Endogenous Endophthalmitis—The Clinical Significance of the Primary Source of Infection
Abstract
:1. Background
2. Primary Source of Infection
2.1. Pyogenic Liver Abscess
2.2. Endocarditis
2.3. Meningitis
2.4. Urinary Tract Infection, Urological and Gastrointestinal Interventions
2.5. Soft Tissue Infections
2.6. Osteomyelitis
2.7. Intravenous Drug Use
2.8. Indwelling Central Venous Access Devices (CVAD)
3. Diagnostic Challenges
4. Treatment Possibilities
5. Complications
6. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Durand, M.L. Bacterial and Fungal Endophthalmitis. Clin. Microbiol. Rev. 2017, 30, 597–613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheu, S.-J. Endophthalmitis. Korean J. Ophthalmol. 2017, 31, 283–289. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steeples, L.R.; Jones, N.P. Staphylococcal endogenous endophthalmitis in association with pyogenic vertebral osteomyelitis. Eye 2016, 30, 152–155. [Google Scholar] [CrossRef] [PubMed]
- Jackson, T.L.; Paraskevopoulos, T.; Georgalas, I. Systematic review of 342 cases of endogenous bacterial endophthalmitis. Surv. Ophthalmol. 2014, 59, 627–635. [Google Scholar] [CrossRef] [PubMed]
- Arevalo, J.F.; Jap, A.; Chee, S.P.; Zeballos, D.G. Endogenous endophthalmitis in the developing world. Int. Ophthalmol. Clin. 2010, 50, 173–187. [Google Scholar] [CrossRef]
- Streilein, J.W. Ocular immune privilege: The eye takes a dim but practical view of immunity and inflammation. J. Leukoc. Biol. 2003, 74, 179–185. [Google Scholar] [CrossRef]
- Livingston, E.T.; Mursalin, M.H.; Callegan, M.C. A pyrrhic victory: The PMN response to ocular bacterial infections. Microorganisms 2019, 7, 537. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, D.P.; Brent, B.D.; Orillac, R.; Baber, W.B.; Mayeux, P.A. A natural history study of experimental stuphylococcus epidermidis endophthalmitis. Curr. Eye Res. 1993, 12, 907–912. [Google Scholar] [CrossRef]
- Sekiguchi, T.; Inaba, A. Endogenous endophthalmitis following streptococcus pneumoniae meningitis. Intern. Med. 2015, 54, 2401–2404. [Google Scholar] [CrossRef] [Green Version]
- Safneck, J.R. Endophthalmitis: A review of recent trends. Saudi J. Ophthalmol. 2012, 26, 181–189. [Google Scholar] [CrossRef] [Green Version]
- Yoshida, M.; Yokokura, S.; Nishida, T.; Mochizuki, K.; Suzuki, T.; Maruyama, K.; Otomo, T.; Nishiguchi, K.M.; Kunikata, H.; Nakazawa, T. Endogenous endophthalmitis caused by group B streptococcus; Case reports and review of 35 reported cases. BMC Ophthalmol. 2020, 20, 126. [Google Scholar] [CrossRef] [PubMed]
