Acute-Phase Inflammatory Reaction Predicts CMR Myocardial Scar Pattern and 2-Year Mortality in STEMI Patients Undergoing Primary PCI
Abstract
:1. Introduction
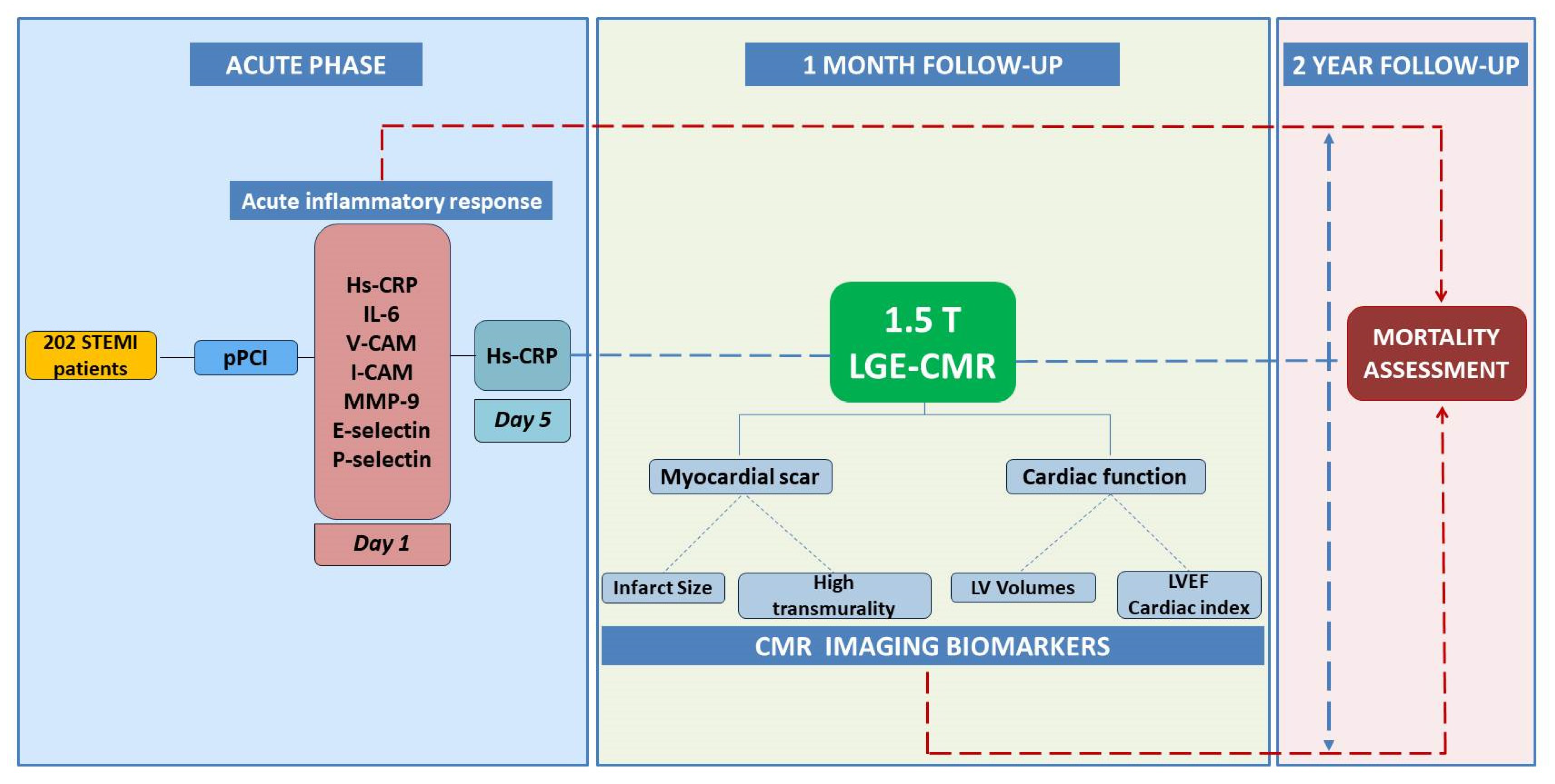
2. Materials and Methods
2.1. Study Population
2.2. Study Procedures
2.2.1. Clinical and Angiographic Characteristics
2.2.2. Serum Biomarkers
2.2.3. CMR Imaging
2.2.4. Statistical Analysis
3. Results
3.1. Demographics and Clinical Characteristics
3.2. Angiographic Findings
3.3. Serum Biomarkers
3.4. CMR Parameters
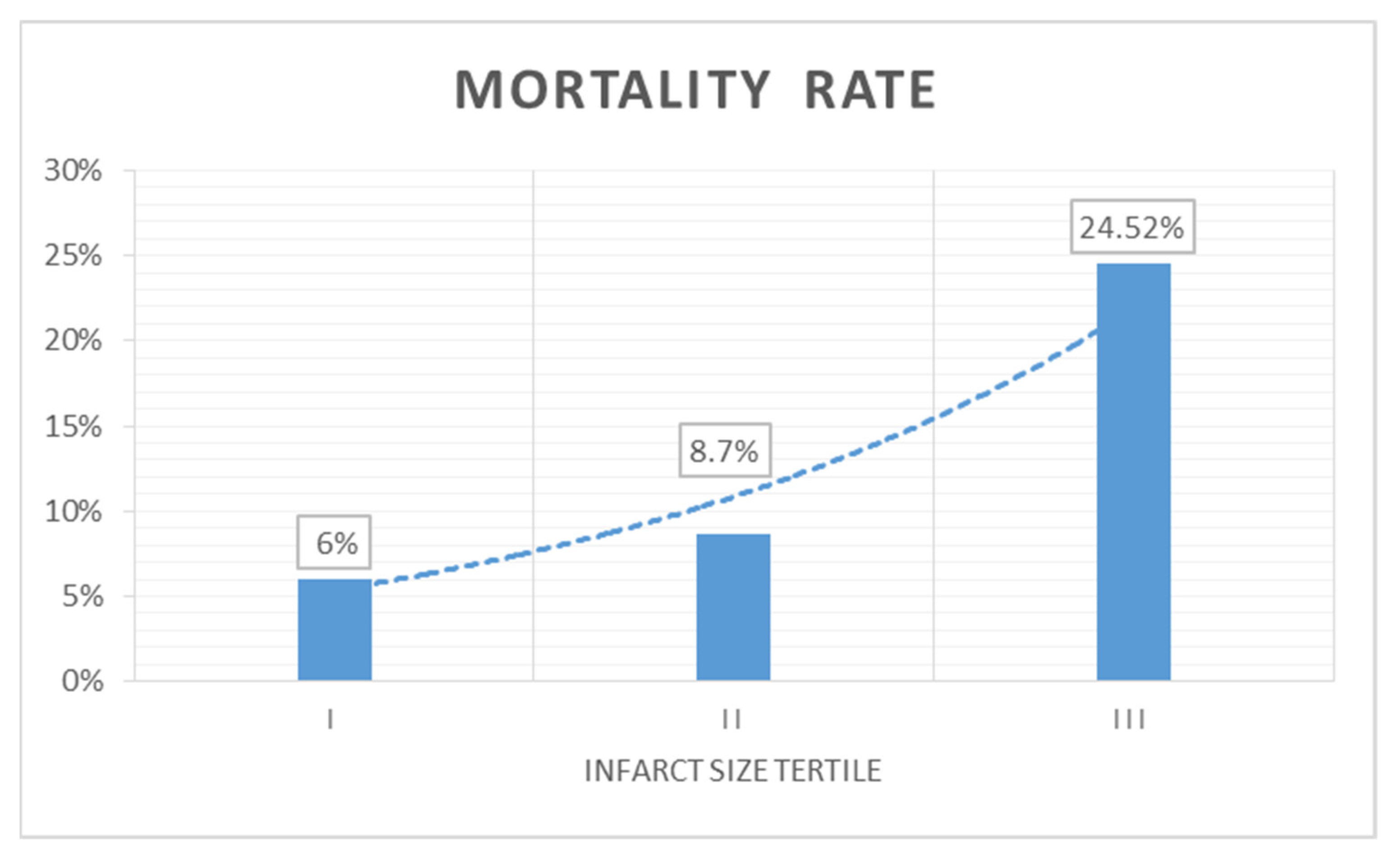
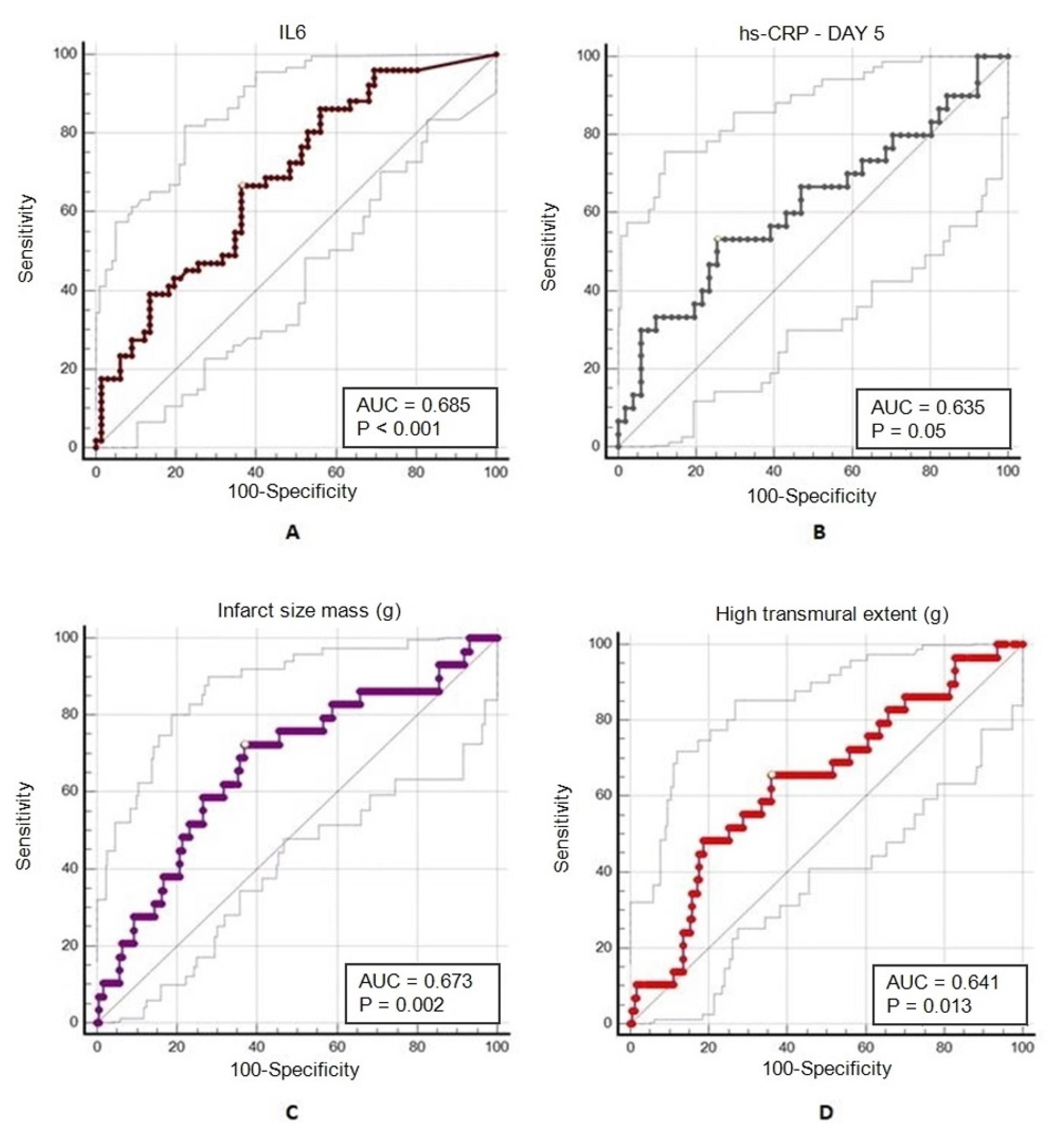
3.5. Predictors of 2-Year Mortality
4. Discussion
4.1. Future Clinical Perspectives
4.2. Study Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Timmis, A.; Townsend, N.; Gale, C.P.; Torbica, A.; Lettino, M.; Petersen, S.E.; Mossialos, E.A.; Maggioni, A.P.; Kazakiewicz, D.; May, H.T.; et al. European Society of Cardiology: Cardiovascular Disease Statistics 2019. Eur. Heart J. 2020, 41, 12–85. [Google Scholar] [CrossRef]
- García-García, C.; Ribas, N.; Recasens, L.L.; Meroño, O.; Subirana, I.; Fernández, A.; Pérez, A.; Miranda, F.; Tizón-Marcos, H.; Martí-Almor, J.; et al. In-hospital prognosis and long-term mortality of STEMI in a reperfusion network. "Head to head" analysis: Invasive reperfusion vs optimal medical therapy. BMC Cardiovasc. Disord. 2017, 17, 139. [Google Scholar] [CrossRef] [Green Version]
- Doost Hosseiny, A.; Moloi, S.; Chandrasekhar, J.; Farshid, A. Mortality pattern and cause of death in a long-term follow-up of patients with STEMI treated with primary PCI. Open. Heart 2016, 3, e000405. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, F.; Butrymovich, V.; Kelbæk, H.; Wachtell, K.; Helqvist, S.; Kastrup, J.; Holmvang, L.; Clemmensen, P.; Engstrøm, T.; Grande, P.; et al. Short- and long-term cause of death in patients treated with primary PCI for STEMI. J. Am. Coll. Cardiol. 2014, 64, 2101–2108. [Google Scholar] [CrossRef]
