IGNITE Status Epilepticus Survey: A Nationwide Interrogation about the Current Management of Status Epilepticus in Germany
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Participants and Number of Cases
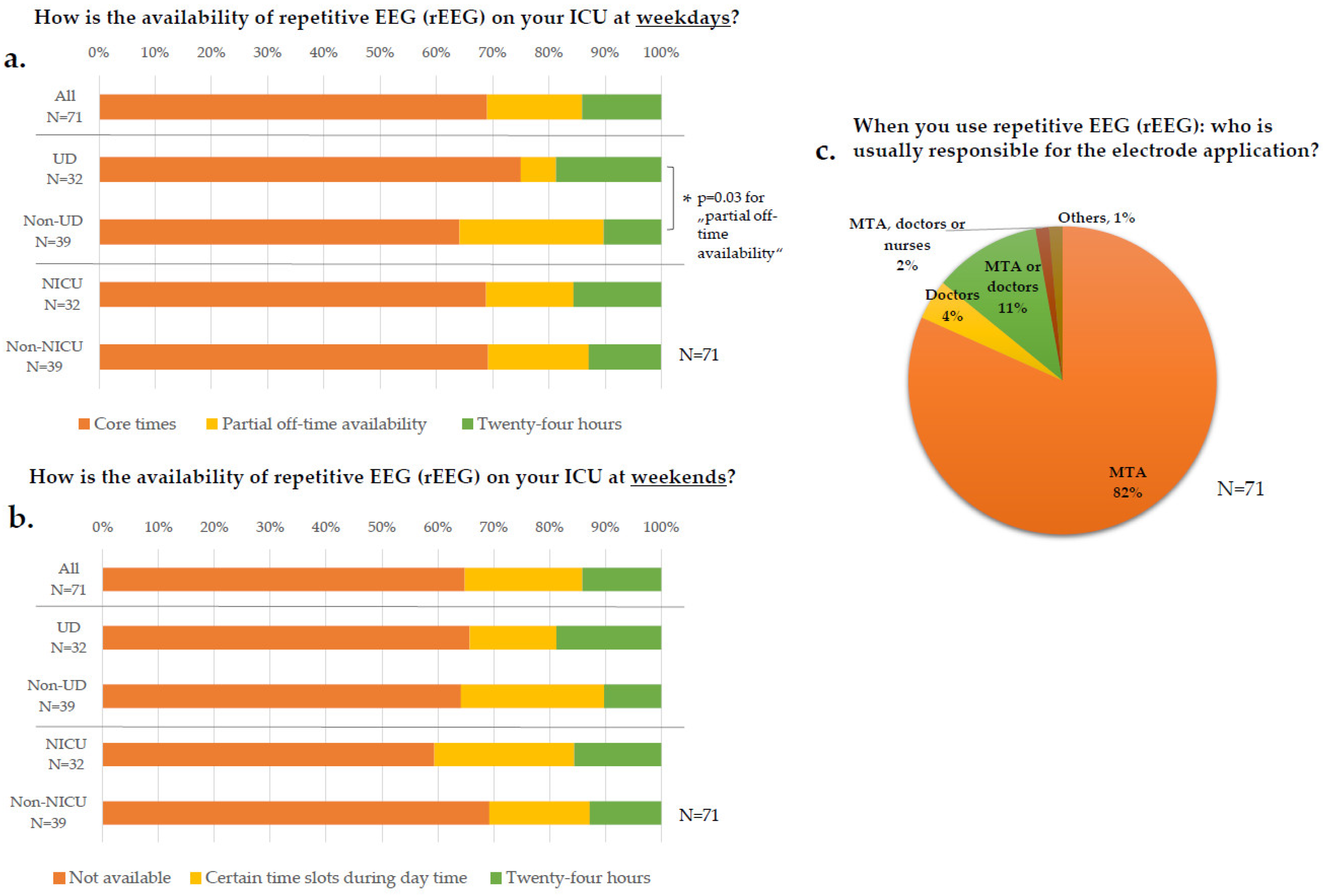
3.2. EEG Diagnostics
3.3. Therapy
3.3.1. Standard Operating Procedures (SOP)
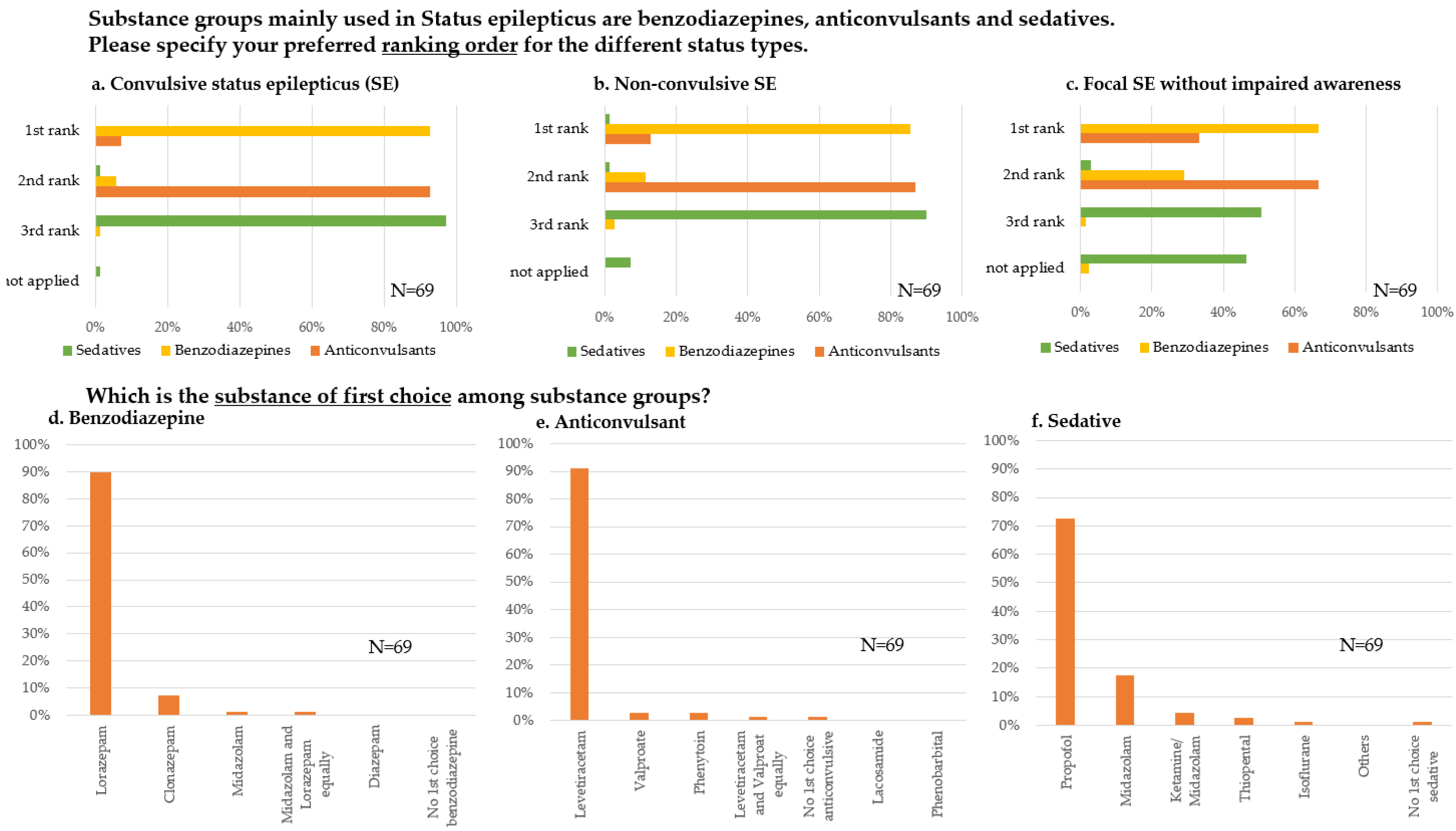
3.3.2. Substance Preferences and Sequences
3.4. Network Structures and Established Follow-Up Evaluation
4. Discussion
4.1. Participating Departments
4.2. EEG Diagnostics
4.3. Therapy
4.4. Organizational Structures
4.5. Limitations
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Trinka, E.; Cock, H.; Hesdorffer, D.; Rossetti, A.; Scheffer, I.; Shinnar, S.; Shorvon, S.; Lowenstein, D.H. A definition and classification of status epilepticus—Report of the ILAE Task Force on Classification of Status Epilepticus. Epilepsia 2015, 56, 1515–1523. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, F.; Weber, J.; Dohmen, C.; Hamer, H.M.; Holtkamp, M.; Jahnke, K. Status epilepticus im Erwachsenenalter, S2k-Leitlinie. In Deutsche Gesellschaft für Neurologie (Hrsg.), Leitlinien für Diagnostik und Therapie in der Neurologie; 2020; Available online: www.dgn.org/leitlinien (accessed on 29 January 2022).
- Abend, N.S.; Dlugos, D.J.; Hahn, C.; Hirsch, L.; Herman, S. Use of EEG Monitoring and Management of Non-Convulsive Seizures in Critically Ill Patients: A Survey of Neurologists. Neurocrit. Care 2010, 12, 382–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gavvala, J.; Abend, N.; Laroche, S.; Hahn, C.; Herman, S.T.; Claassen, J.; Macken, M.; Schuele, S.; Gerard, E.; the Critical Care EEG Monitoring Research Consortium (CCEMRC). Continuous EEG monitoring: A survey of neurophysiologists and neurointensivists. Epilepsia 2014, 55, 1864–1871. [Google Scholar] [CrossRef] [PubMed]
