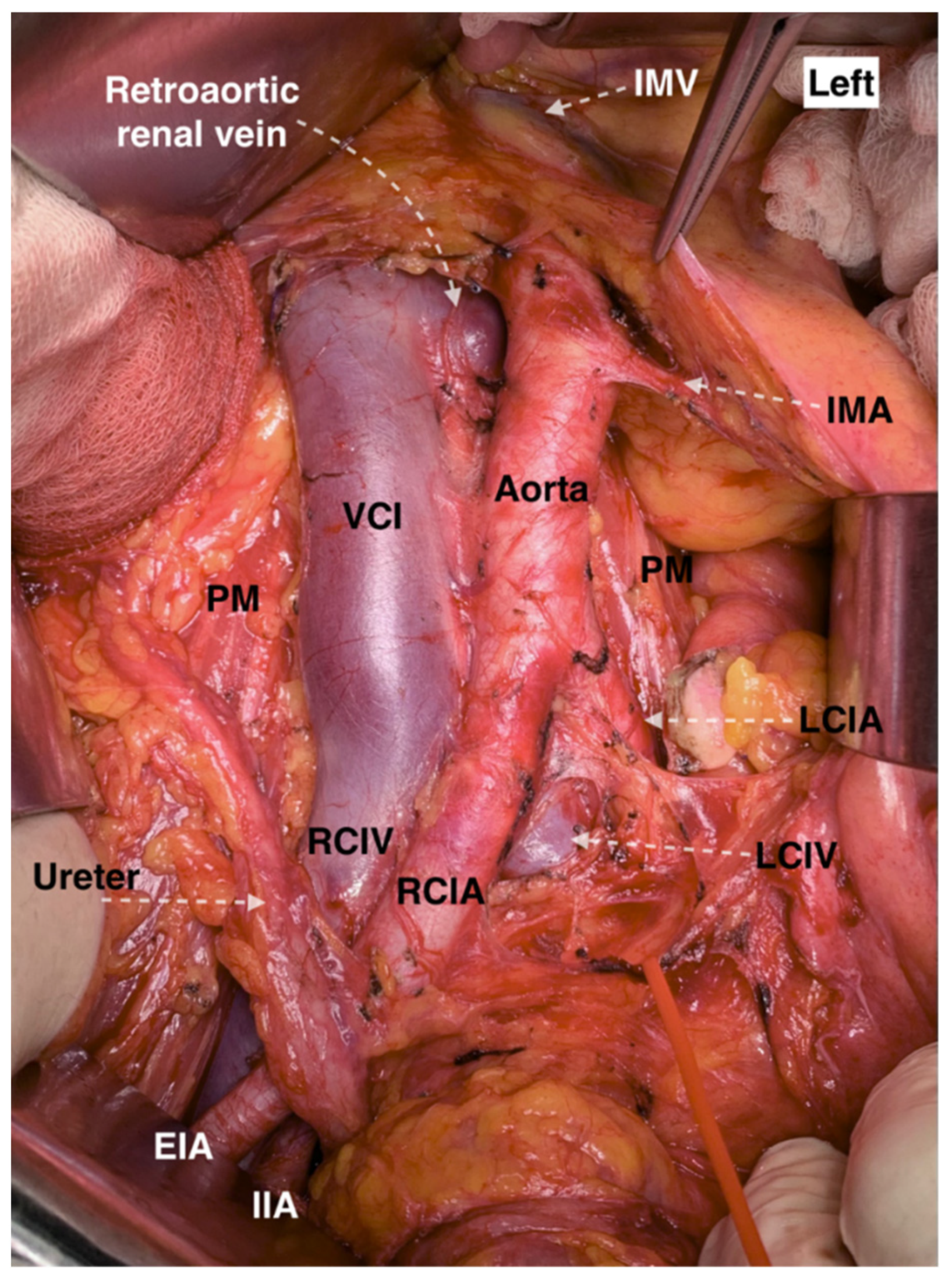
9.2.5. Retroaortic Left Renal Vein
Surgical Considerations
9.2.6. Retropelvic Tributary of the Left Renal Vein
Surgical Considerations
Incidence of Iatrogenic Injury of RVs Variations
9.3. Abdominal Aorta Variations
9.4. Inferior Vena Cava Anatomy
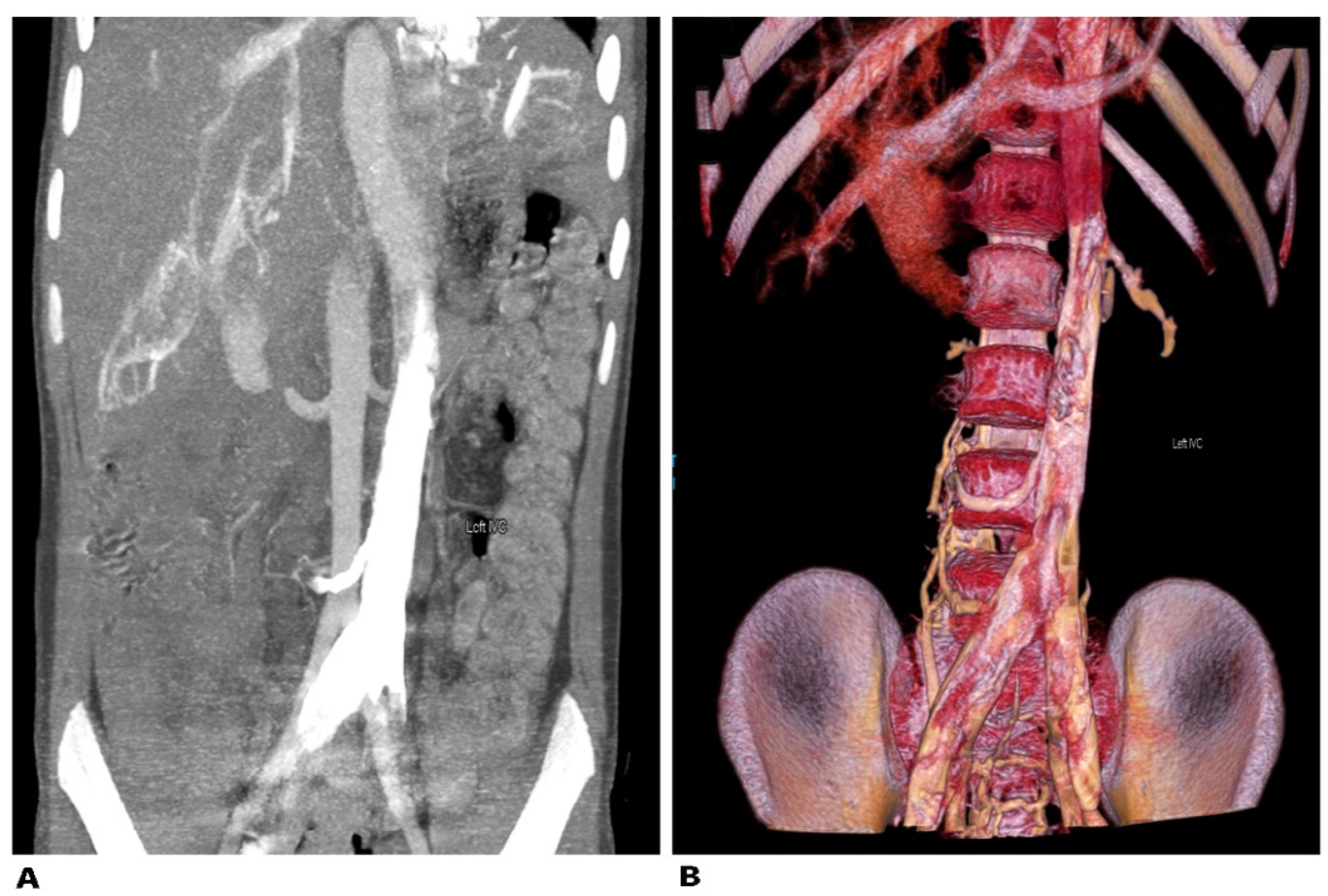
9.4.1. Left Sided Inferior Vena Cava
Surgical Considerations
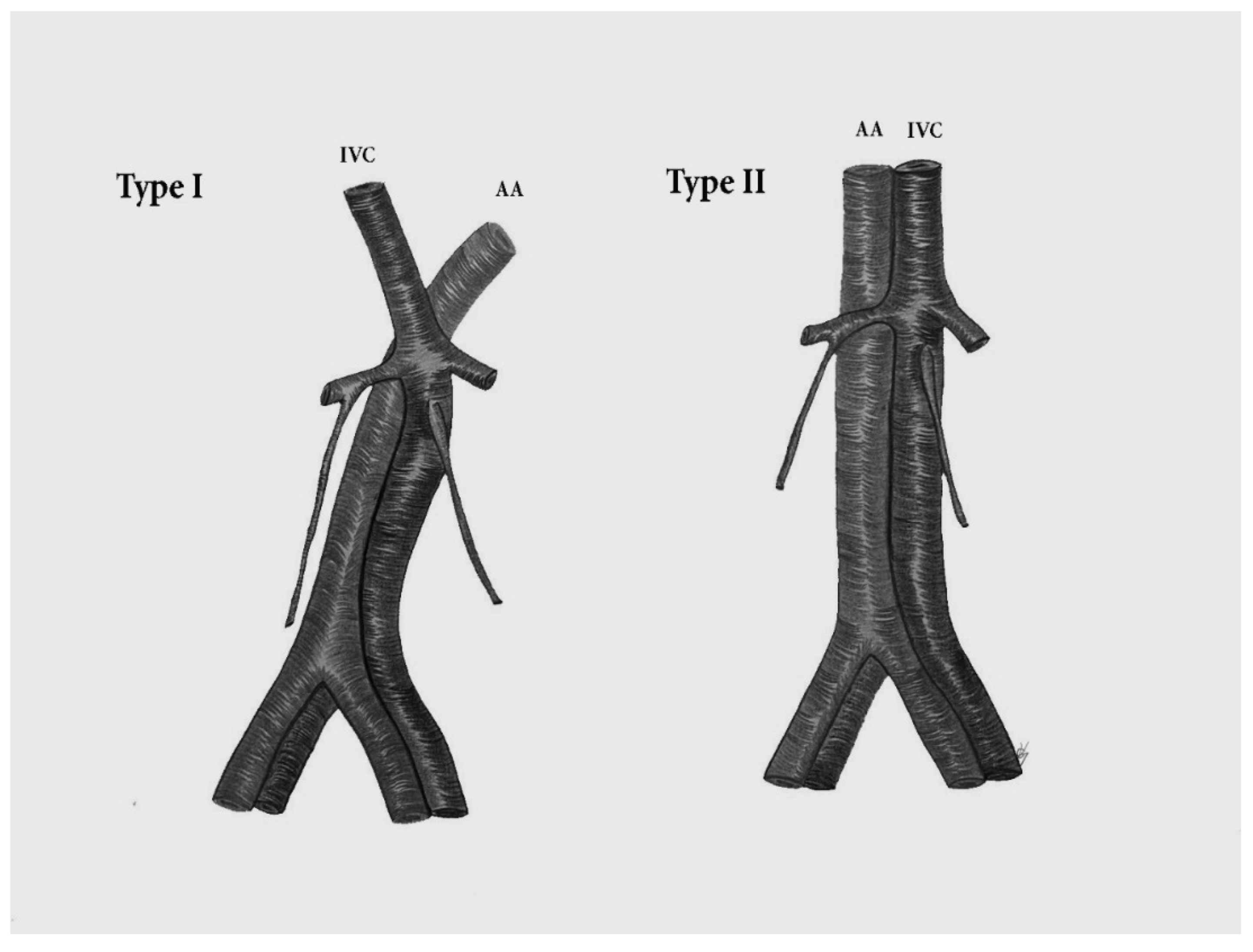
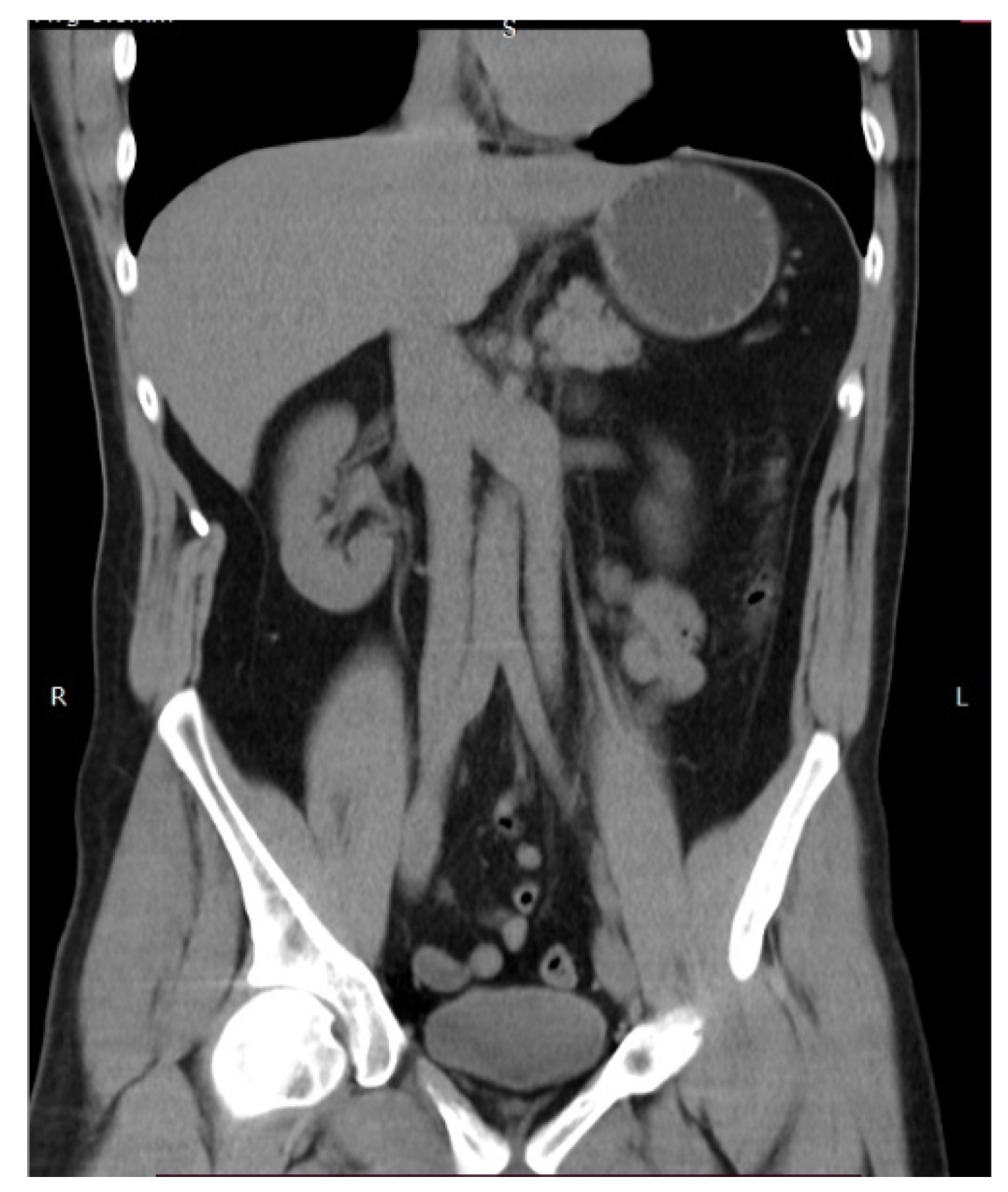
9.4.2. Duplication of the Inferior Vena Cava
Surgical Considerations
9.4.3. Marsupial Inferior Vena Cava
Surgical Considerations
9.5. Ovarian Vessels—Ovarian Arteries Anatomy
9.5.1. Ovarian Artery Variations
Variations in the Level of Aortic Origin and Position
Origin Variations of the OAs
9.6. Ovarian Veins Anatomy
9.6.1. Ovarian Veins Variations
9.6.2. Surgical Considerations
9.7. Inferior Mesenteric Artery Anatomy
9.7.1. Inferior Mesenteric Artery Variations
9.7.2. Surgical Considerations
9.8. Lumbar Arteries Anatomy
9.8.1. Lumbar Arteries Variations
9.8.2. Surgical Considerations
9.9. Lumbar Veins Anatomy
9.9.1. Lumbar Veins Variations
9.9.2. Surgical Considerations
10. Preventing Iatrogenic Injury of Variant Vessels