- Qian, G.; Jin, R.J.; Fu, Z.H.; Yang, Y.Q.; Su, H.L.; Dong, W.; Guo, J.; Jing, J.; Guo, Y.L.; Chen, Y.D. Development and validation of clinical risk score to predict the cardiac rupture in patients with STEMI. Am. J. Emerg. Med. 2017, 35, 589–593. [Google Scholar] [CrossRef]
- Fox, K.A.; Dabbous, O.H.; Goldberg, R.J.; Pieper, K.S.; Eagle, K.A.; Van de Werf, F.; Avezum, A.; Goodman, S.G.; Flather, M.D.; Anderson, F.A., Jr.; et al. Prediction of risk of death and myocardial infarction in the six months after presentation with acute coronary syndrome: Prospective multinational observational study (GRACE). BMJ 2006, 333, 1091. [Google Scholar] [CrossRef] [Green Version]
- Tibaut, M.; Caprnda, M.; Kubatka, P.; Sinkovič, A.; Valentova, V.; Filipova, S.; Gazdikova, K.; Gaspar, L.; Mozos, I.; Egom, E.E.; et al. Markers of Atherosclerosis: Part 1—Serological Markers. Heart Lung. Circ. 2019, 28, 667–677. [Google Scholar] [CrossRef]
- Westman, P.C.; Lipinski, M.J.; Luger, D.; Waksman, R.; Bonow, R.O.; Wu, E.; Epstein, S.E. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 2050–2060. [Google Scholar] [CrossRef]
- Ortolani, P.; Marzocchi, A.; Marrozzini, C.; Palmerini, T.; Saia, F.; Taglieri, N.; Baldazzi, F.; Silenzi, S.; Bacchi-Reggiani, M.L.; Guastaroba, P.; et al. Predictive value of high sensitivity C-reactive protein in patients with ST-elevation myocardial infarction treated with percutaneous coronary intervention. Eur. Heart J. 2008, 29, 1241–1249. [Google Scholar] [CrossRef] [Green Version]