- Pillai, G.S.; Remadevi, K.K.; Anilkumar, V.; Radhakrishnan, N.; Rasheed, R.; Ravindran, G.C. Clinical profile and outcome of endogenous endophthalmitis at a quaternary referral centre in south India. Indian J. Ophthalmol. 2020, 68, 827–833. [Google Scholar] [PubMed]
- Chung, K.S.; Kim, Y.K.; Song, Y.G.; Kim, C.O.; Han, S.H.; Chin, B.S.; Gu, N.S.; Jeong, S.J.; Baek, J.H.; Choi, J.Y.; et al. Clinical review of endogenous endophthalmitis in Korea: A 14-year review of culture positive cases of two large hospitals. Yonsei Med. J. 2011, 52, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Bjerrum, S.S.; la Cour, M. 59 eyes with endogenous endophthalmitis–causes, outcomes and mortality in a Danish population between 2000 and 2016. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017, 255, 2023–2027. [Google Scholar] [CrossRef] [PubMed]
- Lou, B.; Sun, Y.; Lin, J.; Yuan, Z.; He, L.; Long, C.; Lin, X. Clinical Features of Endogenous Endophthalmitis Secondary to Minimally Invasive Upper Urinary Tract Calculus Removal. Ocul. Immunol. Inflamm. 2020. [Google Scholar] [CrossRef]
- Chien, K.H.; Huang, K.H.; Chung, C.H.; Hsieh, Y.H.; Liang, C.M.; Chang, Y.H.; Weng, T.H.; Chien, W.C. The impact of diabetes mellitus medication on the incidence of endogenous endophthalmitis. PLoS ONE 2020, 15, e0227442. [Google Scholar] [CrossRef]
- Vaziri, K.; Pershing, S.; Albini, T.A.; Moshfeghi, D.M.; Moshfeghi, A.A. Risk factors predictive of endogenous endophthalmitis among hospitalized patients with hematogenous infections in the United States. Am. J. Ophthalmol. 2015, 159, 498–504. [Google Scholar] [CrossRef]
- Danielescu, C.; Anton, N.; Stanca, H.T.; Munteanu, M. Endogenous Endophthalmitis: A Review of Case Series Published between 2011 and 2020. J. Ophthalmol. 2020, 2020, 8869590. [Google Scholar] [CrossRef]
- Lardière-Deguelte, S.; Ragot, E.; Amroun, K.; Piardi, T.; Dokmak, S.; Bruno, O.; Appere, F.; Sibert, A.; Hoeffel, C.; Sommacale, D.; et al. Hepatic abscess: Diagnosis and management. J. Visc. Surg. 2015, 152, 231–243. [Google Scholar] [CrossRef]
- Roediger, R.; Lisker-Melman, M. Pyogenic and Amebic Infections of the Liver. Gastroenterol. Clin. N. Am. 2020, 49, 361–377. [Google Scholar] [CrossRef]
- Serraino, C.; Elia, C.; Bracco, C.; Rinaldi, G.; Pomero, F.; Silvestri, A.; Melchio, R.; Fenoglio, L.M. Characteristics and management of pyogenic liver abscess: A European experience. Medicine 2018, 97, e0628. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.J.; Tao, Z.; Wu, H.L. Etiology and clinical manifestations of bacterial liver abscess: A study of 102 cases. Medicine 2018, 97, e12326. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, X.; Di, Y. Surgery combined with antibiotics for the treatment of endogenous endophthalmitis caused by liver abscess. BMC Infect. Dis. 2020, 20, 661. [Google Scholar] [CrossRef] [PubMed]
