Adrenal Gland Irradiation Causes Fatigue Accompanied by Reactive Changes in Cortisol Levels
Abstract
:1. Introduction
2. Materials and Methods
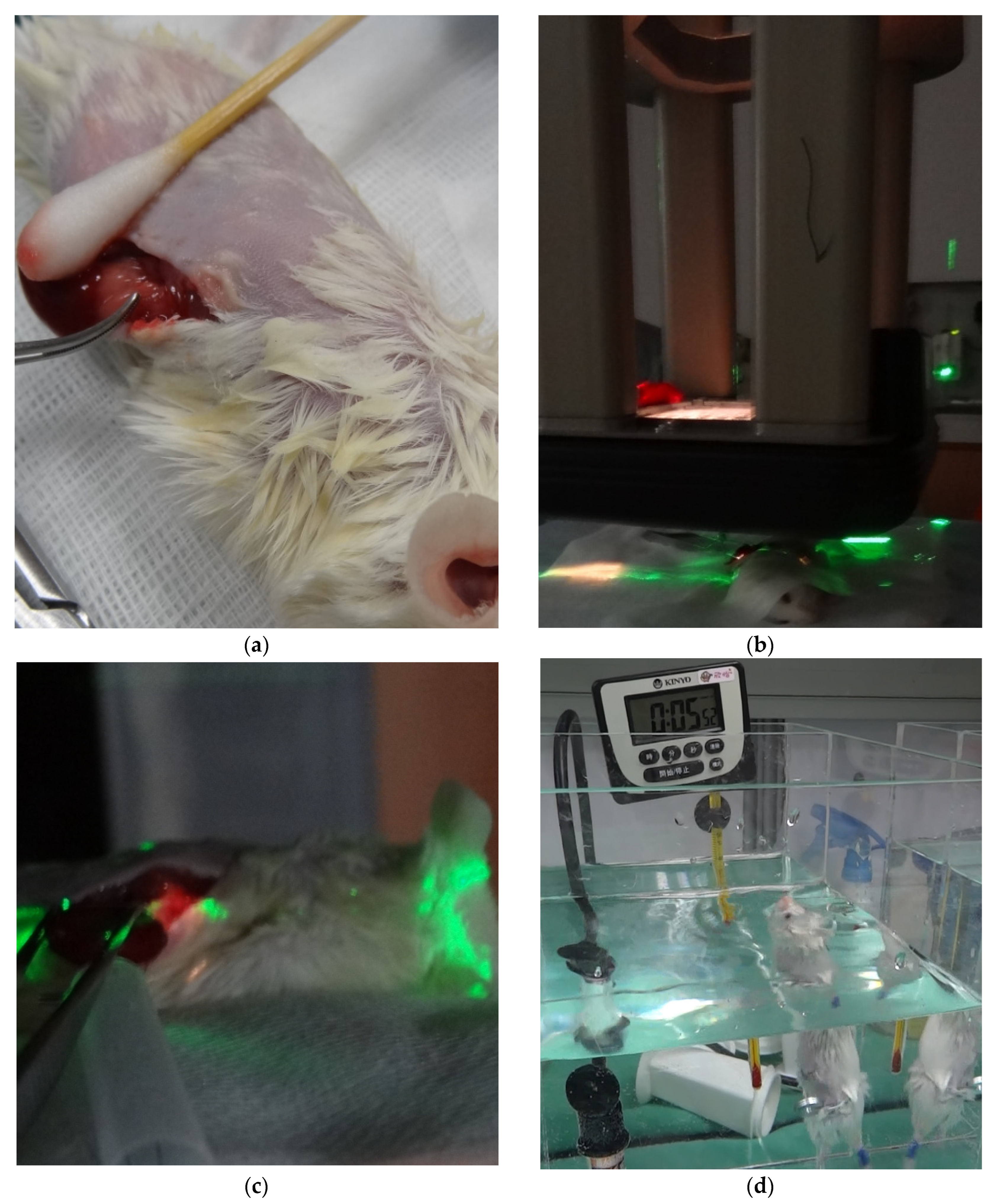
2.1. Experimental Animal Model
2.1.1. Experimental Animals
2.1.2. Blood Analysis
2.1.3. RT Technique
2.1.4. Swimming Endurance Test
2.1.5. Percentage Change (Δ)
2.1.6. Histopathology
2.2. Clinical Observation—Preliminary Data
2.2.1. Patients
2.2.2. Blood Analysis
2.2.3. RT Technique
2.2.4. Fatigue Severity Scale
2.2.5. Ethical Statement
2.3. Statistical Analysis
3. Results
3.1. Experimental Animal Model
3.1.1. Blood Analysis
3.1.2. Swimming Endurance Time
3.1.3. Histopathology
3.2. Clinical Observation—Preliminary Data
3.2.1. Patient Characteristics
3.2.2. RT Dosimetry
3.2.3. Blood Analysis
3.2.4. Fatigue Severity Scale
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Berger, A.M.; Mooney, K.; Alvarez-Perez, A.; Breitbart, W.S.; Carpenter, K.M.; Cella, D.; Cleeland, C.; Dotan, E.; Eisenberger, M.A.; Escalante, C.P. Cancer-related fatigue, version 2.2015. J. Natl. Compr. Cancer Netw. 2015, 13, 1012–1039. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Mitchell, S.A.; Jacobsen, P.B.; Pirl, W.F. Screening, evaluation, and management of cancer-related fatigue: Ready for implementation to practice? CA Cancer J. Clin. 2015, 65, 190–211. [Google Scholar] [CrossRef] [PubMed]
- Bower, J.E.; Bak, K.; Berger, A.; Breitbart, W.; Escalante, C.P.; Ganz, P.A.; Schnipper, H.H.; Lacchetti, C.; Ligibel, J.A.; Lyman, G.H.; et al. Screening, assessment, and management of fatigue in adult survivors of cancer: An American Society of Clinical oncology clinical practice guideline adaptation. J. Clin. Oncol. 2014, 32, 1840–1850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goedendorp, M.M.; Gielissen, M.F.; Verhagen, C.A.; Peters, M.E.; Bleijenberg, G. Severe fatigue and related factors in cancer patients before the initiation of treatment. Br. J. Cancer 2008, 99, 1408–1414. [Google Scholar] [CrossRef] [Green Version]
- Servaes, P.; Verhagen, C.; Bleijenberg, G. Fatigue in cancer patients during and after treatment: Prevalence, correlates and interventions. Eur. J. Cancer 2002, 38, 27–43. [Google Scholar] [CrossRef]
- Prue, G.; Rankin, J.; Allen, J.; Gracey, J.; Cramp, F. Cancer-related fatigue: A critical appraisal. Eur. J. Cancer 2006, 42, 846–863. [Google Scholar] [CrossRef]
- Jones, J.M.; Olson, K.; Catton, P.; Catton, C.N.; Fleshner, N.E.; Krzyzanowska, M.K.; McCready, D.R.; Wong, R.K.; Jiang, H.; Howell, D. Cancer-related fatigue and associated disability in post-treatment cancer survivors. J. Cancer Surviv. 2016, 10, 51–61. [Google Scholar] [CrossRef]
- Lotfi, C.F.P.; Kremer, J.L.; Dos Santos Passaia, B.; Cavalcante, I.P. The human adrenal cortex: Growth control and disorders. Clinics 2018, 73, e473s. [Google Scholar] [CrossRef]