- Caixeta, A.; Stone, G.W.; Mehran, R.; Lee, E.A.; McLaurin, B.T.; Cox, D.A.; Bertrand, M.E.; Lincoff, A.M.; Moses, J.W.; White, H.D.; et al. Predictive value of C-reactive protein on 30-day and 1-year mortality in acute coronary syndromes: An analysis from the ACUITY trial. J. Thromb. Thrombolysis 2011, 31, 154–164. [Google Scholar] [CrossRef]
- Ye, L.; Bai, H.M.; Jiang, D.; He, B.; Wen, X.S.; Ge, P.; Zhang, D.Y. Combination of eosinophil percentage and high-sensitivity C-reactive protein predicts in-hospital major adverse cardiac events in ST-elevation myocardial infarction patients undergoing primary percutaneous coronary intervention. J. Clin. Lab. Anal. 2020, 34, e23367. [Google Scholar] [CrossRef]
- Fanola, C.L.; Morrow, D.A.; Cannon, C.P.; Jarolim, P.; Lukas, M.A.; Bode, C.; Hochman, J.S.; Goodrich, E.L.; Braunwald, E.; O’Donoghue, M.L. Interleukin-6 and the Risk of Adverse Outcomes in Patients After an Acute Coronary Syndrome: Observations From the SOLID-TIMI 52 (Stabilization of Plaque Using Darapladib-Thrombolysis in Myocardial Infarction 52) Trial. J. Am. Heart Assoc. 2017, 6, e005637. [Google Scholar] [CrossRef] [Green Version]
- Frangogiannis, N.G. The inflammatory response in myocardial injury, repair, and remodelling. Nat. Rev. Cardiol. 2014, 11, 255–265. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; MacFadyen, J.G.; Glynn, R.J.; Bradwin, G.; Hasan, A.A.; Rifai, N. Comparison of interleukin-6, C-reactive protein, and low-density lipoprotein cholesterol as biomarkers of residual risk in contemporary practice: Secondary analyses from the Cardiovascular Inflammation Reduction Trial. Eur. Heart J. 2020, 41, 2952–2961. [Google Scholar] [CrossRef] [Green Version]