- Pentecost, G.S.; Kesterson, J. Pyogenic liver abscess and endogenous endophthalmitis secondary toKlebsiella pneumoniae. Am. J. Emerg. Med. 2021, 41, 264.e1–264.e3. [Google Scholar] [CrossRef] [PubMed]
- Hussain, I.; Ishrat, S.; Ho, D.C.; Khan, S.R.; Veeraraghavan, M.A.; Palraj, B.R.; Molton, J.S.; Abid, M.B. Endogenous endophthalmitis in Klebsiella pneumoniae pyogenic liver abscess: Systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 101, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Martel, A.; Butet, B.; Ramel, J.C.; Martiano, D.; Baillif, S. Prise en charge médicale d’un abcès sous-rétinien à Klebsiella pneumoniae avec récupération visuelle sans recours à la vitrectomie. J. Fr. Ophtalmol. 2017, 40, 876–881. [Google Scholar] [CrossRef] [PubMed]
- Harris, E.W.; D’Amico, D.J.; Bhisitkul, R.; Priebe, G.P.; Petersen, R. Bacterial subretinal abscess: A case report and review of the literature. Am. J. Ophthalmol. 2000, 129, 778–785. [Google Scholar] [CrossRef]
- Villasmil, R.J.; Lattanzio, N.; Burns, K.; Alkayali, T. More Than Meets the Eye: Infective Endocarditis Presenting as Endogenous Endophthalmitis. Cureus 2021, 13, e14745. [Google Scholar] [CrossRef]
- Chen, K.J.; Sun, M.H.; Chen, Y.P.; Wang, N.K.; Wu, W.C.; Lai, C.C. Endogenous Endophthalmitis Caused by Infective Endocarditis in East Asia. Ophthalmol. Retin. 2019, 3, 382–384. [Google Scholar] [CrossRef]
- Chu, V.H. Endocarditis. JAMA-J. Am. Med. Assoc. 2018, 320, 102. [Google Scholar] [CrossRef] [Green Version]
- Nakata, M.; Mashidori, T.; Higa, N.; Manita, M.; Chibana, N.; Tabata, K. Infective endocarditis with no underlying disease for which bacterial endophthalmitis have been the first symptom. Intern. Med. 2020, 59, 2061–2065. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.J.; Seo, S.W.; Park, J.M.; Chung, I.Y. Bilateral endophthalmitis as the initial presentation of bacterial meningitis. Korean J. Ophthalmol. 2009, 23, 321–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torii, H.; Miyata, H.; Sugisaka, E.; Ichikawa, Y.; Shinoda, K.; Inoue, M. Bilateral endophthalmitis in a patient with bacterial meningitis caused by Streptococcus pneumoniae. Ophthalmologica 2008, 222, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qureshi, A.; Ashworth, J.; Sharma, V.; Ivanova, T. Favourable outcome in paediatric endogenous endophthalmitis secondary to Neisseria meningitidis following pars plana vitrectomy. BMJ Case Rep. 2020, 13, e233133. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-Y. Neonatal candidiasis associated with meningitis and endophthalmitis. Pediatr. Int. 1994, 36, 261–265. [Google Scholar] [CrossRef] [PubMed]