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kostov, S.; Kornovski, Y.; Slavchev, S.; Ivanova, Y.; Dzhenkov, D.; Dimitrov, N.; Yordanov, A. Pelvic Lymphadenectomy in Gynecologic Oncology—Significance of Anatomical Variations. Diagnostics 2021, 11, 89. [Google Scholar] [CrossRef] [PubMed]
- Emons, G. Significance of Lymph Node Dissection in Gynecological Oncology. Oncol. Res. Treat. 2014, 37, 500–504. [Google Scholar] [CrossRef] [PubMed]
- Bogani, G.; Dowdy, S.C.; Cliby, W.A.; Ghezzi, F.; Rossetti, D.; Mariani, A. Role of pelvic and para-aortic lymphadenectomy in endometrial cancer: Current evidence. J. Obstet. Gynaecol. Res. 2014, 40, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Pomel, C.; Naik, R.; Martinez, A.; Ferron, G.; Nassif, J.; Dauplat, J.; Jeyarajah, A. Systematic (complete) para-aortic lymphadenectomy: Description of a novel surgical classification with technical and anatomical considerations. BJOG 2012, 119, 249–253. [Google Scholar] [CrossRef] [PubMed]
- Fowler, J.; Backes, F. Pelvic and Paraaortic Lymphadenectomy in Gynecologic Cancers Up to Date 2020. Available online: https://www.uptodate.com/contents/pelvic-and-paraaortic-lymphadenectomy-in-gynecologic-cancers (accessed on 16 May 2021).
- Rouviere, H.; Delmas, A.; Delmas, V. Anatomia Humana Descriptiva Topografica Functional; Masson: Barcelona, Spain, 2005; p. 258. [Google Scholar]