- Kowoll, C.M.; Dohmen, C.; Kahmann, J.; Dziewas, R.; Schirotzek, I.; Sakowitz, O.W.; Bösel, J.; for the Initiative of German NeuroIntensive Trial Engagement (IGNITE). Standards of Scoring, Monitoring, and Parameter Targeting in German Neurocritical Care Units: A National Survey. Neurocrit. Care 2013, 20, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Hilkman, D.M.W.; Van Mook, W.N.K.A.; Mess, W.H.; Van Kranen-Mastenbroek, V.H.J.M. The Use of Continuous EEG Monitoring in Intensive Care Units in The Netherlands: A National Survey. Neurocrit. Care 2018, 29, 195–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossetti, A.O.; Schindler, K.; Sutter, R.; Rüegg, S.; Zubler, F.; Novy, J.; Oddo, M.; Warpelin-Decrausaz, L.; Alvarez, V. Continuous vs. Routine Electroencephalogram in Critically Ill Adults with Altered Consciousness and No Recent Seizure. JAMA Neurol. 2020, 77, 1225–1232. [Google Scholar] [CrossRef] [PubMed]
- Herman, S.T.; Abend, N.S.; Bleck, T.; Chapman, K.E.; Drislane, F.W.; Emerson, R.G.; Gerard, E.E.; Hahn, C.; Husain, A.M.; Kaplan, P.W.; et al. Consensus Statement on Continuous EEG in Critically Ill Adults and Children, Part I. J. Clin. Neurophysiol. 2015, 32, 87–95. [Google Scholar] [CrossRef] [Green Version]
- Herman, S.T.; Abend, N.S.; Bleck, T.; Chapman, K.E.; Drislane, F.W.; Emerson, R.G.; Gerard, E.E.; Hahn, C.; Husain, A.M.; Kaplan, P.W.; et al. Consensus Statement on Continuous EEG in Critically Ill Adults and Children, Part II. J. Clin. Neurophysiol. 2015, 32, 96–108. [Google Scholar] [CrossRef] [Green Version]
- Beniczky, S.; Hirsch, L.; Kaplan, P.W.; Pressler, R.; Bauer, G.; Aurlien, H.; Brøgger, J.C.; Trinka, E. Unified EEG terminology and criteria for nonconvulsive status epilepticus. Epilepsia 2013, 54, 28–29. [Google Scholar] [CrossRef]
- Gaspard, N.; Foreman, B.P.; Alvarez, V.; Kang, C.C.; Probasco, J.C.; Jongeling, A.C.; Meyers, E.; Espinera, A.; Haas, K.F.; Schmitt, S.E.; et al. New-onset refractory status epilepticus. Neurology 2015, 85, 1604–1613. [Google Scholar] [CrossRef] [Green Version]
