An Assessment of Selected Molecular and Biochemical Markers of the Folate Pathway as Potential Risk Factors for Fetal Trisomy 21 during the First Trimester of Pregnancy in the Polish Population
Abstract
:1. Introduction
2. Aims
3. Materials and Methods
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nicolaides, K.H. The 11–13+6 Weeks Scan; Fetal Medicine Foundation: London, UK, 2004; pp. 7–42. [Google Scholar]
- Nicolaides, K.H. Screening for fetal aneuploidies at 11 to 13 weeks. Prenat. Diagn. 2011, 31, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Santorum, M.; Wright, D.; Syngelaki, A.; Karagioti, N.; Nicolaides, K.H. Accuracy of first-trimester combined test in screening for trisomies 21, 18 and 13. Ultrasound Obstet. Gynecol. 2017, 49, 714–720. Available online: Wileyonlinelibrary.com (accessed on 20 February 2022). [CrossRef] [PubMed] [Green Version]
- Ziolkowska, K.; Dydowicz, P.; Sobkowski, M.; Tobola-Wrobel, K.; Wysocka, E.; Pietryga, M. The clinical usefulness of biochemical (free β-hCG, PAPP-A) and ultrasound (nuchal translucency) parameters in prenatal screening of trisomy 21 in the first trimester Of pregnancy. Ginekol. Pol. 2019, 90, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Hobbs, C.A.; Sherman, L.; Yi, P.; Hopkins, S.E.; Torfs, C.P.; Hine, R.J.; Pogribna, M.; Rozen, R.; James, S.J. Polymorphisms in genes involved in folate metabolism as maternal risk factors for Down Syndrome. Am. J. Hum. Genet. 2000, 67, 623–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppede, F.; Marini, G.; Bargagna, S.; Stuppia, L.; Minichilli, F.; Fontana, I.; Colognato, R.; Astrea, G.; Palka, G.; Migliore, L. Foate gene polymorphisms and the risk of Down syndrome pregnancies in young italian women. Am. J. Med. Genet. Part A 2006, 140, 1083–1091. [Google Scholar] [CrossRef]
- Wang, S.; Qiao, F.; Feng, L.; Lv, J. Polymorphisms in genes involved in folate metabolism as maternal risk factors for Down syndrome in China. J. Zhejiang Univ. Sci. B 2008, 9, 93–99. [Google Scholar] [CrossRef] [Green Version]
- Pavarino, E.C.; Zampieri, B.L.; Biselli, J.M.; Goloni Bertollo, E.M. Abnormal folate metabolism and maternal risk for Down syndrome. In Genetics and Etiology of Down Syndrome; InTech: Rijeka, Croacia, 2011; pp. 97–120. [Google Scholar] [CrossRef] [Green Version]
- Fintelman-Rodrigues, N.; Corrêa, J.C.; Santos, J.M.; Pimentel, M.M.G.; Santos-Rebouças, C.B. Investigation of CBS, MTR, RFC-1 And TC polymorphisms as maternal risk factors for Down syndrome. Dis. Markers 2009, 26, 155–161. [Google Scholar] [CrossRef]