- Paño, B.; Sebastià, C.; Ripoll, E.; Paredes, P.; Salvador, R.; Buñesch, L.; Nicolau, C. Pathways of lymphatic spread in gynecologic malignancies. Radiographics 2015, 35, 916–945. [Google Scholar] [CrossRef]
- Panici, P.B.; Angioli, R. Role of lymphadenectomy in ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2002, 16, 529–551. [Google Scholar] [CrossRef]
- Cibula, D.; Abu-Rustum, N.R. Pelvic lymphadenectomy in cervical cancer–surgical anatomy and proposal for a new classification system. Gynecol. Oncol. 2010, 116, 33–37. [Google Scholar] [CrossRef]
- Singh, K.; Fares, R. Pelvic and Para-aortic Lymphadenectomy in Gynecologic Cancers. In Gynecologic and Obstetric Surgery: Challenges and Management Options, 1st ed.; Coomarasamy, A., Shafi, M., Davila, G.W., Chan, K.K., Eds.; John Wiley & Sons Inc.: New York, NY, USA, 2016; pp. 408–411. [Google Scholar]
- Selçuk, İ.; Öz, M.; Toyran, A.; Tatar, İ.; Engin-Üstün, Y.; Demiryürek, D. Para-aortic lymphadenectomy: Step by step surgical education video. J. Turk. Ger. Gynecol. Assoc. 2021, 22, 253–254. [Google Scholar] [CrossRef]
- Available online: https://medtube.net/gynecology/medical-videos/19848-open-para-aortic-lymphadenectomy (accessed on 10 June 2021).