- Quintyn, J.C.; Poupelin, S.; Fajoles-Vasseneix, C.; Brasseur, G. Meningococcus endophthalmitis without meningitidis. J. Fr. Ophtalmol. 2006, 29, e24. [Google Scholar]
- Balaskas, K.; Potamitou, D. Endogenous endophthalmitis secondary to bacterial meningitis from Neisseria Meningitidis: A case report and review of the literature. Cases J. 2009, 2, 149. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.K.; Leo, S.W.; Edwards, C.J.; Koh, E.T. Primary Meningococcal Arthritis and Endogenous Endophthalmitis: A Case Report. Ann. Acad. Med. Singap. 2003, 32, 706–709. [Google Scholar]
- Nicolle, L.E. Urinary Tract Infection. Crit. Care Clin. 2013, 29, 699–715. [Google Scholar] [CrossRef]
- Margo, C.E.; Mames, R.N.; Guy, J.R. Endogenous Klebsiella Endophthahnitis: Report of Two Cases and Review of the Literature. Ophthalmology 1994, 101, 1298–1301. [Google Scholar] [CrossRef]
- Lin, H.-H.; Chien, C.-C.; Fang, J.-T.; Lai, R.-H.; Huang, C.-C. Unusual clinical presentation of klebsiella pneumoniae induced endogenous endophthalmitis and xanthogranulomatous pyelonephritis in a non-nephrolithiasis and non-obstructive urinary tract. Ren. Fail. 2002, 24, 659–665. [Google Scholar] [CrossRef] [Green Version]
- Corredores, J.; Hemo, I.; Jaouni, T.; Habot-Wilner, Z.; Kramer, M.; Shulman, S.; Jabaly-Habib, H.; Al-Talbishi, A.; Halpert, M.; Averbukh, E.; et al. Endogenous fungal endophthalmitis: Risk factors, clinical course, and visual outcome in 13 patients. Int. J. Ophthalmol. 2021, 14, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Muda, R.; Vayavari, V.; Subbiah, D.; Ishak, H.; Adnan, A.; Mohamed, S.O. Endogenous endophthalmitis: A 9-year retrospective study at a tertiary referral hospital in Malaysia. J. Ophthalmic Inflamm. Infect. 2018, 8, 14. [Google Scholar] [CrossRef] [PubMed]
- Makusha, L.P.; Young, C.R.; Agarwal, D.R.; Pucar, D. Bilateral End-Organ Endophthalmitis in Setting of Serratia marcescens Urosepsis on 18F-FDG PET/CT. Clin. Nucl. Med. 2020, 45, E141–E143. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Eliott, D. The emergence of Klebsiella pneumoniae endogenous endophthalmitis in the USA: Basic and clinical advances. J. Ophthalmic Inflamm. Infect. 2013, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Ghoraba, H.H.; Ellakwa, A.F.; Elgemai, E.M.; Mansour, H.O.; Heikal, M.A. Results of pars plana vitrectomy for the management of endogenous fungal endophthalmitis after urinary tract procedures. Retin. Cases Br. Rep. 2017, 11, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Tsujinaka, T.; Kambayashi, J.; Imaoka, M.; Iwanaga, T.; Mori, T. A multi-institutional study for prevalence, diagnosis and treatment of deep fungal infection in gastrointestinal surgery. Nippon Geka Gakkai Zasshi 1994, 95, 217–223. [Google Scholar]