- Coppede, F. The complex relationship between folate/homocysteine metabolism and risk of Down syndrome. Mutat. Res./Rev. Mutat. Res. 2009, 682, 54–70. [Google Scholar] [CrossRef]
- Rai, V.; Yadav, U.; Kumar, P.; Yadav, S.K.; Mishra, O.P. Maternal methylenetetrahydrofolate rductase C677T polymorphism and Down syndrome risk: A meta-analysis from 34 studies. PLoS ONE 2014, 9, e108552. [Google Scholar] [CrossRef]
- Czeczot, H. Kwas foliowy w fizjologii i patologii Folic acid in physiology and pathology. Postepy Hig. I Med. Dosw. 2008, 62, 405–419. [Google Scholar]
- Laskowska-Klita, T.; Chełchowska, M.; Ambroszkiewicz, J.; Gajewska, J. Kwas foliowy—Rola w metabolizmie komórki. Bromat. Chem. Toksykol. XLV 2012, 2, 144–151. [Google Scholar]
- Czechowicz, P.; Małodobra-Mazur, M.; Lebioda, A.; Jonkisz, A.; Dobosz, T.; Śmigiel, R. Polymorphisms of the MTHFR gene in mothers of children with trisomy 21 (Down syndrome) in a Polish population. Adv. Clin. Exp. Med. 2020, 29, 251–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppede, F.; Grossi, E.; Migheli, F.; Migliore, L. Polymorphisms in folate -metabolizing genes, chromosome damage, and risk of Down syndrome in Italian women: Identification of key factors using artificial neural networks. BMC Med. Genom. 2010, 3, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scala, I.; Granese, B.; Sellitto, M.; Salomè, S.; Sammartino, A.; Pepe, A.; Mastroiacovo, P.; Sebastio, G.; Andria, G. Analysis of seven maternal polymorphisms of genes involved in homocysteine/folate metabolism and risk of Down syndrome offspring. Genet. Med. 2006, 8, 409–416. [Google Scholar] [CrossRef] [Green Version]
- Coppede, F.; Bosco, P.; Lorenzoni, V.; Denaro, M.; Anello, G.; Antonucci, I.; Barone, C.; Stuppia, L.; Romano, C.; Migliore, L. The MTRR 66A>G polymorphism and maternal risk of birth of a child with Down syndrome in Caucasian women: A case-control study and a meta-analysis. Mol. Biol. Rep. 2014, 41, 5571–5583. [Google Scholar] [CrossRef]
- Kaur, A.; Kaur, A. Maternal MTHFR polymorphism (677 C–T) and risk of Down’s syndrome child: Meta-analysis. J. Genet. 2016, 95, 505–513. [Google Scholar] [CrossRef]
- Oussalah, A.; Levy, J.; Filhine-Tresarrieu, P.; Namour, F.; Guéant, J. Association of TCN2 rs1801198 c.776G.C polymorphism with markers of one-carbon metabolism and related diseases: A systematic review and meta-analysis of genetic association studies. Am. J. Clin. Nutr. 2017, 106, 1142–1156. [Google Scholar] [CrossRef] [Green Version]