- Shantsila, E.; Tapp, L.D.; Wrigley, B.J.; Montoro-García, S.; Lip, G.Y. Receptors to interleukin-6 and adhesion molecules on circulating monocyte subsets in acute myocardial infarction. Thromb. Haemost. 2013, 110, 340–348. [Google Scholar] [CrossRef] [Green Version]
- Lai, S.L.; Marín-Juez, R.; Stainier, D.Y.R. Immune responses in cardiac repair and regeneration: A comparative point of view. Cell. Mol. Life Sci. 2019, 76, 1365–1380. [Google Scholar] [CrossRef] [Green Version]
- Ong, S.B.; Hernández-Reséndiz, S.; Crespo-Avilan, G.E.; Mukhametshina, R.T.; Kwek, X.Y.; Cabrera-Fuentes, H.A.; Hausenloy, D.J. Inflammation following acute myocardial infarction: Multiple players, dynamic roles, and novel therapeutic opportunities. Pharmacol. Ther. 2018, 186, 73–87. [Google Scholar] [CrossRef]
- Hamed, G.M.; Fattah, M.F. Clinical Relevance of matrix metalloproteinase 9 in patients with acute coronary syndrome. Clin. Appl. Thromb. Hemost. 2015, 21, 705–711. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.J.; Wu, E.; Rafael, A.; Chen, E.L.; Parker, M.A.; Simonetti, O.; Klocke, F.J.; Bonow, R.O.; Judd, R.M. The use of contrast-enhanced magnetic resonance imaging to identify reversible myocardial dysfunction. N. Engl. J. Med. 2000, 343, 1445–1453. [Google Scholar] [CrossRef]
- Moulin, K.; Viallon, M.; Romero, W.; Chazot, A.; Mewton, N.; Isaaz, K.; Croisille, P. MRI of Reperfused Acute Myocardial Infarction Edema: ADC Quantification versus T1 and T2 Mapping. Radiology 2020, 295, 542–549. [Google Scholar] [CrossRef]
- Stiermaier, T.; Thiele, H.; Eitel, I. Early myocardial edema after acute myocardial infarction is stable and not bimodal in humans—Evidence from a large CMR multicenter study. Int. J. Cardiol. 2017, 246, 87–89. [Google Scholar] [CrossRef]