- Ansari, A.S.; Lai, K.; Tejwani, D. An unexpected case of iatrogenic Escherichia coli endogenous pan-endopthalmitis after ERCP-associated biliary sepsis: A case report. Eur. J. Ophthalmol. 2019, 29, 4–9. [Google Scholar] [CrossRef]
- Richter, J.C.; Waldegg, G.; Richter, K.K. Rotes auge und visusstörung zwei monate nach ösophagusresektion mit kompliziertem postoperativem verlauf. Praxis 2008, 97, 1091–1095. [Google Scholar] [CrossRef]
- Pizango, O.; Tejeda, E.; Buendia, M.; Lujan, S. Bilateral endogenous ophthalmitis due to Candida glabrata after complicated bariatric surgery. Arch. Soc. Esp. Oftalmol. 2014, 89, 282–285. [Google Scholar] [CrossRef]
- Armesto, E.; Cubillas, M.; Antón, S. Bacterial endogenous endophthalmitis after colonoscopy. Rev. Esp. Enferm. Dig. 2018, 110, 601. [Google Scholar] [CrossRef] [PubMed]
- Bystritsky, R.; Chambers, H. Cellulitis and soft tissue infections. Ann. Intern. Med. 2018, 168, ITC17–ITC31. [Google Scholar] [CrossRef]
- Sim, Y.R.; Lee, Y.J.; Park, S.W.; Kim, S.H.; Choi, J.H.; Choi, J.Y.; Kim, M.J.; Sohn, J.W.; Ahn, J.; Yoon, Y.K. Infective endocarditis presenting as endogenous endophthalmitis secondary to Streptococcus agalactiae in a healthy adult: Case report and literature review. Infect. Chemother. 2017, 49, 286–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghiam, B.K.; Israelsen, P.; Wang, A.; Grob, S.; Esfahani, M.R. Klebsiella pneumoniae endogenous endophthalmitis presenting as orbital cellulitis. GMS Ophthalmol. Cases 2019, 9, Doc30. [Google Scholar]
- Etcheverria, E. Recognizing and treating five common dermatologic conditions seen in primary care. JAAPA Off. J. Am. Acad. Physician Assist. 2020, 33, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, J.; Flynn, H.W.; Kuriyan, A.E.; Dubovy, S.; Miller, D. Endophthalmitis caused by Klebsiella species. Retina 2014, 34, 1875–1881. [Google Scholar] [CrossRef] [Green Version]
- Lew, P.D.P.; Waldvogel, P.F.A. Osteomyelitis. Lancet 2004, 364, 369–379. [Google Scholar] [CrossRef]
- Hatzenbuehler, J.; Pulling, T.J. Diagnosis and Management of Osteomyelitisof Osteomyelitis. Am. Fam. Physician 2011, 84, 1027–1033. [Google Scholar]
- Christensen, S.R.; Hansen, A.B.E.; la Cour, M.; Fledelius, H.C. Bilateral endogenous bacterial endophthalmitis: A report of four cases. Acta Ophthalmol. Scand. 2004, 82, 306–310. [Google Scholar] [CrossRef]