- Gaspard, N.; Hirsch, L. Pitfalls in ictal EEG interpretation: Critical care and intracranial recordings. Neurology 2012, 80, S26–S42. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.H.; Sherill, G.C.; Sinha, S.R.; Swisher, C. A Trial of Real-Time Electrographic Seizure Detection by Neuro-ICU Nurses Using a Panel of Quantitative EEG Trends. Neurocrit. Care 2019, 31, 312–320. [Google Scholar] [CrossRef] [PubMed]
- Rosenow, F.; Weber, J.; on behalf of Deutsche Gesellschaft für Neurologie (DGN). S2k-Leitlinie: Status Epilepticus im Erwachsenenalter. Nervenarzt 2021, 92, 1002–1030. [Google Scholar] [CrossRef] [PubMed]
- Alldredge, B.K.; Gelb, A.M.; Isaacs, S.M.; Corry, M.D.; Allen, F.; Ulrich, S.; Gottwald, M.D.; O’Neil, N.; Neuhaus, J.M.; Segal, M.R.; et al. A Comparison of Lorazepam, Diazepam, and Placebo for the Treatment of Out-of-Hospital Status Epilepticus. N. Engl. J. Med. 2001, 345, 631–637. [Google Scholar] [CrossRef] [PubMed]
- Prasad, K.; Al-Roomi, K.; Krishnan, P.R.; Sequeira, R.; Al-Roomi, K. Anticonvulsant therapy for status epilepticus. Cochrane Database Syst. Rev. 2005, 4, CD003723. [Google Scholar] [CrossRef]
- Kellinghaus, C.; Rossetti, A.O.; Trinka, E.; Lang, N.; May, T.W.; Unterberger, I.; Rüegg, S.; Sutter, R.; Strzelczyk, A.; Tilz, C.; et al. Factors predicting cessation of status epilepticus in clinical practice: Data from a prospective observational registry (SENSE). Ann. Neurol. 2019, 85, 421–432. [Google Scholar] [CrossRef] [Green Version]
- Beuchat, I.; Novy, J.; Rossetti, A.O. Newer Antiepileptic Drugs in Status Epilepticus: Prescription Trends and Outcomes in Comparison with Traditional Agents. CNS Drugs 2017, 31, 327–334. [Google Scholar] [CrossRef]
- Kellinghaus, C.; Rossetti, A.O.; Trinka, E.; Lang, N.; Unterberger, I.; Rüegg, S.; Tilz, C.; Uzelac, Z.; Rosenow, F. SENSE registry for status epilepticus. Epilepsia 2018, 59, 150–154. [Google Scholar] [CrossRef]