- Larose, E.; Rodés-Cabau, J.; Pibarot, P.; Rinfret, S.; Proulx, G.; Nguyen, C.M.; Déry, J.P.; Gleeton, O.; Roy, L.; Noël, B.; et al. Predicting late myocardial recovery and outcomes in the early hours of ST-segment elevation myocardial infarction traditional measures compared with microvascular obstruction, salvaged myocardium, and necrosis characteristics by cardiovascular magnetic resonance. J. Am. Coll. Cardiol. 2010, 55, 2459–2469. [Google Scholar] [CrossRef] [Green Version]
- Bulluck, H.; Hammond-Haley, M.; Weinmann, S.; Martinez-Macias, R.; Hausenloy, D.J. Myocardial Infarct Size by CMR in Clinical Cardioprotection Studies: Insights From Randomized Controlled Trials. JACC. Cardiovasc. Imaging 2017, 10, 230–240. [Google Scholar] [CrossRef]
- Kendziora, B.; Dewey, M. Prognostic value of the myocardial salvage index measured by T2-weighted and T1-weighted late gadolinium enhancement magnetic resonance imaging after ST-segment elevation myocardial infarction: A systematic review and meta-regression analysis. PLoS ONE 2020, 15, e0228736. [Google Scholar] [CrossRef]
- Eitel, I.; Stiermaier, T.; Lange, T.; Rommel, K.P.; Koschalka, A.; Kowallick, J.T.; Lotz, J.; Kutty, S.; Gutberlet, M.; Hasenfuß, G.; et al. Cardiac Magnetic Resonance Myocardial Feature Tracking for Optimized Prediction of Cardiovascular Events Following Myocardial Infarction. JACC Cardiovasc. Imaging 2018, 11, 1433–1444. [Google Scholar] [CrossRef]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D. Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction. Fourth Universal Definition of Myocardial Infarction. J. Am. Coll. Cardiol. 2018, 72, 2231–2264. [Google Scholar] [CrossRef]
- Lino, D.O.C.; Freitas, I.A.; Meneses, G.C.; Martins, A.M.C.; Daher, E.F.; Rocha, J.H.C.; Silva Junior, G.B. Interleukin-6 and adhesion molecules VCAM-1 and ICAM-1 as biomarkers of post-acute myocardial infarction heart failure. Braz. J. Med. Biol. Res. 2019, 52, e8658. [Google Scholar] [CrossRef]