- Mavrakanas, T.A.; de Haller, R.; Philippe, J. Endogenous Endophthalmitis in a Patient with Diabetes and Foot Osteomyelitis. Can. J. Diabetes 2015, 39, 18–20. [Google Scholar] [CrossRef] [Green Version]
- Bloom, C.; Thomson, E.C.; Main, J. An elderly lady with sudden blindness and a sore foot. J. Infect. 2006, 52, e53–e54. [Google Scholar] [CrossRef] [PubMed]
- Carr, J.M.; Hagan, G.; Guest, P.; Gompertz, S. A "not so superficial" skin infection in a patient with diabetes. BMJ Case Rep. 2012, 2012, bcr2012007062. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, K.; Tarumoto, N.; Tachibana, H.; Hanabusa, A.; Sakai, J.; Yokota, K.; Mimura, T.; Maesaki, S. Endogenous endophthalmitis secondary to septic arthritis caused by group A Streptococcus infection: A case report and literature review. J. Infect. Chemother. 2020, 26, 128–131. [Google Scholar] [CrossRef]
- Degenhardt, L.; Peacock, A.; Colledge, S.; Leung, J.; Grebely, J.; Vickerman, P.; Stone, J.; Cunningham, E.B.; Trickey, A.; Dumchev, K.; et al. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: A multistage systematic review. Lancet Glob. Health 2017, 5, e1192–e1207. [Google Scholar] [CrossRef] [Green Version]
- Tirpack, A.R.; Duker, J.S.; Baumal, C.R. An outbreak of endogenous fungal endophthalmitis among intravenous drug abusers in New England. AMA Ophthalmol. 2017, 135, 534–540. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.N.; Rescigno, R.J.; Zarbin, M.A.; Langer, P.; Bhagat, N. Endogenous endophthalmitis associated with intravenous drug abuse. Retina 2014, 34, 1460–1465. [Google Scholar] [CrossRef]
- Uppuluri, A.; Zarbin, M.A.; Bhagat, N. Endogenous endophthalmitis in patients with intravenous opioid use: Demographics and associated comorbidities. Int. Ophthalmol. 2021, 41, 1513–1520. [Google Scholar] [CrossRef]
- Becker, J.B.; Hu, M. Sex differences in drug abuse. Front. Neuroendocrinol. 2008, 29, 36–47. [Google Scholar] [CrossRef] [Green Version]
- Tuon, F.F.; de Almeida, G.M.D.; Costa, S.F. Central venous catheter-associated fungemia due to Rhodotorula spp.—A systematic review. Med. Mycol. 2007, 45, 441–447. [Google Scholar] [CrossRef] [Green Version]
- Bisbe, J.; Miro, J.M.; Latorre, X.; Moreno, A.; Mallolas, J.; Gatell, J.M.; de la Bellacasa, J.P.; Soriano, E. Disseminated candidiasis in addicts who use brown heroin: Report of 83 cases and review. Clin. Infect. Dis. 1992, 15, 910–923. [Google Scholar] [CrossRef]