- Kapur, J.; Elm, J.; Chamberlain, J.M.; Barsan, W.; Cloyd, J.; Lowenstein, D.; Shinnar, S.; Conwit, R.; Meinzer, C.; Cock, H.; et al. Randomized Trial of Three Anticonvulsant Medications for Status Epilepticus. N. Engl. J. Med. 2019, 381, 2103–2113. [Google Scholar] [CrossRef]
- Costa, A.-M.; Lucchi, C.; Malkoç, A.; Rustichelli, C.; Biagini, G. Relationship between Delta Rhythm, Seizure Occurrence and Allopregnanolone Hippocampal Levels in Epileptic Rats Exposed to the Rebound Effect. Pharmaceuticals 2021, 14, 127. [Google Scholar] [CrossRef]
- Sutter, R.; De Marchis, G.M.; Semmlack, S.; Fuhr, P.; Rüegg, S.; Marsch, S.; Ziai, W.C.; Kaplan, P.W. Anesthetics and Outcome in Status Epilepticus: A Matched Two-Center Cohort Study. CNS Drugs 2017, 31, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Marchi, N.A.; Novy, J.; Faouzi, M.; Stähli, C.; Burnand, B.; Rossetti, A.O. Status Epilepticus. Crit. Care Med. 2015, 43, 1003–1009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schubert, J.; Brämer, D.; Huttner, H.B.; Gerner, S.T.; Fuhrer, H.; Melzer, N.; Dik, A.; Prüss, H.; Ly, L.-T.; Fuchs, K.; et al. Management and prognostic markers in patients with autoimmune encephalitis requiring ICU treatment. Neurol.-Neuroimmunol. Neuroinflamm. 2019, 6, e514. [Google Scholar] [CrossRef]
- Shorvon, S.; Ferlisi, M. The outcome of therapies in refractory and super-refractory convulsive status epilepticus and recommendations for therapy. Brain 2012, 135, 2314–2328. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| All (N = 83) | Uni-Department (UD, N = 36) | Non-Uni-Department (Non-UD, N = 47) | Sig | NICU (N = 38) | Non-NICU (N = 45) | Sig | |
|---|---|---|---|---|---|---|---|
| ICU patient population (N, %) | |||||||
| Only neurological | 29 (35%) | 17 (47%) * | 12 (26%) * | p = 0.040 + | 28/38 (74%) | 1/45 (2%) * | p < 0.001 + |
| Only neurosurgical | 2 (2%) | 2 (6%) | 0 (0%) | n.s. | 2/38 (5%) | 0/45 (0%) | n.s. |