- Chen, R.Z.; Liu, C.; Zhou, P.; Tan, Y.; Sheng, Z.X.; Li, J.N.; Zhou, J.Y.; Wu, Y.; Yang, Y.M.; Song, L.; et al. Associations between postprocedural D-dimer, hs-CRP, LDL-C levels and prognosis of acute myocardial infarction patients treated by percutaneous coronary intervention. Zhonghua Xin Xue Guan Bing Za Zhi 2020, 48, 359–366. [Google Scholar] [CrossRef]
- Al Aseri, Z.A.; Habib, S.S.; Marzouk, A. Predictive value of high sensitivity C-reactive protein on progression to heart failure occurring after the first myocardial infarction. Vasc. Health Risk. Manag. 2019, 15, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Morariu, M.; Márton, E.; Mester, A.; Rațiu, M.; Benedek, I. Association Between Acute Inflammatory Response and Infarct Size in Stemi Patients Undergoing Primary PCI. J. Cardiovasc. Emerg. 2018, 4, 140–146. [Google Scholar] [CrossRef] [Green Version]
- Kristono, G.A.; Holley, A.S.; Lakshman, P.; Brunton-O’Sullivan, M.M.; Harding, S.A.; Larsen, P.D. Association between inflammatory cytokines and long-term adverse outcomes in acute coronary syndromes: A systematic review. Heliyon 2020, 6, e03704. [Google Scholar] [CrossRef]
- Opincariu, D.; Rodean, I.; Rat, N.; Hodas, R.; Benedek, I.; Benedek, T. Systemic Vulnerability, as Expressed by I-CAM and MMP-9 at Presentation, Predicts One Year Outcomes in Patients with Acute Myocardial Infarction-Insights from the VIP Clinical Study. J. Clin. Med. 2021, 10, 3435. [Google Scholar] [CrossRef]
- Stone, G.W.; Selker, H.P.; Thiele, H.; Patel, M.R.; Udelson, J.E.; Ohman, E.M.; Maehara, A.; Eitel, I.; Granger, C.B.; Jenkins, P.L.; et al. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J. Am. Coll. Cardiol. 2016, 67, 1674–1683. [Google Scholar] [CrossRef]