- Uccella, S.; Ghezzi, F.; Casarin, J.; Glaser, G.; Mariani, A. Chapter 9—Hysterectomy with Pelvic and Paraaortic Lymphadenectomy. In Principles of Gynecologic Oncology Surgery; Ramirez, R.T., Frumovitz, M., Abu-Rustum, N.R., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 122–140. [Google Scholar] [CrossRef]
- Benedetti Panici, P.; Basile, S.; Angioli, R. Pelvic and aortic lymphadenectomy in cervical cancer: The standardization of surgical procedure and its clinical impact. Gynecol. Oncol. 2009, 113, 284–290. [Google Scholar] [CrossRef]
- Ouldamer, L.; Fichet-Djavadian, S.; Marret, H.; Barillot, I.; Body, G. Upper margin of para-aortic lymphadenectomy in cervical cancer. Acta Obstet. Gynecol. Scand. 2012, 91, 893–900. [Google Scholar] [CrossRef]
- Díaz-Feijoo, B.; Franco, S.; Torné, A.; Benito, V.; Hernández, A.; Lago, V.; Rovira, R.; Acosta, Ú.; Agustí, N.; Gil-Moreno, A.; et al. SEGO Spain-GOG Group. Implications of extraperitoneal paraaortic lymphadenectomy to the left renal vein in locally advanced cervical cancer. A Spanish multicenter study. Gynecol. Oncol. 2020, 158, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Llueca, A.; Escrig, J.; Gil-Moreno, A.; Benito, V.; Hernández, A.; Díaz-Feijoo, B.; Spain-Gynecologic Oncology Group (GOG) Working Group. The extent of aortic lymphadenectomy in locally advanced cervical cancer impacts on survival. J. Gynecol. Oncol. 2021, 32, e4. [Google Scholar] [CrossRef] [PubMed]
- Ercoli, A.; Delmas, V.; Iannone, V.; Fagotti, A.; Fanfani, F.; Corrado, G.; Ferrandina, G.; Scambia, G. The lymphatic drainage of the uterine cervix in adult fresh cadavers: Anatomy and surgical implications. Eur. J. Surg. Oncol. 2010, 36, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Kraima, A.C.; Derks, M.; Smit, N.N.; Van Munsteren, J.C.; Van der Velden, J.; Kenter, G.G.; De Ruiter, M.C. Lymphatic drainage pathways from the cervix uteri: Implications for radical hysterectomy? Gynecol. Oncol. 2014, 132, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Bakkum-Gamez, J.N. Lymphadenectomy in the Management of Gynecologic Cancer. Clin. Obstet. Gynecol. 2019, 62, 749–755. [Google Scholar] [CrossRef]
- Kleppe, M.; Kraima, A.C.; Kruitwagen, R.F.; Van Gorp, T.; Smit, N.N.; van Munsteren, J.C.; De Ruiter, M.C. Understanding Lymphatic Drainage Pathways of the Ovaries to Predict Sites for Sentinel Nodes in Ovarian Cancer. Int. J. Gynecol. Cancer 2015, 25, 1405–1414. [Google Scholar] [CrossRef]
- Takeshima, N.; Hirai, Y.; Umayahara, K.; Fujiwara, K.; Takizawa, K.; Hasumi, K. Lymph node metastasis in ovarian cancer: Difference between serous and non-serous primary tumors. Gynecol. Oncol. 2005, 99, 427–431. [Google Scholar] [CrossRef]
- Wisner, K.P.; Gupta, S.; Ahmad, S.; Holloway, R.W. Indications and techniques for robotic pelvic and para-aortic lymphadenectomy in gynecologic oncology. J. Surg. Oncol. 2015, 112, 782–789. [Google Scholar] [CrossRef]
- Harter, P.; Sehouli, J.; Lorusso, D.; Reuss, A.; Vergote, I.; Marth, C.; Kim, J.-W.; Raspagliesi, F.; Lampe, B.; Aletti, G.; et al. A Randomized Trial of Lymphadenectomy in Patients with Advanced Ovarian Neoplasms. N. Engl. J. Med. 2019, 380, 822–832. [Google Scholar] [CrossRef]
- Fotopoulou, C.; Planchamp, F.; Aytulu, T.; Chiva, L.; Cina, A.; Ergönül, Ö.; Fagotti, A.; Haidopoulos, D.; Hasenburg, A.; Hughes, C.; et al. European Society of Gynaecological Oncology guidelines for the peri-operative management of advanced ovarian cancer patients undergoing debulking surgery. Int. J. Gynecol. Cancer 2021, 31, 1199–1206. [Google Scholar] [CrossRef]
- Cibula, D.; Dostalek, L.; Hillemanns, P.; Scambia, G.; Jarkovsky, J.; Persson, J.; Raspagliesi, F.; Novak, Z.; Jaeger, A.; Capilna, M.E.; et al. Completion of radical hysterectomy does not improve survival of patients with cervical cancer and intraoperatively detected lymph node involvement: ABRAX international retrospective cohort study. Eur. J. Cancer 2021, 143, 88–100. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.; Standring, S.; Hrold Ellis, H.; Berkovitz, B. Gray’s Anatomy: The Anatomical Basis of Clinical Practice, 39th ed.; Elsevier Churchill Livingstone Edinburgh: New York, NY, USA, 2005; pp. 2171–2175. [Google Scholar]
- Tubbs, R.S.; Shoja, M.M.; Loukas, M. Bergman’s Comprehensive Encyclopedia of Human Anatomic Variation; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 669–693, 884–889. [Google Scholar]
- Gardner, S. An Accessory Left Renal Artery: A Case Report. Austin. J. Anat. 2015, 2, 1041. [Google Scholar]
- Leslie, S.W.; Sajjad, H. Anatomy, Abdomen and Pelvis, Renal Artery; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Ozkan, U.; Oğuzkurt, L.; Tercan, F.; Kizilkiliç, O.; Koç, Z.; Koca, N. Renal artery origins and variations: Angiographic evaluation of 855 consecutive patients. Diagn. Interv. Radiol. 2006, 12, 183–186. [Google Scholar] [PubMed]
- Gümüş, H.; Bükte, Y.; Ozdemir, E.; Cetinçakmak, M.G.; Tekbaş, G.; Ekici, F.; Onder, H.; Uyar, A. Variations of renal artery in 820 patients using 64-detector CT-angiography. Ren. Fail. 2012, 34, 286–290. [Google Scholar] [CrossRef]
- van Baalen, J.M.; van Bockel, H.J.; Overbosch, E.H. Thoracic origin of right renal artery. J. Vasc. Surg. 1994, 19, 762–763. [Google Scholar] [CrossRef][Green Version]
- Ichikawa, T.; Iino, M.; Koizumi, J.; Hara, T.; Kazama, T.; Sekiguchi, T.; Hashimoto, J.; Nakamura, M.; Imai, Y. A case of right renal artery originating from the thoracic aorta. Jpn. J. Radiol. 2014, 32, 716–720. [Google Scholar] [CrossRef]
- Gadabanahalli, K.; Bhat, V. Thoracic Origin of Single Right Renal Artery: Some Interesting Facets. Int. J. Angiol. 2017, 26, 264–266. [Google Scholar] [CrossRef]