- Stanisławska-Sachadyn, A.; Woodside, J.V.; Sayers, C.M.; Yarnell, J.W.; Young, I.S.; Evans, A.E.; Mitchell, L.E.; Whitehead, A.S. The transcobalamin (TCN2) 776C4G polymorphism affects homocysteine concentrations among subjects with low vitamin B12 status. Eur. J. Clin. Nutr. 2010, 64, 1338–1343. [Google Scholar] [CrossRef] [Green Version]
- Anjali Haloi, A.; Das, D. Vitamin B12 Gene Polymorphisms and Chronic Diseases. J. Nutr. Disord. Ther. 2014, 4, 149. [Google Scholar] [CrossRef]
- Katarzyńska, J. Potencjał aplikacyjny witaminy B12 i jej analogów. Instytut Chemii Organicznej, Wydział Chemiczny, Politechnika Łódzka. Eliksir 2016, 2, 11–18. [Google Scholar]
- Moczulska, H.; Pesz, K.; Gach, A.; Borowiec, M.; Sieroszewski, P.; Sąsiadek, M.; Jakubowski, L.; Wielgoś, M. Stanowisko ekspertów Polskiego Towarzystwa Genetyki Człowieka i Polskiego Towarzystwa Ginekologów i Położników w sprawie zlecania i interpretacji wyników badań pod kątem wariantów genetycznych w genie MTHFR. (MTHFR genetic testing: Recommendations of the Polish Society of Gynecologists and Obstetricians and the Polish Human Genetics Society). Ginekol. Perinatol. Prakt. Med. 2017, 2, 234–238. [Google Scholar]
- Coppede, F. The genetics of folate metabolism and maternal risk of birth of a child with Down syndrome and associated congenital heart defects. Front. Genet. 2015, 6, 223. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.R.J.; Vergani, N.; Galdieri, L.C.; Picarelli Ribeiro Porto, M.; Bragagnolo Longhitano, S.; Brunoni, D.; D’Almeida, V.; Alvarez Perez, A.B. Relationship between polymorphisms in genes involved in homocysteine metabolism and maternal risk for Down syndrome in Brazil. Am. J. Med. Genet. A 2005, 135, 263–267. [Google Scholar] [CrossRef]
- Sukla, K.K.; Jaiswal, S.K.; Rai, A.K.; Mishra, O.P.; Gupta, V.; Kumar, A.; Raman, R. Role of folate-homocysteine pathway gene polymorphisms and nutritional cofactors in Down syndrome: A triad study. Hum. Reprod. 2015, 30, 1982–1993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liao, Y.P.; Bao, M.S.; Liu, C.Q.; Liu, H.; Zhng, D. Folate gene polymorphism and the risk of Down syndrome pregnancies in young Chinese women. Yi Chuan 2010, 32, 461–466. [Google Scholar] [CrossRef] [PubMed]