- Poowanawittayakom, N.; Dutta, A.; Stock, S.; Touray, S.; Ellison, R.T.; Levitz, S.M. Reemergence of intravenous drug use as risk factor for Candidemia, Massachusetts, USA. Emerg. Infect. Dis. 2018, 24, 631–637. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Breazzano, M.P.; Jonna, G.; Nathan, N.R.; Nickols, H.H.; Agarwal, A. Endogenous Serratia marcescens panophthalmitis: A case series. Am. J. Ophthalmol. Case Rep. 2019, 16, 100531. [Google Scholar] [CrossRef]
- Pasula, S.; Trivedi, V.; Loshe, E.; Chandrasekar, P. Serratia marcescens infection-associated loss of vision: A case report in a patient with a history of intravenous drug use. Am. J. Case Rep. 2021, 22, e929116. [Google Scholar] [CrossRef] [PubMed]
- Ullman, A.J.; Marsh, N.; Mihala, G.; Cooke, M.; Rickard, C.M. Complications of central venous access devices: A systematic review. Pediatrics 2015, 136, e1331–e1344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khanna, V.; Mukhopadhayay, C.; KE, V.; Verma, M.; Dabke, P. Evaluation of Central Venous Catheter Associated Blood Stream Infections: A Microbiological Observational Study. J. Pathog. 2013, 2013, 936864. [Google Scholar] [CrossRef] [PubMed]
- Ruhnke, M.; Rickerts, V.; Cornely, O.A.; Buchheidt, D.; Glöckner, A.; Heinz, W.; Höhl, R.; Horré, R.; Karthaus, M.; Kujath, P.; et al. Diagnosis and therapy of Candida infections: Joint recommendations of the German Speaking Mycological Society and the Paul-Ehrlich-Society for Chemotherapy. Mycoses 2011, 54, 279–310. [Google Scholar] [CrossRef] [PubMed]
- Berman, A.J.; del Priore, L.V.; Fischer, C.K. Endogenous Ochrobactrum anthropi endophthalmitis. Am. J. Ophthalmol. 1997, 123, 560–562. [Google Scholar] [CrossRef]
- Equi, R.A.; Green, W.R. Endogenous Serratia marcescens endophthalmitis with dark hypopyon: A case report and review. Surv. Ophthalmol. 2001, 46, 259–268. [Google Scholar] [CrossRef]
- Cunningham, E.T.; Flynn, H.W.; Relhan, N.; Zierhut, M. Endogenous Endophthalmitis. Ocul. Immunol. Inflamm. 2018, 26, 491–495. [Google Scholar]
- Davis, J.L. Diagnostic dilemmas in retinitis and endophthalmitis. Eye 2012, 26, 194–201. [Google Scholar] [CrossRef]
- Regan, K.A.; Radhakrishnan, N.S.; Hammer, J.D.; Wilson, B.D.; Gadkowski, L.B.; Iyer, S.S.R. Endogenous Endophthalmitis: Yield of the diagnostic evaluation. BMC Ophthalmol. 2020, 20, 138. [Google Scholar] [CrossRef]
- Zhao, M.; Yu, Y.; Liu, W. Vitreous biopsy under air: Technique, complications, and volume outcomes. Ophthalmic Surg. Lasers Imaging Retin. 2019, 50, 365–370. [Google Scholar] [CrossRef] [Green Version]