- Xing, Y.; Lerario, A.M.; Rainey, W.; Hammer, G.D. Development of adrenal cortex zonation. Endocrinol. Metab. Clin. N. Am. 2015, 44, 243–274. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.; Greco, S.; Nguyen, H.H.; Ho, J.T.; Lewis, J.G.; Torpy, D.J.; Inder, W.J. Plasma, salivary and urinary cortisol levels following physiological and stress doses of hydrocortisone in normal volunteers. BMC Endocr. Disord. 2014, 14, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho, A.E.; Chapman, K.E. The anti-inflammatory and immunosuppressive effects of glucocorticoids, recent developments and mechanistic insights. Mol. Cell. Endocrinol. 2011, 335, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.; McQueen, A.; Chen, T.C.; Wang, J.C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv. Exp. Med. Biol. 2015, 872, 99–126. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.J.; Djurhuus, C.B.; Gravholt, C.H.; Iversen, P.; Christiansen, J.S.; Schmitz, O.; Weeke, J.; Jorgensen, J.O.; Moller, N. Effects of cortisol on carbohydrate, lipid, and protein metabolism: Studies of acute cortisol withdrawal in adrenocortical failure. J. Clin. Endocrinol. Metab. 2007, 92, 3553–3559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hannibal, K.E.; Bishop, M.D. Chronic stress, cortisol dysfunction, and pain: A psychoneuroendocrine rationale for stress management in pain rehabilitation. Phys. Ther. 2014, 94, 1816–1825. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Kim, E.; Choi, M.H. Technical and clinical aspects of cortisol as a biochemical marker of chronic stress. BMB Rep. 2015, 48, 209–216. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, S.; Miao, Y.L.; Jiao, G.Z.; Sun, M.J.; Li, H.; Lin, J.; Luo, M.J.; Tan, J.H. Dynamics and correlation of serum cortisol and corticosterone under different physiological or stressful conditions in mice. PLoS ONE 2015, 10, e0117503. [Google Scholar] [CrossRef] [PubMed]
- Thurston, J.H.; Hauhart, R.E. Effect of momentary stress on brain energy metabolism in weanling mice: Apparent use of lactate as cerebral metabolic fuel concomitant with a decrease in brain glucose utilization. Metab. Brain Dis. 1989, 4, 177–186. [Google Scholar] [CrossRef]
- Nakamura, K.; Aoike, A.; Hosokawa, T.; Rokutan, K.; Koyama, K.; Nishi, Y.; Yoshida, A.; Kawai, K. Effect of food-restriction stress on immune response in mice. J. Neuroimmunol. 1990, 30, 23–29. [Google Scholar] [CrossRef]
- Won, S.J.; Lin, M.T. Thermal stresses reduce natural killer cell cytotoxicity. J. Appl. Physiol. 1995, 79, 732–737. [Google Scholar] [CrossRef]