- Zampieri, B.L.; Biselli, J.M.; Goloni-Bertollo, E.M.; Vannucchib, H.; Carvalhoc, V.M.; Cordeirod, J.A.; Pavarinoa, E.C. Maternal risk for Down syndrome is modulated by genes involved in folate metabolism. Dis. Markers 2012, 32, 73–81. [Google Scholar] [CrossRef]
- Pandey, S.K.; Mohanty, P.K.; Polipalli, S.K.; Polipalli, S.K.; Kapoor, S. Genetic polymorphisms of MTHFR (677T and 1298C) and homocysteine metabolism as maternal risk factor for Down’s syndrome patients in north indian population. Int. J. Pharma Bio Sci. 2013, 4, 249–256. [Google Scholar]
- Tayeb, M.T. The methylenetetrahydrofolate reductase gene variant (C677T) in risk mothers with Down syndrome among Saudi population. Egypt. J. Med. Hum. Genet. 2012, 13, 263–268. [Google Scholar] [CrossRef] [Green Version]
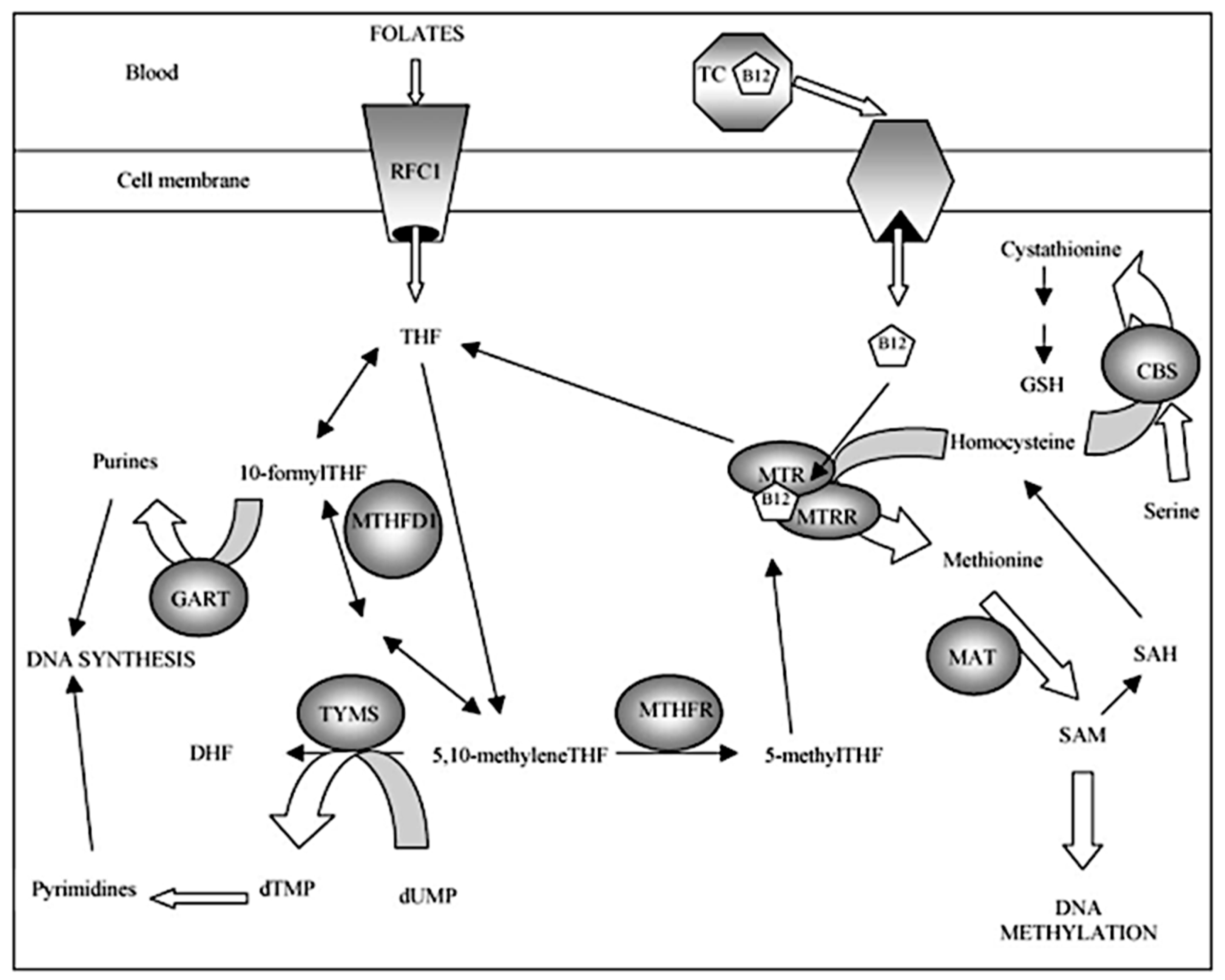
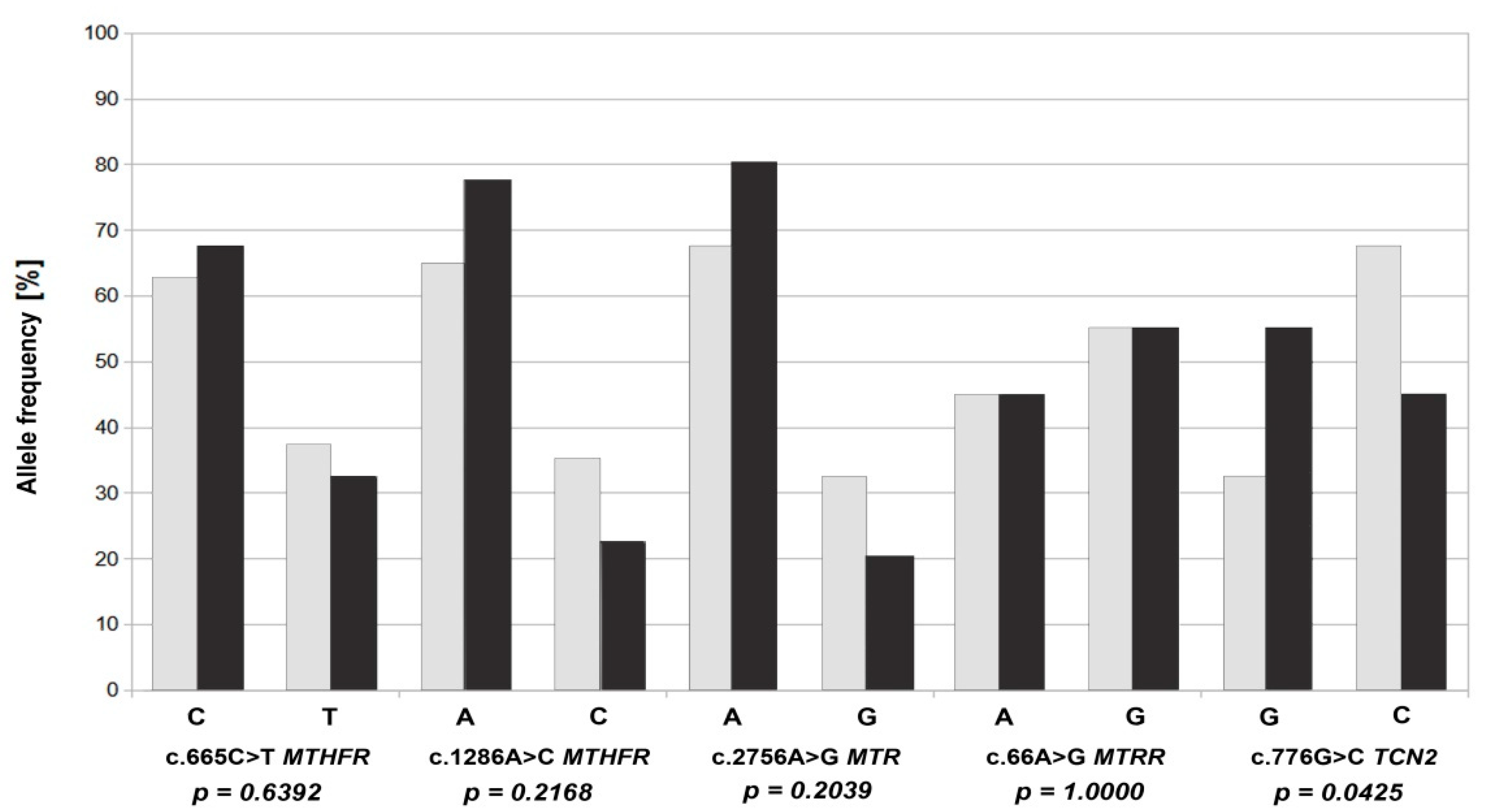
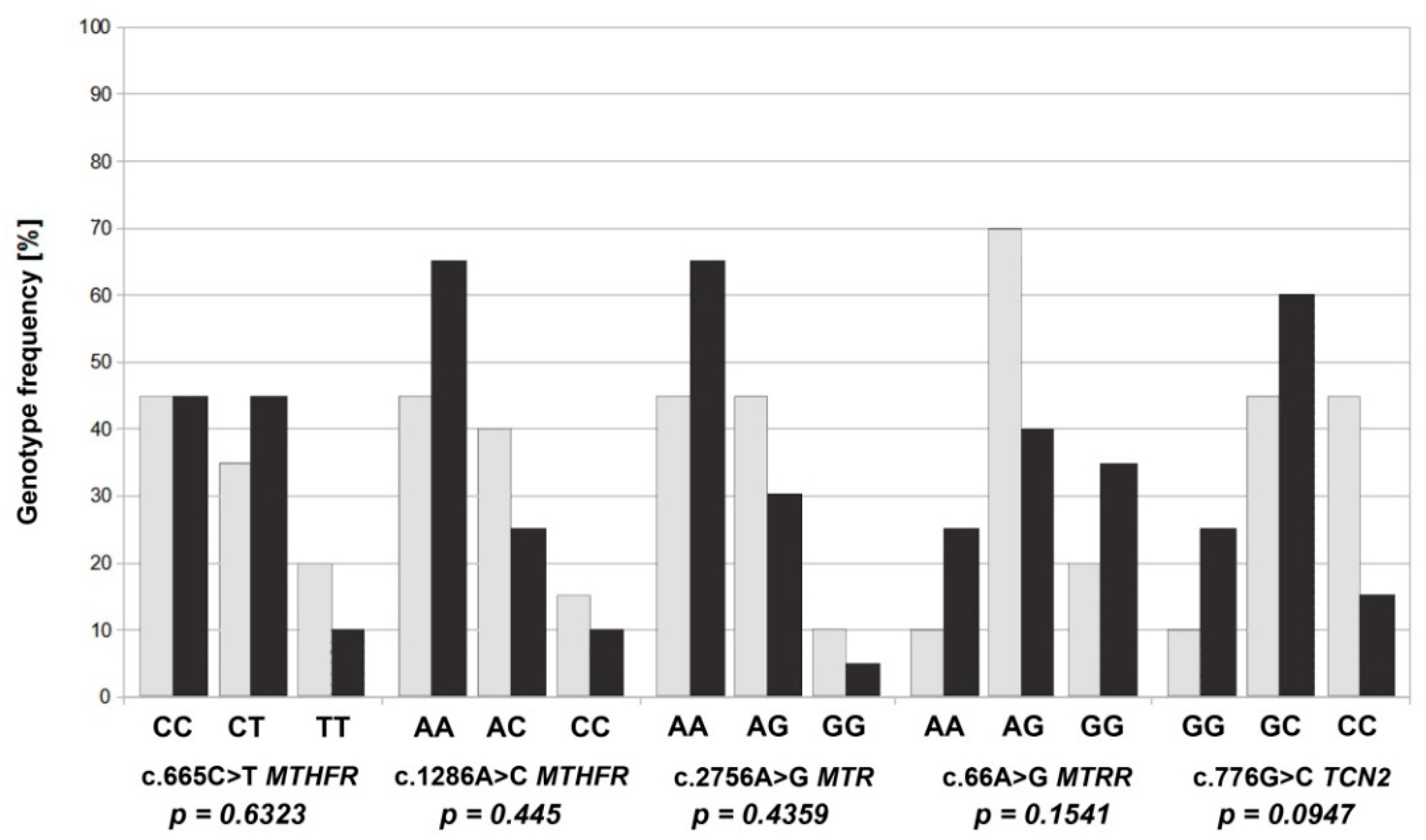
| Groups | Allele | N | p-Value |
|---|---|---|---|
| c.665C>T MTHFR (rs1801131) | |||
| Study | C | 27 | 0.639 |
| Study | T | 13 | |
| Control | C | 25 | |
| Control | T | 15 | |
| c.1286A>C MTHFR (rs1801133) | |||
| Study | A | 31 | 0.217 |
| Study | C | 9 | |
| Control | A | 26 | |
| Control | C | 14 | |
| c.2756A>G MTR (rs1805087) | |||
| Study | A | 32 | 0.204 |
| Study | G | 8 | |
| Control | A | 27 | |
| Control | G | 13 | |
| c.66A>G MTRR (rs1801394) | |||
| Study | A | 18 | 1.000 |
| Study | G | 22 | |
| Control | A | 18 | |
| Control | G | 22 | |
| c.776G>C TCN2 (rs1801198) | |||
| Study | G | 29 | 0.042 |
| Study | C | 11 | |
| Control | G | 13 | |
| Control | C | 27 | |
| Parameters | Control Group | Study Group | p-Value |
|---|---|---|---|
| Patient’s age (years) | 38 ± 4 | 36 ± 6 | 0.3062 |
| BMI (kg/m2) | 24.4 ± 4.6 | 23.8 ± 3.5 | 0.6263 |
| HCY (µmol/L) | 6.176 ± 1.378 | 7.137 ± 2.360 | 0.126 |
| Vit. B12 (pg/mL) | 57.434 ± 15.632 | 49.203 ± 14.335 | 0.0907 |
| Folate SER (ng/mL) | 15.075 (6.3–26.9) | 12.725 (5.49–62.6) | 0.0818 |
| Folate RBC (ng/mL) | 319.545 ± 46.518 | 358.31 ± 86.228 | >0.05 |
| MMA (ng/mL) | 6.013 (0.56–34.98) | 1.105 (0.188–80.113) | <0.0001 |
| PAPP-A MoM | 1.14 (0.41–1.98) | 0.336 (1.32–1.857) | 0.019 |
| free β-hCG MoM | 1.1 (0.46–2.51) | 2.64 (0.59–9.76) | 0.0029 |
| Test Parameters | Genetic Variants of the Folate Pathway | p-Value | |
|---|---|---|---|
| Reference Homozygote | Hetero- and Homozygous Alternative | ||
| 665C>T MTHFR | |||
| CC (n = 18) | CT + TT (n = 22) | ||
| Age (years) | 35 ± 6 | 39 ± 3 | 0.0103 |