- Anson, B.J.; McVay, C.B. The topographical positions and the mutual relations of the visceral branches of the abdominal aorta. A study of 100 consecutive cadavers. Anat. Record 1936, 67, 7–15. [Google Scholar] [CrossRef]
- Halloul, Z.; Meyer, F.; Buerger, T. Ectopic vascularization of the right kidney by a contralateral origin of the main renal artery from the left common iliac artery: Report of a case. Surg. Today 2001, 31, 371–373. [Google Scholar] [CrossRef]
- Kruyt, R. Vascularization of left kidney by single vessel originating from splenic artery. Urology 1992, 39, 487–489. [Google Scholar] [CrossRef]
- Garti, I.; Nissenkorn, I.; Lerner, M. Common origin of inferior mesenteric and main renal artery. Eur. Urol. 1986, 12, 215–216. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, R.; Frasson, F.; Pistolesi, G.F. Normal angiographic patterns of the inferior mesenteric artery. Radiol. Clin. Biol. 1971, 40, 153–174. [Google Scholar] [PubMed]
- Sarlon-Bartoli, G.; Magnan, P.E.; Lépidi, H.; Bartoli, M.A. Celiomesenteric and renal common trunk associated with distal thoracic aorta coarctation and three saccular aneurysms. J. Vasc. Surg. 2014, 59, 1432. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mazzeo, C.; Viscosi, F.; Badessi, G.; Cucinotta, E. A critical view of safety in left colectomy surgery: A case of renal artery injury. Int. J. Surg. Case Rep. 2021, 83, 106035. [Google Scholar] [CrossRef] [PubMed]
- Satyapal, K.S.; Haffejee, A.A.; Singh, B.; Ramsaroop, L.; Robbs, J.V.; Kalideen, J.M. Additional renal arteries: Incidence and morphometry. Surg. Radiol. Anat. 2001, 23, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Tardo, D.T.; Briggs, C.; Ahern, G.; Pitman, A.; Sinha, S. Anatomical variations of the renal arterial vasculature: An Australian perspective. J. Med. Imaging Radiat. Oncol. 2017, 61, 643–649. [Google Scholar] [CrossRef]
- Saldarriaga, B.; Pérez, A.F.; Ballesteros, L.E. A direct anatomical study of additional renal arteries in a Colombian mestizo population. Folia Morphol. (Warsz.) 2008, 67, 129–134. [Google Scholar]
- Dogra, A.; Chauhan, R.S.; Sharma, S.; Partap, A.; Diwan, Y.; Chawla, K.; Negi, K.; Rana, S.; Diwan, D. Variations of renal arteries on 64 slice multidetector computed tomography. J. Anat. Soc. India 2017, 66, 20–25. [Google Scholar] [CrossRef]
- Ravery, V.; Cussenot, O.; Desgrandchamps, F.; Teillac, P.; Martin-Bouyer, Y.; Lassau, J.P.; Le Duc, A. Variations in arterial blood supply and the risk of hemorrhage during percutaneous treatment of lesions of the pelviureteral junction obstruction: Report of a case of testicular artery arising from an inferior polar renal artery. Surg. Radiol. Anat. 1993, 15, 355–359. [Google Scholar] [CrossRef]
- Kommuru, H.; Sree Lekha, D.; Jothi, S.S.; Rajeswararao, N.; Sujatha, N. Presence of renal artery variations and its surgical correlation. Int. J. Clin. Med. Res. 2012, 3, 176–179. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, I.; Babu, C.S.; Gupta, K.K.; Gupta, O.P. Clinical insight into the precaval right renal artery: A multidetector row computed tomography angiographic study. ISRN Anat. 2013, 2013, 250950. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, R.F. Unusual origins of renal arteries. Radiology 1972, 102, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Eitan, R.; Abu-Rustum, N.R.; Walker, J.L.; Barakat, R.R. Ligation of an anatomic variant of renal vasculature during laparoscopic periaortic lymph node dissection: A cause of postoperative renal infarction. Gynecol. Oncol. 2003, 91, 416–420. [Google Scholar] [CrossRef]
- Benedetti-Panici, P.; Maneschi, F.; Scambia, G.; Greggi, S.; Mancuso, S. Anatomic abnormalities of the retroperitoneum encountered during aortic and pelvic lymphadenectomy. Am. J. Obstet. Gynecol. 1994, 170, 111–116. [Google Scholar] [CrossRef]
- Lee, W.M.; Choi, J.S.; Bae, J.; Jung, U.S.; Eom, J.M. Encountering the Accessory Polar Renal Artery during Laparoscopic Para-Aortic Lymphadenectomy. J. Minim. Invasive Gynecol. 2018, 25, 10–11. [Google Scholar] [CrossRef]
- Madsen, M.; Gorostidi, M.; Ruiz, R.; Jaunarena, I.; Cobas, P.; Lekuona, A. Accessory polar renal artery not pre-operatively visualized at extra-peritoneal para-aortic lymphadenectomy. Int. J. Gynecol. Cancer 2019, 29, 1226–1227. [Google Scholar] [CrossRef]
- Loukas, M.; Aparicio, S.; Beck, A.; Calderon, R.; Kennedy, M. Rare case of right accessory renal artery originating as a common trunk with the inferior mesenteric artery: A case report. Clin. Anat. 2005, 18, 530–535. [Google Scholar] [CrossRef]
- Chakravarthi, K.K. Unilateral multiple variations of renal, phrenic, suprarenal, inferior mesenteric and gonadal arteries. J. Nat. Sci. Biol. Med. 2014, 5, 173–175. [Google Scholar] [CrossRef]
- Gülsün, M.; Balkanci, F.; Cekirge, S.; Deger, A. Pelvic kidney with an unusual blood supply: Angiographic findings. Surg. Radiol. Anat. 2000, 22, 59–61. [Google Scholar] [CrossRef]
- Dretler, S.P.; Olsson, C.; Pfister, R.C. The anatomic, radiologic and clinical characteristics of the pelvic kidney: An analysis of 86 cases. J. Urol. 1971, 105, 623–627. [Google Scholar] [CrossRef]
- Yeh, B.M.; Coakley, F.V.; Meng, M.V.; Breiman, R.S.; Stoller, M.L. Precaval right renal arteries: Prevalence and morphologic associations at spiral, C.T. Radiology 2004, 230, 429–433. [Google Scholar] [CrossRef] [PubMed]
- Kose, M.F.; Turan, T.; Karasu, Y.; Gundogdu, B.; Boran, N.; Tulunay, G. Anomalies of major retroperitoneal vascular structure. Int. J. Gynecol. Cancer 2011, 21, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Sabouri, S.; Hosseini, A.; Shivaei, S.S. Retro-Aortic Inverted Left Renal Vein: A Rare Anomaly Found in a Renal Donor. Iran J. Radiol. 2015, 12, e11374. [Google Scholar] [CrossRef] [PubMed]