| Interdisciplinary | 52 (63%) | 17 (47%) * | 35 (75%) * | p = 0.011 + | 8/38 (21%) | 44/45 (98%) * | p < 0.001 + |
| N = 83 | N = 36 | N = 47 | N = 38 | N = 45 | |||
| Specialty of ICU (department association, N, %) | |||||||
| Neurology | 34 (41%) | 20 (56%) * | 14 (30%) * | p = 0.018 + | 34/38 (90%) * | 0/45 (0%) * | p < 0.001 + |
| Neurosurgery | 4 (5%) | 4 (11%) * | 0 (0) * | p = 0.019 + | 4/38 (11%) * | 0/45 (0%) * | p = 0.026 + |
| Anesthesiology | 15 (18%) | 1 (3%) * | 14 (30%) * | p = 0.002 + | 0/38 (0%) * | 15/45 (33%) * | p < 0.001 + |
| Internal Medicine | 8 (10%) | 2 (6%) | 6 (13%) | n.s. | 0/38 (0%) * | 8/45 (18%) * | p = 0.006 + |
| Interdisciplinary | 22 (27%) | 9 (25%) | 13 (28%) | n.s. | 0/38 (0%) * | 22/45 (49%) * | p < 0.001 + |
| N = 83 | N = 36 | N = 47 | N = 38 | N = 45 | |||
| Median ICU bed size (range) | 12 (5–40) | 12 (6–35) | 12 (5–40) | n.s. | 12 (5–28) * | 16 (6–40) * | p = 0.002 # |
| N = 83 | N = 36 | N = 47 | N = 38 | N = 45 | |||
| Number of neurological patients per month (N, %) | |||||||
| <1–10 | 22 (27%) | 3 (8%) * | 19 (40%) * | p = 0.001 + | 0 (0%) * | 22 (49%) * | p < 0.001 + |
| 11–30 | 29 (35%) | 13 (36%) | 16 (34%) | n.s. | 13 (36%) | 16 (34%) | n.s. |
| >30 | 32 (39%) | 20 (56%) * | 12 (26%) * | p = 0.005 + | 25 (66%) * | 7 (16%) * | p < 0.001 + |
| N = 83 | N = 36 | N = 47 | N = 38 | N = 45 | |||
| Estimated number of SE in 2018 per department (median N, range) | 35 (3–250) | 45 (3–250) | 30 (4–150) | n.s. | 50 (3–200) | 30 (4–250) | n.s. |
| N = 71 | N = 32 | N = 39 | N = 32 | N = 39 | |||
| Estimated percentage of SE types in 2018 (median N, range) | |||||||
| Generalized | 30 (1–85) | 30 (5–80) | 25 (1–85) | n.s. | 30 (5–80) | 30 (1–85) | n.s. |
| Non-convulsive | 40 (5–90) | 40 (20–85) | 40 (5–90) | n.s. | 42.5 (20–90) | 40 (5–85) | n.s. |
| Focal | 20 (0–50) | 17.5 (0–50) | 20 (0–50) | n.s. | 19.5 (0–40) | 20 (0–50) | n.s. |
| Absence | 1 (0–20) | 0.5 (0–20) | 1 (0–20) | n.s. | 2 (0–20) | 0 (0–20) | n.s. |