| BMI (kg/m2) | 24.1908 ± 3.8361 | 24.0482 ± 4.2881 | 0.9133 |
| HCY (µmol/L) | 6.6311 ± 2.1191 | 6.6782 ± 1.8876 | 0.9412 |
| Vit. B12 (pg/mL) | 51.5600 ± 13.6861 | 54.7586 ± 16.8176 | 0.5199 |
| Folate SER (ng/mL) | 18.3706 ± 12.9428 | 12.9659 ± 5.0261 | 0.0792 |
| Folate RBC (ng/mL) | 355.3 ± 93.1147 | 325.5318 ± 41.9387 | 0.1867 |
| MMA (ng/mL) | 2.9700 (0.188–34.98) | 2.5415 (0.54–80.113) | 0.7442 |
| PAPPA MoM | 0.7481 ± 0.5053 | 0.9019 ± 0.5985 | 0.3919 |
| free β-hCG MoM | 2.5843 ± 2.8672 | 2.1161 ± 1.7018 | 0.5252 |
| 1286A>C MTHFR | |||
| AA (n = 22) | AC + CC (n = 18) | ||
| Age (years) | 38 ± 6 | 36 ± 5 | 0.4193 |
| BMI (kg/m2) | 24.4863 ± 24.4863 | 23.6553 ± 3.9584 | 0.5245 |
| HCY (µmol/L) | 6.7941 ± 2.1459 | 6.4894 ± 1.7751 | 0.6326 |
| Vit. B12 (pg/mL) | 50.5186 ± 15.1741 | 56.7422 ± 15.3513 | 0.2070 |
| Folate SER (ng/mL) | 12.41 (5.49–62.6) | 15.3450 (7.77–26.9) | 0.0240 |
| Folate RBC (ng/mL) | 335.3318 ± 51.3728 | 343.3222 ± 89.7874 | 0.7258 |
| MMA (ng/mL) | 1.8965 (0.401–80.113) | 6.8645 (0.188–34.98) | 0.3994 |
| PAPPA MoM | 0.9259 ± 0.6447 | 0.7189 ± 0.4163 | 0.2474 |
| free β-hCG MoM | 2.1468 ± 1.7383 | 2.5468 ± 2.8461 | 0.5875 |
| c.2756A>G MTR | |||
| AA (n = 22) | AG + GG (n = 18) | ||
| Age (years) | 36 ± 6 | 39 ± 4 | 0.0478 |
| BMI (kg/m2) | 23.9457 ± 3.9884 | 24.3160 ± 4.2084 | 0.7772 |
| HCY (µmol/L) | 6.2777 ± 1.9589 | 7.1206 ± 1.9338 | 0.1814 |
| Vit. B12 (pg/mL) | 51.7832 ± 16.2295 | 55.1967 ± 14.5083 | 0.4921 |
| Folate SER (ng/mL) | 16.0636 ± 12.1031 | 14.5844 ± 5.7885 | 0.6374 |
| Folate RBC (ng/mL) | 344.6273 ± 81.3418 | 331.9611 ± 5.6091 | 0.5778 |
| MMA (ng/mL) | 6.1350 (0.188–80.113) | 1.0695 (0.401–60.816) | 0.1656 |
| PAPPA MoM | 0.857 ± 0.591 | 0.8031 ± 0.5278 | 0.7652 |
| free β-hCG MoM | 2.6648 ± 2.583 | 1.9137 ± 1.8318 | 0.3060 |
| c.66A>G MTRR | |||
| AA (n = 7) | AG + GG (n = 33) | ||
| Age (years) | 35 ± 6 | 37 ± 5 | 0.3695 |
| BMI (kg/m2) | 22.676 ± 2.6449 | 24.4170 ± 4.2464 | 0.3065 |
| HCY (µmol/L) | 6.5586 ± 1.9924 | 6.6779 ± 1.9944 | 0.8864 |
| Vit. B12 (pg/mL) | 50.4229 ± 18.5376 | 53.9336 ± 14.8871 | 0.5899 |
| Folate SER (ng/mL) | 13.68 (7.32–26.76) | 14.00 (5.49–62.6) | 0.9291 |
| Folate RBC (ng/mL) | 373.5857 ± 113.02 | 331.5758 ± 57.6266 | 0.1538 |
| MMA (ng/mL) | 6.56 (0.54–34.93) | 2.72 (0.188–80.113) | 0.7086 |
| PAPPA MoM | 0.5631 ± 0.4976 | 0.8899 ± 0.5589 | 0.1613 |
| free β-hCG MoM | 3.0783 ± 3.1022 | 2.1674 ± 2.0932 | 0.3436 |
| c.776 C>G TCN2 | |||