- Kinoshita, M.; Nakashima, H.; Nakashima, M.; Koga, M.; Toda, H.; Koiwai, K.; Morimoto, Y.; Miyazaki, H.; Saitoh, D.; Suzuki, H.; et al. The reduced bactericidal activity of neutrophils as an incisive indicator of water-immersion restraint stress and impaired exercise performance in mice. Sci. Rep. 2019, 9, 4562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayada, K.; Tadano, T.; Endo, Y. Gnawing behavior of a mouse in a narrow cylinder: A simple system for the study of muscle activity, fatigue, and stress. Physiol. Behav. 2002, 77, 161–166. [Google Scholar] [CrossRef]
- Chen, Y.; Kong, L.D.; Xia, X.; Kung, H.F.; Zhang, L. Behavioral and biochemical studies of total furocoumarins from seeds of Psoralea corylifolia in the forced swimming test in mice. J. Ethnopharmacol. 2005, 96, 451–459. [Google Scholar] [CrossRef]
- Podsevatkin, V.G.; Kiriukhina, S.V.; Podsevatkin, D.V.; Podsevatkina, S.V.; Blinov, D.S. Dynamics of the behavioral response and cortisole level caused by the combined action of mexidole, diazepam, thymogen, and hyperbaric oxygenation in mice under immobilization stress conditions. Eksp. Klin. Farmakol. 2008, 71, 22–25. [Google Scholar] [PubMed]
- Zhang, S.Y.; Wang, J.Z.; Li, J.J.; Wei, D.L.; Sui, H.S.; Zhang, Z.H.; Zhou, P.; Tan, J.H. Maternal restraint stress diminishes the developmental potential of oocytes. Biol. Reprod. 2011, 84, 672–681. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, W.L. The Hypothalamic-Pituitary-Adrenal Axis: A Brief History. Horm. Res. Paediatr. 2018, 89, 212–223. [Google Scholar] [CrossRef]
- Boggero, I.A.; Hostinar, C.E.; Haak, E.A.; Murphy, M.L.M.; Segerstrom, S.C. Psychosocial functioning and the cortisol awakening response: Meta-analysis, P-curve analysis, and evaluation of the evidential value in existing studies. Biol. Psychol. 2017, 129, 207–230. [Google Scholar] [CrossRef] [Green Version]
- Oster, H.; Challet, E.; Ott, V.; Arvat, E.; de Kloet, E.R.; Dijk, D.J.; Lightman, S.; Vgontzas, A.; Van Cauter, E. The Functional and Clinical Significance of the 24-Hour Rhythm of Circulating Glucocorticoids. Endocr. Rev. 2017, 38, 3–45. [Google Scholar] [CrossRef]
- Matsumoto, K.; Ishihara, K.; Tanaka, K.; Inoue, K.; Fushiki, T. An adjustable-current swimming pool for the evaluation of endurance capacity of mice. J. Appl. Physiol. 1996, 81, 1843–1849. [Google Scholar] [CrossRef] [Green Version]
- Hjollund, N.H.; Andersen, J.H.; Bech, P. Assessment of fatigue in chronic disease: A bibliographic study of fatigue measurement scales. Health Qual. Life Outcomes 2007, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Valko, P.O.; Bassetti, C.L.; Bloch, K.E.; Held, U.; Baumann, C.R. Validation of the fatigue severity scale in a Swiss cohort. Sleep 2008, 31, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- Casamassima, F.; Livi, L.; Masciullo, S.; Menichelli, C.; Masi, L.; Meattini, I.; Bonucci, I.; Agresti, B.; Simontacchi, G.; Doro, R. Stereotactic radiotherapy for adrenal gland metastases: University of Florence experience. Int. J. Radiat. Oncol. Biol. Phys. 2012, 82, 919–923. [Google Scholar] [CrossRef] [PubMed]