- Hostiuc, S.; Rusu, M.C.; Negoi, I.; Dorobanțu, B.; Grigoriu, M. Anatomical variants of renal veins: A meta-analysis of prevalence. Sci. Rep. 2019, 9, 10802. [Google Scholar] [CrossRef]
- Yi, S.Q.; Ueno, Y.; Naito, M.; Ozaki, N.; Itoh, M. The three most common variations of the left renal vein: A review and meta-analysis. Surg. Radiol. Anat. 2012, 34, 799–804. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, L.; Yang, Z.; Zhou, H.; Tang, G. Classification of the renal vein variations: A study with multidetector computed tomography. Surg. Radiol. Anat. 2015, 37, 667–675. [Google Scholar] [CrossRef]
- Gillot, C. La veine renale gauche. Anat. Clin. 1978, 1, 135–155. [Google Scholar] [CrossRef]
- Kuzan, T.Y.; Kuzan, B.N.; Telli, T.A.; Tüney, D. Evaluation of the frequency of left renal vein variations in computed tomography and its relationship with cancer development. Folia Morphol. 2020, 79, 793–798. [Google Scholar] [CrossRef]
- Hsieh, C.L.; Tiao, W.M.; Chou, Y.H.; Tiu, C.M. Retroaortic Left Renal Vein: Three Case Reports. J. Med. Ultrasound 2012, 20, 115–118. [Google Scholar] [CrossRef]
- Gupta, A.; Gupta, R.; Rikki, S. Congenital variations of renal veins: Embryological background and clinical implications. J. Clin. Diagn. Res. 2011, 6, 1140–1143. [Google Scholar]
- Kyung, D.S.; Lee, J.H.; Shin, D.Y.; Kim, D.K.; Choi, I.J. The double retro-aortic left renal vein. Anat. Cell Biol. 2012, 45, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Koc, Z.; Ulusan, S.; Tokmak, N.; Oguzkurt, L.; Yildirim, T. Double retroaortic left renal veins as a possible cause of pelvic congestion syndrome: Imaging findings in two patients. Br. J. Radiol. 2006, 79, e152–e155. [Google Scholar] [CrossRef] [PubMed]
- Terayama, H.; Yi, S.Q.; Shoji, S.; Tanaka, O.; Kanazawa, T.; Kosemura, N.; Tamura, M.; Sekiguchi, M.; Naito, M.; Akamatsu, T.; et al. Anomalous connection of the left posterior renal vein with the left ascending lumbar vein in a Japanese cadaver. Folia Morphol. (Warsz.) 2015, 74, 544–547. [Google Scholar] [CrossRef][Green Version]
- Satyapal, K.S.; Kalideen, J.M.; Haffejee, A.A.; Singh, B.; Robbs, J.V. Left renal vein variations. Surg. Radiol. Anat. 1999, 21, 77–81. [Google Scholar] [CrossRef] [PubMed]
- Gyimadu, A.; Salman, M.C.; Karcaaltincaba, M.; Yuce, K. Retroperitoneal vascular aberrations increase the risk of vascular injury during lymphadenectomy in gynecologic cancers. Arch. Gynecol. Obstet. 2012, 286, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Truty, M.J.; Bower, T.C. Congenital anomalies of the inferior vena cava and left renal vein: Implications during open abdominal aortic aneurysm reconstruction. Ann. Vasc. Surg. 2007, 21, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Seyfettinoglu, S.; Khatib, G.; Kucukgoz Gulec, U.; Guzel, A.B.; Bayazit, Y.; Vardar, M.A. lEP980 Gonadal vein graft after retroaortic left renal vein injury during paraaortic lymphadenectomy: A case report. Int. J. Gynecol. Cancer 2019, 29, A519–A520. [Google Scholar]
- Kayhan, A.; Collins, J.; Al-Sadir, J.; Zhu, F.; Oto, A.; Svensson, E.C. A double abdominal aorta with a double inferior vena cava: A human congenital vascular patterning defect. Birth Defects Res. Part A Clin. Mol. Teratol. 2011, 91, 586–589. [Google Scholar] [CrossRef]
- Meuwly, J.Y.; Knopfli, A.S.; Gullo, G. Duplication of abdominal aorta: A very rare congenital anomaly but a common ultrasound artifact. Ultraschall Med. 2011, 32, 233–236. [Google Scholar] [CrossRef]
- Turyna, R.; Kachlik, D.; Feyreisl, J.; Stingl, J.; Baca, V. Anterior retroperitoneal rami: Until now unnamed direct branches of the abdominal aorta. Clin. Anat. 2014, 27, 894–899. [Google Scholar] [CrossRef]
- Coco, D.; Cecchini, S.; Leanza, S.; Viola, M.; Ricci, S.; Campagnacci, R. Inferior Vena Cava Duplication: Incidental Case in a Young Woman. Case Rep. Radiol. 2016, 2016, 3071873. [Google Scholar] [CrossRef] [PubMed]
- Rajabnejad, Y.; Aliakbarian, M.; Rajabnejad, A.; Motie, M.R. Left-Sided Inferior Vena Cava Encountered During Organ Retrieval Surgery: Report of Two Cases. Int. J. Organ Transplant. Med. 2016, 7, 229–232. [Google Scholar] [PubMed]
- Mathews, R.; Smith, P.A.; Fishman, E.K.; Marshall, F.F. Anomalies of the inferior vena cava and renal veins: Embryologic and surgical considerations. Urology 1999, 53, 873–880. [Google Scholar] [CrossRef]
- Bass, J.E.; Redwine, M.D.; Kramer, L.A.; Huynh, P.T.; Harris, J.H. Spectrum of Congenital Anomalies of the Inferior Vena Cava: Cross-sectional Imaging Findings. RadioGraphics 2000, 20, 639–652. [Google Scholar] [CrossRef]
- Lucas, M.F. A Case of Double Inferior Vena Cava. J. Anat. 1916, 51 Pt 1, 69–70. [Google Scholar]
- Chen, H.; Emura, S.; Nagasaki, S.; Kubo, K.Y. Double inferior vena cava with interiliac vein: A case report and literature review. Okajimas Folia Anat. Jpn. 2012, 88, 147–151. [Google Scholar] [CrossRef]
- Natsis, K.; Apostolidis, S.; Noussios, G.; Papathanasiou, E.; Kyriazidou, A.; Vyzas, V. Duplication of the inferior vena cava: Anatomy, embryology and classification proposal. Anat. Sci. Int. 2010, 85, 56–60. [Google Scholar] [CrossRef]
- Morita, S.; Higuchi, M.; Saito, N.; Mitsuhashi, N. Pelvic venous variations in patients with congenital inferior vena cava anomalies: Classification with computed tomography. Acta Radiol. 2007, 48, 974–979. [Google Scholar] [CrossRef]
- Honma, S.; Tokiyoshi, A.; Kawai, K.; Koizumi, M.; Kodama, K. Left inferior vena cava with regressed right inferior vena cava. Anat. Sci. Int. 2008, 83, 173–178. [Google Scholar] [CrossRef]