- Brockhaus, L.; Goldblum, D.; Eggenschwiler, L.; Zimmerli, S.; Marzolini, C. Revisiting systemic treatment of bacterial endophthalmitis: A review of intravitreal penetration of systemic antibiotics. Clin. Microbiol. Infect. 2019, 25, 1364–1369. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.J.; Chen, Y.P.; Chao, A.N.; Wang, N.K.; Wu, W.C.; Lai, C.C.; Chen, T.L. Prevention of evisceration or enucleation in endogenous bacterial panophthalmitis with no light perception and scleral abscess. PLoS ONE 2017, 12, e0169603. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Fu, B.; Lu, C.; Xu, L.; Sun, J. Successful treatment of endogenous endophthalmitis with extensive subretinal abscess: A case report. BMC Ophthalmol. 2018, 18, 238. [Google Scholar] [CrossRef] [PubMed]
- Shikari, H.; Silva, P.S.; Sun, J.K. Complications of intravitreal injections in patients with diabetes. Semin. Ophthalmol. 2014, 29, 276–289. [Google Scholar] [CrossRef] [PubMed]
- Witkin, A.J.; Chang, D.F.; Jumper, J.M.; Charles, S.; Eliott, D.; Hoffman, R.S.; Mamalis, N.; Miller, K.M.; Wykoff, C.C. Vancomycin-Associated Hemorrhagic Occlusive Retinal Vasculitis: Clinical Characteristics of 36 Eyes. Ophthalmology 2017, 124, 583–595. [Google Scholar] [CrossRef] [Green Version]
- Vaziri, K.; Schwartz, S.G.; Kishor, K.; Flunn, H.W., Jr. Endophthalmitis: State of the art. Clin. Ophthalmol. 2015, 9, 95–108. [Google Scholar]
- Lu, X.; Chen, W.; Xia, H.; Zheng, K.; Jin, C.; Ng, D.S.; Chen, H. Atrophy of retinal inner layers is associated with poor vision after endophthalmitis: A spectral domain optical coherence tomography study. Eye 2017, 31, 1488–1495. [Google Scholar] [CrossRef]
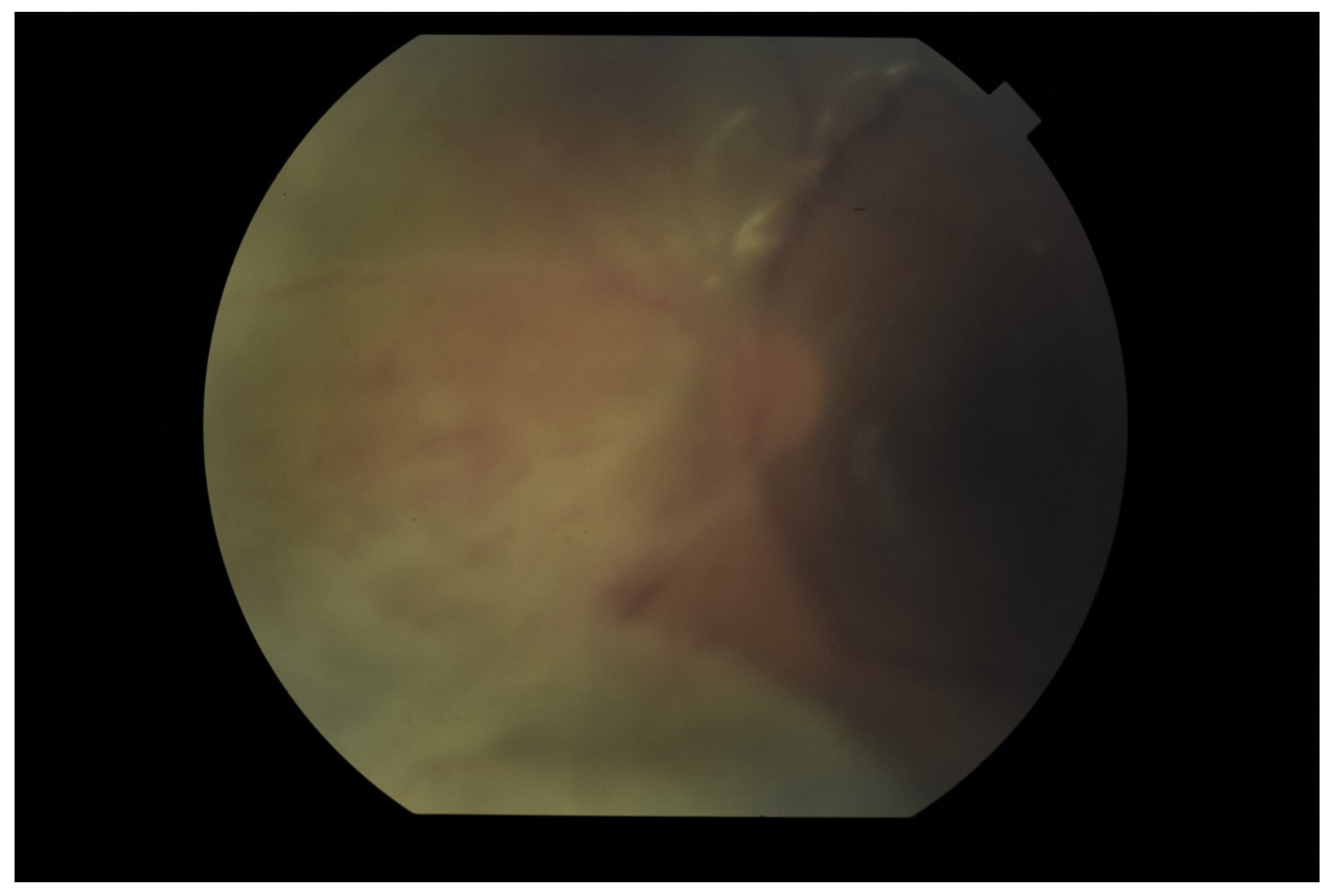




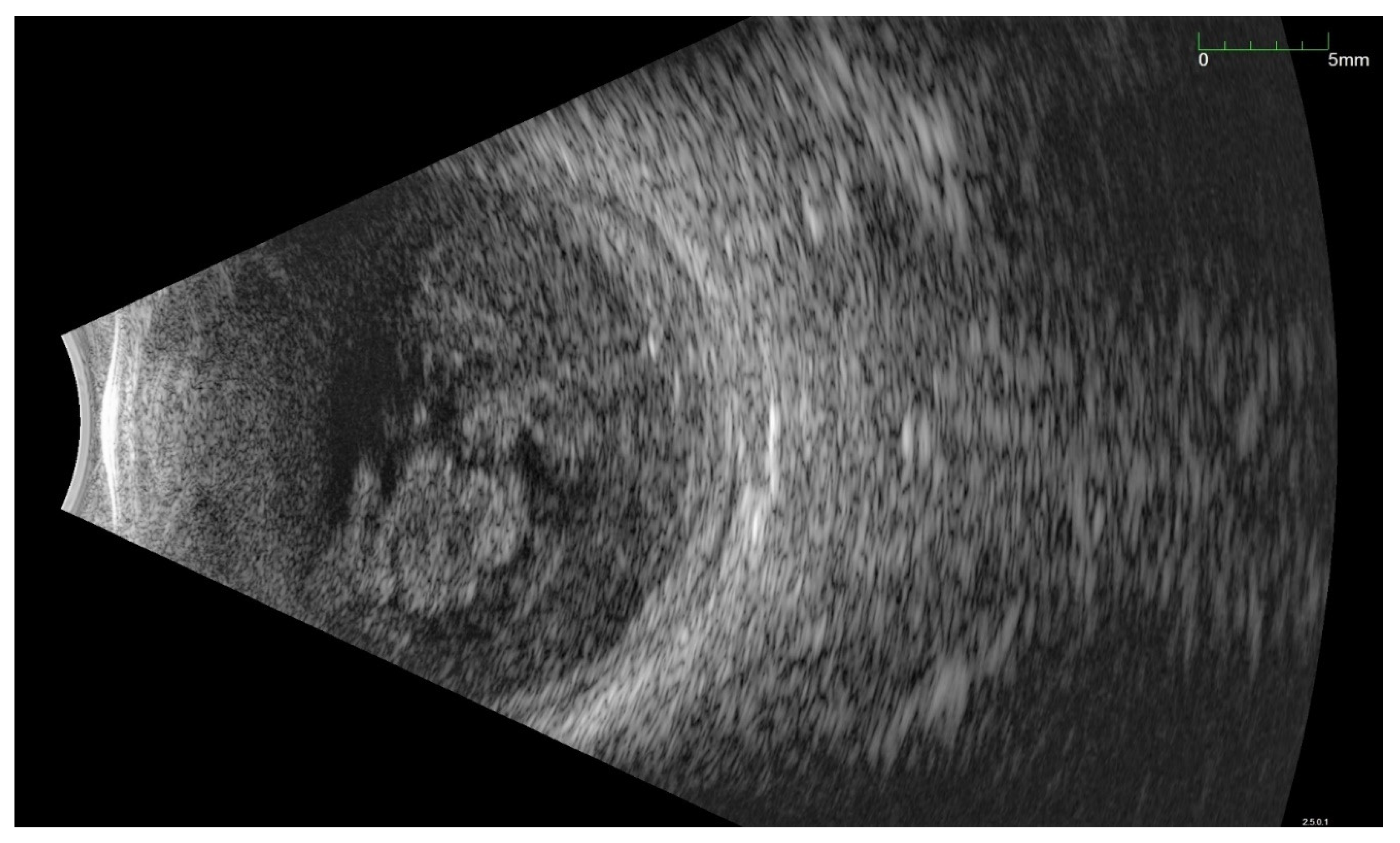

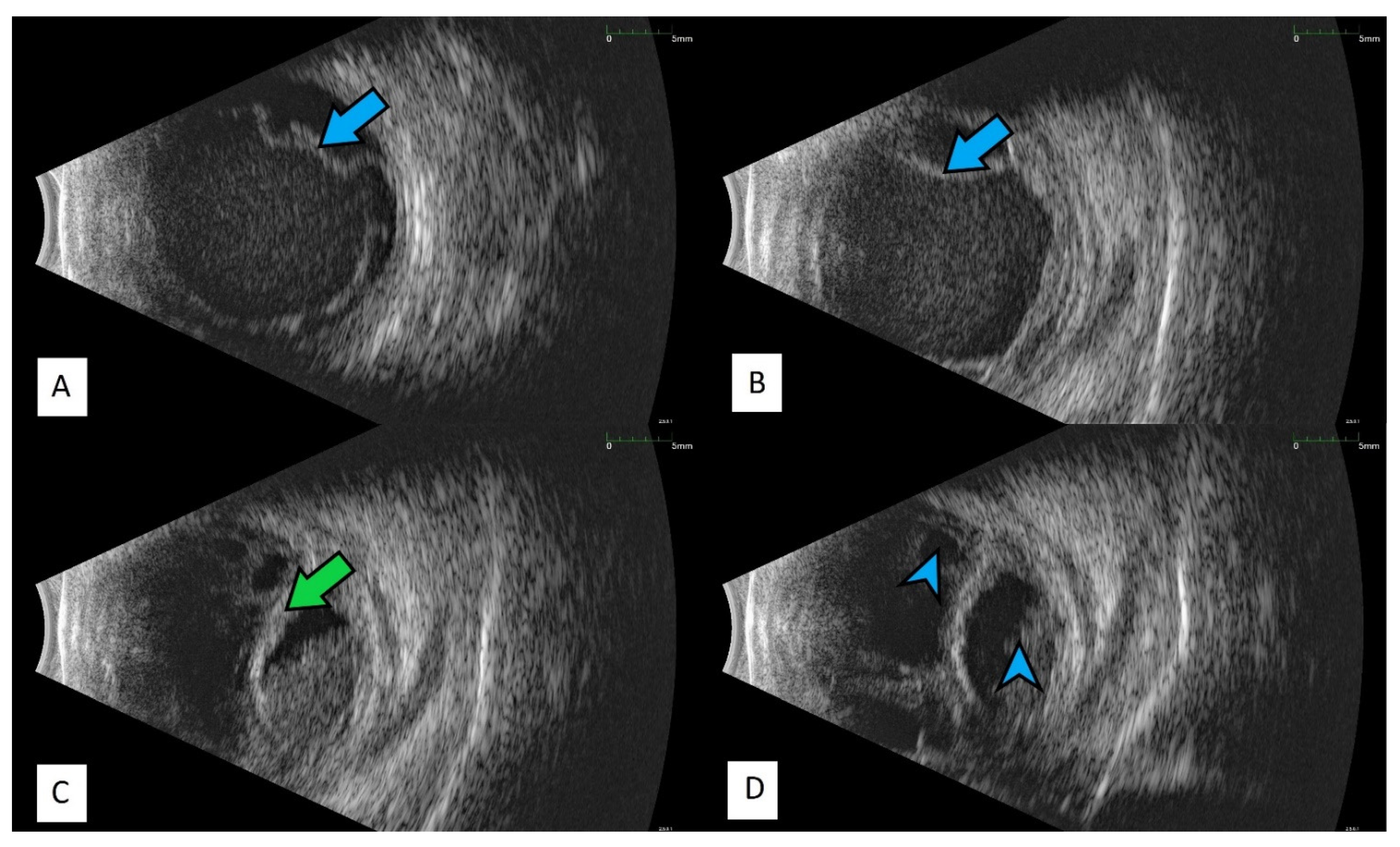








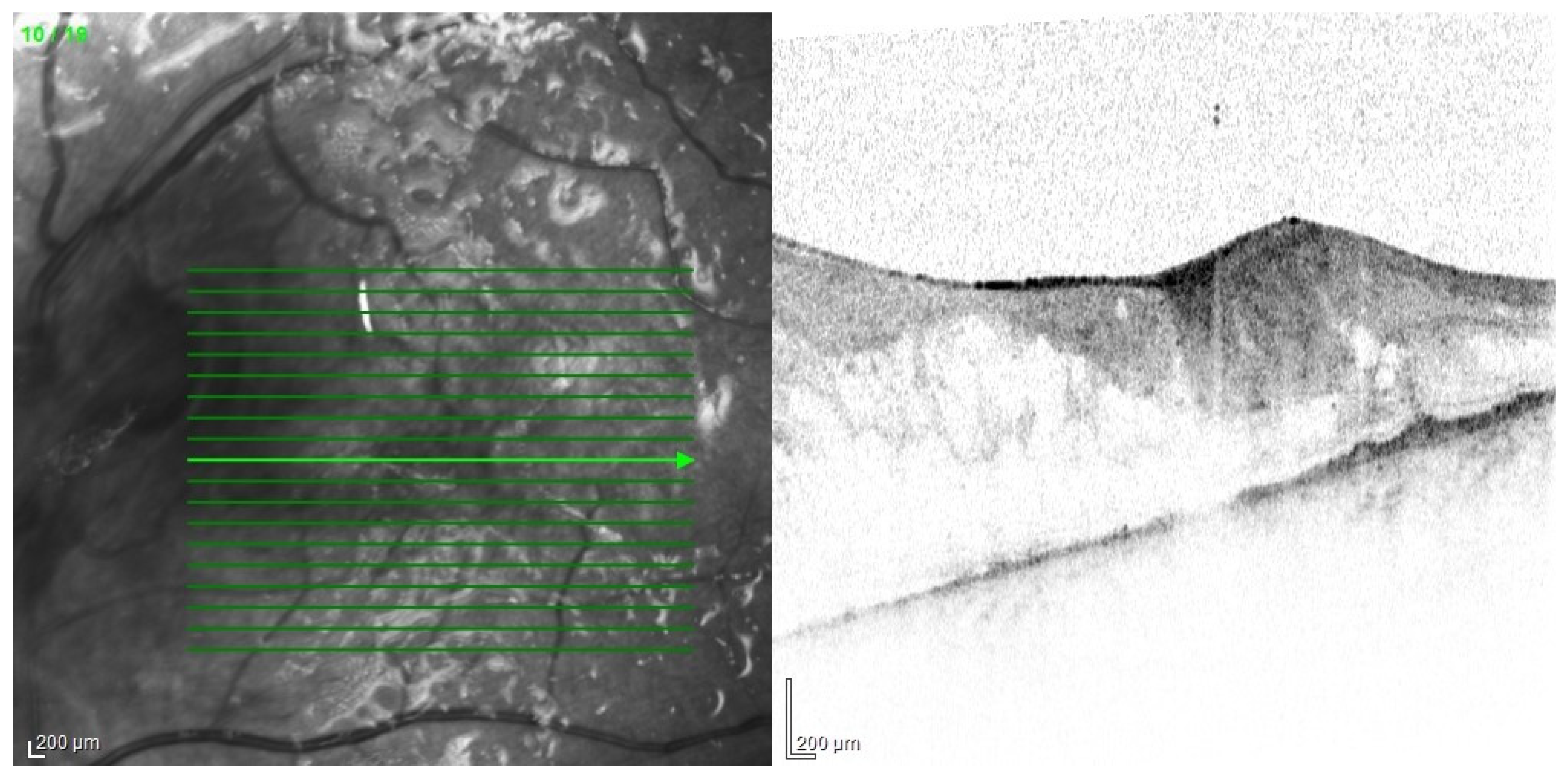

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajdzis, M.; Figuła, K.; Kamińska, J.; Kaczmarek, R. Endogenous Endophthalmitis—The Clinical Significance of the Primary Source of Infection. J. Clin. Med. 2022, 11, 1183. https://doi.org/10.3390/jcm11051183
Gajdzis M, Figuła K, Kamińska J, Kaczmarek R. Endogenous Endophthalmitis—The Clinical Significance of the Primary Source of Infection. Journal of Clinical Medicine. 2022; 11(5):1183. https://doi.org/10.3390/jcm11051183
Chicago/Turabian StyleGajdzis, Małgorzata, Kornelia Figuła, Joanna Kamińska, and Radosław Kaczmarek. 2022. "Endogenous Endophthalmitis—The Clinical Significance of the Primary Source of Infection" Journal of Clinical Medicine 11, no. 5: 1183. https://doi.org/10.3390/jcm11051183
APA StyleGajdzis, M., Figuła, K., Kamińska, J., & Kaczmarek, R. (2022). Endogenous Endophthalmitis—The Clinical Significance of the Primary Source of Infection. Journal of Clinical Medicine, 11(5), 1183. https://doi.org/10.3390/jcm11051183