| N = 71 | N = 32 | N = 39 | N = 32 | N = 39 | |||
| Estimated frequency of SE stages in percent in 2018 (median N, range) | |||||||
| Responsive | 40 (10–80) | 40 (10–70) | 40 (0–80) | n.s. | 40 (0–80) | 40 (8–80) | n.s. |
| Established | 30 (5–62) | 30 (10–60) | 25 (5–62) | n.s. | 30 (5–60) | 30 (10–62) | n.s. |
| Refractory | 15 (4–60) | 15 (5–60) | 20 (4–60) | n.s. | 15 (5–55) | 20 (4–60) | n.s. |
| Super-refractory | 10 (0–50) | 5 (0–50) | 10 (0–50) | n.s. | 10 (0–50) | 10 (0–30) | n.s. |
| N = 71 | N = 32 | N = 39 | N = 32 | N = 39 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowoll, C.M.; Klein, M.; Salih, F.; Fink, G.R.; Stetefeld, H.R.; Onur, O.A.; Malter, M.P.; on behalf of the IGNITE Group. IGNITE Status Epilepticus Survey: A Nationwide Interrogation about the Current Management of Status Epilepticus in Germany. J. Clin. Med. 2022, 11, 1171. https://doi.org/10.3390/jcm11051171
Kowoll CM, Klein M, Salih F, Fink GR, Stetefeld HR, Onur OA, Malter MP, on behalf of the IGNITE Group. IGNITE Status Epilepticus Survey: A Nationwide Interrogation about the Current Management of Status Epilepticus in Germany. Journal of Clinical Medicine. 2022; 11(5):1171. https://doi.org/10.3390/jcm11051171
Chicago/Turabian StyleKowoll, Christina M., Matthias Klein, Farid Salih, Gereon R. Fink, Henning R. Stetefeld, Oezguer A. Onur, Michael P. Malter, and on behalf of the IGNITE Group. 2022. "IGNITE Status Epilepticus Survey: A Nationwide Interrogation about the Current Management of Status Epilepticus in Germany" Journal of Clinical Medicine 11, no. 5: 1171. https://doi.org/10.3390/jcm11051171
APA StyleKowoll, C. M., Klein, M., Salih, F., Fink, G. R., Stetefeld, H. R., Onur, O. A., Malter, M. P., & on behalf of the IGNITE Group. (2022). IGNITE Status Epilepticus Survey: A Nationwide Interrogation about the Current Management of Status Epilepticus in Germany. Journal of Clinical Medicine, 11(5), 1171. https://doi.org/10.3390/jcm11051171