- Tagliafico, A.; Capaccio, E.; Rosenberg, I.; Martinoli, C.; Derchi, L.E. Double right inferior vena cava associated with an anomalous venous ring encircling the right common iliac artery: Report of one case with CT and, U.S. Eur. J. Radiol. Extra 2007, 64, 111–115. [Google Scholar] [CrossRef]
- Nagashima, T.; Lee, J.; Andoh, K.; Itoh, T.; Tanohata, K.; Arai, M.; Inoue, T. Right double inferior vena cava: Report of 5 cases and literature review. J. Comput. Assist. Tomogr. 2006, 30, 642–645. [Google Scholar] [CrossRef] [PubMed]
- Ureyen, I.; Kestel, Z.; Sahin, E.G.; Karalok, A.; Turan, T.; Boran, N.; Tulunay, G. Double vena cava inferior: A report of three cases. Asian Pac. J. Reprod. 2013, 2, 248–250. [Google Scholar] [CrossRef]
- Sousa Gomes, M.; Pardal, C.; Monteiro, C.; Serrano, P. Double inferior vena cava in gynaecological oncology surgery. BMJ Case Rep. 2020, 13, e240361. [Google Scholar] [CrossRef] [PubMed]
- McNeil, J.; Whipp, K.P.; Lambert, H. Unique variant of a double inferior vena cava with interiliac communication: Review of clinical and surgical relevance. Int. J. Anat. Var. 2016, 9, 35–38. [Google Scholar]
- Milani, C.; Constantinou, M.; Berz, D.; Butera, J.N.; Colvin, G.A. Left side inferior vena cava duplication and venous thromboembolism: Case report and review of literature. J. Hematol. Oncol. 2008, 1, 24. [Google Scholar] [CrossRef]
- Senecail, B.; Lefevre, C.; Person, H.; Meriot, P. Radiologic anatomy of duplication of the inferior vena cava: A trap in abdominal imaging. A report of 8 cases. Surg. Radiol. Anat. 1987, 9, 151–157. [Google Scholar] [CrossRef]
- Mao, Y.Q.; Zhu, S.X.; Zhang, W. The iatrogenic injury of double vena cava due to misdiagnosis during the radical nephroureterectomy and cystectomy. World J. Surg. Oncol. 2015, 13, 41. [Google Scholar] [CrossRef][Green Version]
- Reis Filho, A.J.S.; Rocha, M.A.; Lemos, G.R.; Yamauchi, F.I.; Tachibana, A.; Baroni, R.H. Marsupial vena cava mimicking lymph node enlargement on tomography. Einstein (Sao Paulo) 2018, 16, eAI4394. [Google Scholar] [CrossRef]
- Singh, R.; Sieunarine, K. Marsupial inferior vena cava. J. Vasc. Interv. Radiol. 2014, 25, 71. [Google Scholar] [CrossRef]
- Rocha Mde, S.; Lourenço, R.B.; Chang, Y.S.; Gebrim, E.M.; Cerri, G.G. Preaortic iliac confluence (marsupial vena cava): Report of 4 cases. J. Comput. Assist. Tomogr. 2008, 32, 706–709. [Google Scholar] [CrossRef]
- Notkovich, H. Variations of the testicular and ovarian arteries in relation to the renal pedicle. Surg. Gynecol. Obstet. 1956, 103, 487–495. [Google Scholar] [PubMed]
- Shoja, M.M.; Tubbs, R.S.; Shakeri, A.B.; Oakes, W.J. Origins of the gonadal artery: Embryologic implications. Clin. Anat. 2007, 20, 428–432. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Yang, S.B.; Goo, D.E.; Kim, Y.J.; Chang, Y.W.; Lee, J.M. Aberrant ovarian artery arising from the common iliac artery: Case report. Korean J. Radiol. 2013, 14, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.H.; Kim, M.D.; Lee, K.H.; Lee, M.; Lee, M.S.; Won, J.Y.; Park, S.I.; Lee, D.Y. Aberrant ovarian collateral originating from external iliac artery during uterine artery embolization. Cardiovasc. Intervent. Radiol. 2013, 36, 269–271. [Google Scholar] [CrossRef]
- Lee, J.E.; Lee, J.M. Aberrant Ovarian Artery Originating from the Iliolumbar Artery: A Case Report. J. Korean Soc. Radiol. 2016, 74, 339–343. [Google Scholar] [CrossRef]
- Stones, R.W. Pelvic vascular congestion-half a century later. Clin. Obstet. Gynecol. 2003, 46, 831–836. [Google Scholar] [CrossRef]
- Ghosh, A.; Chaudhury, S. A cadaveric study of ovarian veins: Variations, measurements and clinical significance. Anat. Cell Biol. 2019, 52, 385–389. [Google Scholar] [CrossRef]
- Koc, Z.; Ulusan, S.; Oguzkurt, L. Right ovarian vein drainage variant: Is there a relationship with pelvic varices? Eur. J. Radiol. 2006, 59, 465–471. [Google Scholar] [CrossRef]
- Balcerzak, A.; Kwaśniewska, O.; Podgórski, M.; Olewnik, Ł.; Polguj, M. Types of inferior mesenteric artery: A proposal for a new classification. Folia Morphol. (Warsz.) 2021, 80, 827–838. [Google Scholar] [CrossRef]
- Zebrowski, W.; Augustyniak, E.; Zajac, S. Zmienność odejścia i sposobu rozgałezienia tetnicy krezkowej dolnej oraz jej wzajemne zespolenia Variations of origin and branching of the interior mesenteric artery and its anastomoses. Folia Morphol. (Warsz.) 1971, 30, 575–583. [Google Scholar]
- Yoo, S.J.; Ku, M.J.; Cho, S.S.; Yoon, S.P. A case of the inferior mesenteric artery arising from the superior mesenteric artery in a Korean woman. J. Korean Med. Sci. 2011, 26, 1382–1385. [Google Scholar] [CrossRef] [PubMed]
- Lippert, H.; Pabst, R. Arterial Variations in Man: Classification and Frequency, 1st ed.; JF Bergmann Verlag: München, Germany, 1985; pp. 52–53. [Google Scholar]
- Benton, R.S.; Cotter, W.B. A hitherto undocumented variation of the inferior mesenteric artery in man. Anat. Rec. 1963, 145, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Sher, S.; Mukerjee, A.; Skillman, J.J.; Ducksoo, K. Anomalous origin of the inferior artery from common iliac artery. Vas. Endovas. Surg. 1999, 33, 109–112. [Google Scholar]
- Mori, Y.; Ito, I.; Hatashita, S.; Yoshikawa, K. An anomalous case of the mesenteric arteries. Absence of a mesenterica inferior. J. Osaka Med. Coll. 1960, 20, 77–79. [Google Scholar]
- Li, A. High ligation of inferior mesenteric artery in laparoscopic resection of rectal cancer: Is it safe or dangerous? Ann. Laparosc. Endosc. Surg. 2016, 1, 49. [Google Scholar] [CrossRef]
- Kostov, S.; Kornovski, Y.; Ivanova, Y.; Dzhenkov, D.; Stoyanov, G.; Stoilov, S.; Slavchev, S.; Trendafilova, E.; Yordanov, A. Ovarian Carcinosarcoma with Retroperitoneal Para-Aortic Lymph Node Dissemination Followed by an Unusual Postoperative Complication: A Case Report with a Brief Literature Review. Diagnostics 2020, 10, 1073. [Google Scholar] [CrossRef]