- Rudra, S.; Malik, R.; Ranck, M.C.; Farrey, K.; Golden, D.W.; Hasselle, M.D.; Weichselbaum, R.R.; Salama, J.K. Stereotactic body radiation therapy for curative treatment of adrenal metastases. Technol. Cancer Res. Treat. 2013, 12, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wardak, Z.; Meyer, J.; Ghayee, H.; Wilfong, L.; Timmerman, R. Adrenal insufficiency after stereotactic body radiation therapy for bilateral adrenal metastases. Pract. Radiat. Oncol. 2015, 5, e177–e181. [Google Scholar] [CrossRef] [PubMed]
- Chance, W.W.; Nguyen, Q.N.; Mehran, R.; Welsh, J.W.; Gomez, D.R.; Balter, P.; Komaki, R.; Liao, Z.; Chang, J.Y. Stereotactic ablative radiotherapy for adrenal gland metastases: Factors influencing outcomes, patterns of failure, and dosimetric thresholds for toxicity. Pract. Radiat. Oncol. 2017, 7, e195–e203. [Google Scholar] [CrossRef]
- Yuan, B.Y.; Hu, Y.; Zhang, L.; Chen, Y.H.; Dong, Y.Y.; Zeng, Z.C. Radiotherapy for adrenal gland metastases from hepatocellular carcinoma. Clin. Transl. Oncol. 2017, 19, 1154–1160. [Google Scholar] [CrossRef]
- Mohnike, K.; Neumann, K.; Hass, P.; Seidensticker, M.; Seidensticker, R.; Pech, M.; Klose, S.; Streitparth, T.; Garlipp, B.; Benckert, C.; et al. Radioablation of adrenal gland malignomas with interstitial high-dose-rate brachytherapy: Efficacy and outcome. Strahlenther Onkol. 2017, 193, 612–619. [Google Scholar] [CrossRef]
- Konig, L.; Hafner, M.F.; Katayama, S.; Koerber, S.A.; Tonndorf-Martini, E.; Bernhardt, D.; von Nettelbladt, B.; Weykamp, F.; Hoegen, P.; Kluter, S.; et al. Stereotactic body radiotherapy (SBRT) for adrenal metastases of oligometastatic or oligoprogressive tumor patients. Radiat. Oncol. 2020, 15, 30. [Google Scholar] [CrossRef] [Green Version]
- Buergy, D.; Würschmidt, F.; Gkika, E.; Hörner-Rieber, J.; Knippen, S.; Gerum, S.; Balermpas, P.; Henkenberens, C.; Voglhuber, T.; Kornhuber, C. Stereotactic or conformal radiotherapy for adrenal metastases: Patient characteristics and outcomes in a multicenter analysis. Int. J. Cancer 2021, 149, 358–370. [Google Scholar] [CrossRef]
- Burjakow, K.; Fietkau, R.; Putz, F.; Achterberg, N.; Lettmaier, S.; Knippen, S. Fractionated stereotactic radiation therapy for adrenal metastases: Contributing to local tumor control with low toxicity. Strahlenther Onkol. 2019, 195, 236–245. [Google Scholar] [CrossRef]