| CC (n = 12) | CG + GG (n = 28) | ||
| Age (years) | 39 ± 4 | 36 ± 5 | 0.0789 |
| BMI (kg/m2) | 23.6184 ± 4.3182 | 24.3241 ± 3.9778 | 0.6190 |
| HCY (µmol/L) | 6.6708 ± 2.0831 | 6.6511 ± 1.9574 | 0.9772 |
| Vit. B12 (pg/mL) | 54.2075 ± 10.8508 | 52.9386 ± 17.1231 | 0.8145 |
| Folate SER (ng/mL) | 12.465 (7.57–26.9) | 13.84 (5.49–62.6) | 0.9294 |
| Folate RBC (ng/mL) | 337.2167 ± 53.5840 | 339.6607 ± 77.3331 | 0.9214 |
| MMA (ng/mL) | 2.0350 (0.188–80.113) | 4.0950 (0.401–60.816) | 0.5354 |
| PAPPA MoM | 1.0306 ± 0.6020 | 0.7479 ± 0.52949 | 0.1434 |
| free β-hCG MoM | 1.4342 ± 0.8299 | 2.7593 ± 2.5928 | 0.1058 |
| Parameters of Control and Study Groups | c.1286A>C MTHFR | c.665C>T MTHFR | c.2756A>G MTR | c.66A>G MTRR | c.776G>C TCN2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Allele C (n = 23) | Allele T (n = 28) | Allele G (n = 21) | Allele G (n = 44) | Allele C (n = 38) | ||||||
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| HCY (µmol/L) | −0.2074 | 0.6933 | −0.1083 | 0.8407 | 0.6447 | 0.2423 | −0.01039 | 0.9826 | 0.1744 | 0.7329 |
| Vit. B12 (pg/mL) | 4.4357 | 0.2784 | −1.0703 | 0.7979 | 3.1238 | 0.4639 | 1.4485 | 0.6948 | −0.4202 | 0.9155 |
| Folate RBC (ng/mL) | −5.7469 | 0.7563 | −33.782 | 0.0782 | −8.1303 | 0.6743 | 2.1095 | 0.8998 | 21.6294 | 0.2317 |
| Folate SER (ng/mL) | 0.9647 | 0.7065 | −4.1179 | 0.1198 | 0.2742 | 0.9183 | −0.6883 | 0.7665 | 1.243 | 0.6179 |
| MMA (ng/mL) | −2.2179 | 0.6327 | −0.4493 | 0.9247 | −0.08866 | 0.9854 | 0.8206 | 0.845 | 2.6185 | 0.5617 |
| PAPP-A MoM | −0.1469 | 0.3299 | 0.06644 | 0.6627 | 0.005361 | 0.9724 | 0.0173 | 0.8988 | 0.1469 | 0.3148 |
| free β-hCG MoM | 0.0785 | 0.8982 | −0.04186 | 0.9463 | −0.5617 | 0.3762 | 0.3226 | 0.5619 | −0.9328 | 0.12 |
| Parameters of the Study Group | c.1286A>C MTHFR | c.665C>T MTHFR | c.2756A>G MTR | c.66A>G MTRR | c.776G>C TCN2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| AC + CC (n = 7) | CT + TT (n = 11) | AG + GG (n = 7) | AG + GG (n = 15) | GC + CC (n = 15) | ||||||
| Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | Coefficient | p-Value | |
| free β-hCG MoM | 2.1143 | 0.2272 | −0.2669 | 0.8763 | −0.9782 | 0.5254 | −1.113 | 0.5501 | −1.1337 | 0.5389 |
| PAPP-A MoM | −0.08848 | 0.7391 | 0.2388 | 0.3788 | −0.2188 | 0.3663 | 0.1623 | 0.5758 | −0.2902 | 0.3191 |
| MMA (ng/mL) | −1.1728 | 0.929 | 12.1823 | 0.3596 | −6.9839 | 0.5542 | 13.0815 | 0.3485 | 11.9 | 0.4062 |