- Karunanayake, A.L.; Pathmeswaran, A. Anatomical variations of lumbar arteries and their clinical implications: A cadaveric study. ISRN Anat. 2013, 2013, 154625. [Google Scholar] [CrossRef]
- Klemm, P.; Fröber, R.; Köhler, C.; Schneider, A. Vascular anomalies in the paraaortic region diagnosed by laparoscopy in patients with gynaecologic malignancies. Gynecol. Oncol. 2005, 96, 278–282. [Google Scholar] [CrossRef]
- Baniel, J.; Foster, R.S.; Donohue, J.P. Surgical anatomy of the lumbar vessels: Implications for retroperitoneal surgery. J. Urol. 1995, 153, 1422–1425. [Google Scholar] [CrossRef]
- Duan, H.; Liu, P.; Chen, C.; Chen, L.; Li, P.; Li, W.; Gong, S.; Xv, Y.; Chen, R.; Tang, L. Reconstruction of three-dimensional vascular models for lymphadenectomy before surgery. Minim. Invasive Ther. Allied Technol. 2020, 29, 42–48. [Google Scholar] [CrossRef]
- Sahoo, P.K.; Mohanty, R.K. Duplication of inferior vena cava in a case of ovarian carcinoma. J. Anat. Soc. India 2014, 63, S42–S43. [Google Scholar] [CrossRef]
- Hassan, A.; ElKammash, T.; Alam, A. Multidetector computed tomography of renal vasculature. anatomy and normal variants. ZUMJ 2014, 20, 1–13. [Google Scholar] [CrossRef]
- Grewal, K.; Jones, B.; L’Heveder, A.; Jindal, S.; Galazis, N.; Saso, S.; Yazbek, J. The use of intra-operative ultrasound in gynecological surgery: A review. Future Sci. 2021, 7, FSO678. [Google Scholar] [CrossRef] [PubMed]
- Mascilini, F.; Quagliozzi, L.; Moro, F.; Moruzzi, M.C.; Gallotta, V.; Alletti, S.G.; Scambia, G.; Testa, A.C.; Fagotti, A. Role of Intraoperative Ultrasound to Extend the Application of Minimally Invasive Surgery for Treatment of Recurrent Gynecologic Cancer. J. Minim. Invasive Gynecol. 2018, 25, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Ryo, E.; Yasugi, T.; Mizutani, K.; Kita, T.; Takeshita, S.; Ayabe, T. Diagnostic usefulness of intraoperative ultrasonography in avoiding unnecessary para-aortic lymphadenectomy in women with endometrial carcinoma. Int. J. Gynecol. Cancer 2011, 21, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Donadon, M.; Torzilli, G. Intraoperative ultrasound in patients with hepatocellular carcinoma: From daily practice to future trends. Liver Cancer 2013, 2, 16–24. [Google Scholar] [CrossRef]
- Elkerkary, M.A.; Gbr, H.; Shaban, H. Diagnostic accuracy of intraoperative cholangiography for detection of anatomical variations of the biliary system. Egypt J. Surg. 2021, 40, 131–139. [Google Scholar] [CrossRef]









| Vessel Variations | Incidence | Risk of Injury in the Paraaortic Regions | Reported Incidence of Injury |
|---|---|---|---|
| Renal arteries | |||
| Additional renal arteries | 10–50% | Infra/supramesenteric, aortocaval regions | Unknown |
| Accessory renal arteries | Unknown | All regions | Unknown |
| Precaval right renal artery | 0.8–5% | Infra/supramesenteric, aortocaval | Unknown |
| Renal veins | |||
| Additional renal veins | Left—1.3–3.2% Right—20–23% | All regions | Unknown |
| Circumaortic left renal vein | 3.5–7% | Infra/supramesenteric, aortocaval regions | 50% |
| Retroaortic left renal vein | 1.84–6.6% | Infra/supramesenteric, aortocaval regions | 19% |
| Retropelvic tributary of the left renal vein | 30.0–46.4% | Infra/supramesenteric region | Unknown |
| Abdominal aorta | |||
| Double AA | Only few cases described | All regions | Unknown |
| Inferior vena cava | |||
| Left sided IVC | 0.2–0.5% | Infra/supramesenteric region | Unknown |
| Left IVC with regressed right IVC | Less than 10 cases described | Infra/supramesenteric, caudal aortocaval regions | Unknown |
| Right sided duplication of IVC | Approximately 10 cases described | Aortocaval, paracaval | Unknown |
| Bilateral duplication of IVC | 0.2–3% | All regions | Unknown |
| Marsupial IVC | Approximately 20 cases described | Aortocaval, paracaval | Unknown |
| Ovarian vessels | |||
| Ovarian arteries | Unknown | All regions | Unknown |
| Ovarian veins | |||
| LOV draining into the LLVs | 2.2% | Infra/supramesenteric | Unknown |
| ROV draining into the RRV | 8.8–9.9% | Paracaval | Unknown |
| IMA | |||
| IMA arising from SMA | 0.1% | Aortocaval, Infra/supramesenteric regions | Unknown |
| LVs | |||
| LVs draining into the LRV | 17.4–49% | Aortocaval, Infra/supramesenteric | Unknown |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostov, S.; Selçuk, I.; Yordanov, A.; Kornovski, Y.; Yalçın, H.; Slavchev, S.; Ivanova, Y.; Dineva, S.; Dzhenkov, D.; Watrowski, R. Paraaortic Lymphadenectomy in Gynecologic Oncology—Significance of Vessels Variations. J. Clin. Med. 2022, 11, 953. https://doi.org/10.3390/jcm11040953
Kostov S, Selçuk I, Yordanov A, Kornovski Y, Yalçın H, Slavchev S, Ivanova Y, Dineva S, Dzhenkov D, Watrowski R. Paraaortic Lymphadenectomy in Gynecologic Oncology—Significance of Vessels Variations. Journal of Clinical Medicine. 2022; 11(4):953. https://doi.org/10.3390/jcm11040953
Chicago/Turabian StyleKostov, Stoyan, Ilker Selçuk, Angel Yordanov, Yavor Kornovski, Hakan Yalçın, Stanislav Slavchev, Yonka Ivanova, Svetla Dineva, Deyan Dzhenkov, and Rafał Watrowski. 2022. "Paraaortic Lymphadenectomy in Gynecologic Oncology—Significance of Vessels Variations" Journal of Clinical Medicine 11, no. 4: 953. https://doi.org/10.3390/jcm11040953
APA StyleKostov, S., Selçuk, I., Yordanov, A., Kornovski, Y., Yalçın, H., Slavchev, S., Ivanova, Y., Dineva, S., Dzhenkov, D., & Watrowski, R. (2022). Paraaortic Lymphadenectomy in Gynecologic Oncology—Significance of Vessels Variations. Journal of Clinical Medicine, 11(4), 953. https://doi.org/10.3390/jcm11040953