- Scouarnec, C.; Pasquier, D.; Luu, J.; le Tinier, F.; Lebellec, L.; Rault, E.; Lartigau, E.; Mirabel, X. Usefulness of Stereotactic Body Radiation Therapy for Treatment of Adrenal Gland Metastases. Front. Oncol. 2019, 9, 732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Zhu, X.; Fei, J.; Ren, H.; Cao, Y.; Ju, X.; Yuan, Z.; Zhang, H. Short-term outcomes and clinical efficacy of stereotactic body radiation therapy (SBRT) in treatment of adrenal gland metastases from lung cancer. Radiat. Oncol. 2018, 13, 205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, T.L.; Hung, A.Y.; Thomas, C.R.; Wood, L.J. Localized external beam radiation therapy (EBRT) to the pelvis induces systemic IL-1Beta and TNF-alpha production: Role of the TNF-alpha signaling in EBRT-induced fatigue. Radiat. Res. 2016, 185, 4–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villar, S.R.; Ronco, M.T.; Fernández Bussy, R.; Roggero, E.; Lepletier, A.; Manarin, R.; Savino, W.; Perez, A.R.; Bottasso, O. Tumor necrosis factor-α regulates glucocorticoid synthesis in the adrenal glands of Trypanosoma cruzi acutely-infected mice. The role of TNF-R1. PLoS ONE 2013, 8, e63814. [Google Scholar] [CrossRef] [Green Version]
- Judd, A.M.; Call, G.B.; Barney, M.; McILMOIL, C.J.; Balls, A.G.; Adams, A.; Oliveira, G.K. Possible function of IL-6 and TNF as intraadrenal factors in the regulation of adrenal steroid secretion. Ann. N. Y. Acad. Sci. 2000, 917, 628–637. [Google Scholar] [CrossRef]
- Jäättelä, M.; Ilvesmäki, V.; Voutilainen, R.; Stenman, U.-H.; Saksela, E. Tumor necrosis factor as a potent inhibitor of adrenocorticotropin-induced cortisol production and steroidogenic P450 enzyme gene expression in cultured human fetal adrenal cells. Endocrinology 1991, 128, 623–629. [Google Scholar] [CrossRef]
- Van der Meer, M.J.; Hermus, A.R.; Pesman, G.J.; Sweep, C.F. Effects of cytokines on pituitary β-endorphin and adrenal corticosterone release in vitro. Cytokine 1996, 8, 238–247. [Google Scholar] [CrossRef]
- Mitchell, J.; Barbosa, G.; Tsinberg, M.; Milas, M.; Siperstein, A.; Berber, E. Unrecognized adrenal insufficiency in patients undergoing laparoscopic adrenalectomy. Surg. Endosc. 2009, 23, 248–254. [Google Scholar] [CrossRef]
- Aggarwal, S.K.; San Antonio, J.D.; Sokhansanj, A.; Miller, C. Cisplatin-induced peptic ulcers, vagotomy, adrenal and calcium modulation. Anti-Cancer Drugs 1994, 5, 177–193. [Google Scholar] [CrossRef]
- Morrow, G.R.; Hickok, J.T.; Andrews, P.L.; Stern, R.M. Reduction in serum cortisol after platinum based chemotherapy for cancer: A role for the HPA axis in treatment-related nausea? Psychophysiology 2002, 39, 491–495. [Google Scholar] [CrossRef]
- Cornell, R.F.; Kelly, D.F.; Bordo, G.; Carroll, T.B.; Duong, H.T.; Kim, J.; Takasumi, Y.; Thomas, J.P.; Wong, Y.L.; Findling, J.W. Chemotherapy-induced regression of an adrenocorticotropin-secreting pituitary carcinoma accompanied by secondary adrenal insufficiency. Case Rep. Endocrinol. 2013, 2013, 675298. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.R.; Kim, J.H.; Rhee, Y.; Lee, H.; Song, S.E.; Kim, C.; Song, S.; Noh, S.H.; Rha, S.Y. Assessment of adrenal function and health-related quality of life in advanced gastric cancer patients who received first-line chemotherapy. Oncology 2016, 90, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Yeung, S.-C.J.; Chiu, A.C.; Vassilopoulou-Sellin, R.; Gagel, R.F. The endocrine effects of nonhormonal antineoplastic therapy. Endocr. Rev. 1998, 19, 144–172. [Google Scholar] [CrossRef] [PubMed]
- Broersen, L.H.; Pereira, A.M.; Jørgensen, J.O.L.; Dekkers, O.M. Adrenal insufficiency in corticosteroids use: Systematic review and meta-analysis. J. Clin. Endocrinol. Metab. 2015, 100, 2171–2180. [Google Scholar] [CrossRef] [PubMed]
- Habib, G.S. Systemic effects of intra-articular corticosteroids. Clin. Rheumatol. 2009, 28, 749–756. [Google Scholar] [CrossRef] [PubMed]










| Variables | Sham | 0 Gy | p |
|---|---|---|---|
| M ± SD 1 | M ± SD | ||
| Δ Baseline cortisol (%) | −9.6 ± 7.8 | −7.9 ± 8.5 | 0.89 |
| Δ Evening cortisol (%) | 11.4 ± 2.2 | −7.8 ± 11.5 | 0.22 |
| Endurance swimming time (min) | 3.3 ± 0.1 | 3.7 ± 0.3 | 0.26 |
| Variables | 0 Gy | 2 Gy | p |
|---|---|---|---|
| M ± SD 1 | M ± SD | ||
| Δ Baseline cortisol (%) | −7.9 ± 8.5 | −29.9 ± 3.7 | 0.04 * |
| Δ Evening cortisol (%) | −7.8 ± 11.5 | −21.2 ± 6.5 | 0.35 |
| Endurance swimming time (min) | 3.7 ± 0.3 | 1.7 ± 0.6 | 0.02 * |
| Diagnosis | Age | PS 2 | Stage | Pre-RT Treatment 3 | Chemotherapy 4 | Post-RT Treatment 3 | Pre-RT Hb (g/dL) | Pre-RT Alb (g/dL) |
|---|---|---|---|---|---|---|---|---|
| Esophageal cancer | 58 | 0 | IIB | OP | Weekly CDDP (30) | - | 12.6 | 3.5 |
| Esophageal cancer | 52 | 0 | IIIC | - | Weekly CDDP (20) + Taxol (50) | OP | 13.7 | 4 |
| HCC | 31 | 0 | IIIC | OP | - | - | 10.6 | 4.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.-M.; Chi, C.-W.; Wu, P.-S.; Tai, H.-C.; Chien, M.-N.; Chen, Y.-J. Adrenal Gland Irradiation Causes Fatigue Accompanied by Reactive Changes in Cortisol Levels. J. Clin. Med. 2022, 11, 1214. https://doi.org/10.3390/jcm11051214
Huang Y-M, Chi C-W, Wu P-S, Tai H-C, Chien M-N, Chen Y-J. Adrenal Gland Irradiation Causes Fatigue Accompanied by Reactive Changes in Cortisol Levels. Journal of Clinical Medicine. 2022; 11(5):1214. https://doi.org/10.3390/jcm11051214
Chicago/Turabian StyleHuang, Yu-Ming, Chih-Wen Chi, Pao-Shu Wu, Hung-Chi Tai, Ming-Nan Chien, and Yu-Jen Chen. 2022. "Adrenal Gland Irradiation Causes Fatigue Accompanied by Reactive Changes in Cortisol Levels" Journal of Clinical Medicine 11, no. 5: 1214. https://doi.org/10.3390/jcm11051214
APA StyleHuang, Y.-M., Chi, C.-W., Wu, P.-S., Tai, H.-C., Chien, M.-N., & Chen, Y.-J. (2022). Adrenal Gland Irradiation Causes Fatigue Accompanied by Reactive Changes in Cortisol Levels. Journal of Clinical Medicine, 11(5), 1214. https://doi.org/10.3390/jcm11051214