| Folate RBC (ng/mL) | −35.5647 | 0.3331 | −104.3238 | 0.0105 | −20.4323 | 0.5289 | −42.7235 | 0.2679 | 128.4654 | 0.0046 |
| Folate SER (ng/mL) | −4.4338 | 0.5391 | −11.5471 | 0.1217 | −3.7273 | 0.5623 | 1.5842 | 0.8324 | 8.9313 | 0.2579 |
| HCY (µmol/L) | 0.06287 | 0.9637 | −1.0299 | 0.4589 | 1.3878 | 0.2712 | 0.8271 | 0.5689 | 2.213 | 0.152 |
| Vit. B12 (pg/mL) | 0.01619 | 0.9984 | 11.2406 | 0.1781 | 9.1704 | 0.2176 | 1.8014 | 0.8316 | −6.6602 | 0.4497 |
| Patient with T21 in the Fetus | c.665C>T MTHFR | c.1286A>C MTHFR | c.2756A>G MTR | c.66A>G MTRR | c.776G>C TCN2 | Number of Alternate Genotypic Variants | PAPP-A MoM | β-hCG MoM | Age | BMI |
|---|---|---|---|---|---|---|---|---|---|---|
| No. 1 | CC | CC | AG | AG | GG | 3 | 0.31 | 3.74 | 37 | 23 |
| No. 2 | CT | AC | AG | AG | GC | 5 | 0.28 | 8.37 | 36 | 20 |
| No. 3 | TT | AA | AG | AA | GC | 3 | 0.42 | 3.25 | 36 | 23 |
| No. 4 | CT | AA | AA | AA | GC | 2 | 0.13 | 3.2 | 36 | 25 |
| No. 5 | CT | AA | AA | GG | GC | 3 | 0.3 | 3.63 | 39 | 27 |
| No. 6 | CC | AC | AA | AA | GC | 2 | 0.34 | 9.76 | 32 | 18 |
| Group | Number of Alternate Genotypes. Median (Min–Max.) | p-Value |
|---|---|---|
| Study | 3 (1–5) | 0.0215 |
| Control | 4 (2–4) |
| Parameters | Number of Alternate Genotypes | |
|---|---|---|
| Coefficient | p-Value | |
| HCY (µmol/L) | 0.3119 | 0.3513 |
| Vit. B12 (pg/mL) | 4.2411 | 0.1005 |
| Folate SER (ng/mL) | −11.843 | 0.3216 |
| Folate RBC (ng/mL) | −0.9576 | 0.5625 |
| MMA (ng/mL) | 1.0863 | 0.7121 |
| PAPPA MoM | 0.03181 | 0.7437 |
| free β-hCG MoM | −0.6527 | 0.0976 |
| Variants of the Studied Genes | The Arrangement of Alleles and Genotypes in the Analyzed Variants | OR | (95% CI) | p-Value |
|---|---|---|---|---|
| c.665C>T MTHFR | T vs. C | OR = 0.7143 | 95% CI = 0.2815−1.8125 | p = 0.4788 |
| TT vs. CC | OR = 0.5625 | 95% CI = 0.0803−3.9392 | p = 0.5623 | |
| CT vs. CC | OR = 1.4464 | 95% CI = 0.3668−5.7042 | p = 0.598 | |
| TT + CT vs. CC | OR = 1.5195 | 95% CI = 0.4255−5.4266 | p = 0.519 | |
| TT vs. CT + CC | OR = 0.4444 | 95% CI = 0.0716−2.7599 | p = 0.3841 | |
| c.1286A>C MTHFR | C vs. A. | OR = 0.5392 | 95% CI = 0.2011−1.4458 | p = 0.2196 |
| CC vs. AA | OR = 0.4615 | 95% CI = 0.0637−3.3456 | p = 0.4442 | |
| AC vs. AA | OR = 0.4327 | 95% CI = 0.1063−1.7615 | p = 0.2422 | |
| CC + AC vs. AA | OR = 0.4406 | 95% CI = 0.1234−1.5734 | p = 0.2069 | |
| CC vs. AC + AA | OR = 0.6296 | 95% CI = 0.0934−4.2437 | p = 0.6346 | |
| c.2756A>G MTR | G vs. A | OR = 0.5192 | 95% CI = 0.1874−1.4383 | p = 0.2074 |
| GG vs. AA | OR = 0.3462 | 95% CI = 0.0271−4.4178 | p = 0.4142 | |
| AG vs. AA | OR = 0.4615 | 95% CI = 0.1211−1.7586 | p = 0.2573 | |
| CC + AC vs. AA | OR = 0.4406 | 95% CI = 0.1234−1.5734 | p = 0.2069 | |
| CC vs. AC + AA | OR = 0.4737 | 95% CI = 0.0394−5.6879 | p = 0.5557 | |
| c.66A>G MTRR | G vs. A. | OR = 1.000 | 95% CI = 0.4144−2.4132 | p = 1.000 |
| GG vs. AG | OR = 4.375 | 95% CI = 0.8817−21.7077 | p = 0.0709 | |
| GG vs. AA | OR = 0.700 | 95% CI = 0.0902−5.4320 | p = 0.7330 | |
| AG vs. AA | OR = 0.2286 | 95% CI = 0.0357−1.4620 | p = 0.119 | |
| GG + AG vs. AA | OR = 0.3333 | 95% CI = 0.0564−1.9712. | p = 0.2257 | |
| GG vs. AG + AA | OR = 2.1538 | 95% CI = 0.5155−9.0000 | p = 0.293 | |
| c.776G>C TCN2 | C vs. G. | OR = 0.3939 | 95% CI = 0.1588−0.9775 | p = 0.0445 |
| CC vs. GC | OR = 0.25 | 95% CI = 0.522−1.1976 | p = 0.0829 | |
| CC vs. GG | OR = 0.1333 | 95% CI = 0.0164−1.0853 | p = 0.0596 | |
| GC vs. GG | OR = 0.5333 | 95%CI = 0.0836−3.4044 | p = 0.5063 | |
| CC + GC vs. GG | OR = 0.3333 | 95% CI = 0.0564−1.9712 | p = 0.2257 | |
| CC vs. GC + GG | OR = 0.2157 | 95% CI = 0.0476−0.9772 | p = 0.0466 | |
| c.665C>T MTHFR + c.66A>G MTRR | CT + GG | OR = 3.3529 | 95% CI = 0.3179−35.3658 | p = 0.3142 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ziółkowska, K.; Toboła-Wróbel, K.; Pietryga, M.; Kasprzak, G.; Jamsheer, A.; Wysocka, E. An Assessment of Selected Molecular and Biochemical Markers of the Folate Pathway as Potential Risk Factors for Fetal Trisomy 21 during the First Trimester of Pregnancy in the Polish Population. J. Clin. Med. 2022, 11, 1190. https://doi.org/10.3390/jcm11051190
Ziółkowska K, Toboła-Wróbel K, Pietryga M, Kasprzak G, Jamsheer A, Wysocka E. An Assessment of Selected Molecular and Biochemical Markers of the Folate Pathway as Potential Risk Factors for Fetal Trisomy 21 during the First Trimester of Pregnancy in the Polish Population. Journal of Clinical Medicine. 2022; 11(5):1190. https://doi.org/10.3390/jcm11051190
Chicago/Turabian StyleZiółkowska, Katarzyna, Kinga Toboła-Wróbel, Marek Pietryga, Grażyna Kasprzak, Aleksander Jamsheer, and Ewa Wysocka. 2022. "An Assessment of Selected Molecular and Biochemical Markers of the Folate Pathway as Potential Risk Factors for Fetal Trisomy 21 during the First Trimester of Pregnancy in the Polish Population" Journal of Clinical Medicine 11, no. 5: 1190. https://doi.org/10.3390/jcm11051190