- Stiermaier, T.; Jobs, A.; de Waha, S.; Fuernau, G.; Pöss, J.; Desch, S.; Thiele, H.; Eitel, I. Optimized Prognosis Assessment in ST-Segment-Elevation Myocardial Infarction Using a Cardiac Magnetic Resonance Imaging Risk Score. Circ. Cardiovasc. Imaging 2017, 10, e006774. [Google Scholar] [CrossRef] [Green Version]
- Eitel, I.; de Waha, S.; Wöhrle, J.; Fuernau, G.; Lurz, P.; Pauschinger, M.; Desch, S.; Schuler, G.; Thiele, H. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2014, 64, 1217–1226. [Google Scholar] [CrossRef]
- Huang, S.; Frangogiannis, N.G. Anti-inflammatory therapies in myocardial infarction: Failures, hopes and challenges. Br. J. Pharmacol. 2018, 175, 1377–1400. [Google Scholar] [CrossRef] [Green Version]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef]
| Infarct Size I (<14.79 g) Tertile (n = 50) | Infarct Size II (14.79–38.89 g) Tertile (n = 102) | Infarct Size III (>38.89 g) Tertile (n = 50) | p-Value | |
|---|---|---|---|---|
| Age (years ± SD) | 64.31 ± 11.71 | 61.58 ± 11.29 | 63.08 ± 10.94 | 0.49 |
| Height (cm) | 168.2 ± 11.39 | 170.9 ± 10.2 | 167.58 ± 9.9 | 0.168 |
| Weight (kg) | 80.74 ± 13.21 | 85.30 ± 10.5 | 83.14 ± 11.75 | 0.378 |
| BMI (kg/m2) | 28.4 ± 4.28 | 28.7 ± 4.14 | 29.4 ± 3.45 | 0.124 |
| BSA (m2) | 1.88 ± 0.45 | 1.96 ± 0.81 | 1.92 ± 0.71 | 0.314 |
| Male gender n (%) | 32 (64%) | 65 (65.7%) | 40 (80%) | 0.104 |
| Hypertension n (%) | 32 (64%) | 60 (58.8%) | 26 (52%) | 0.39 |
| Diabetes mellitus n (%) | 6 (12%) | 15 (14.7%) | 5 (10%) | 0.702 |
| Documented CKD n (%) | 3 (6%) | 6 (5.8%) | 3 (6%) | 0.999 |
| Current smoker n (%) | 23 (46%) | 39 (38.2%) | 21 (42%) | 0.651 |
| Dyslipidaemia n (%) | 17 (34%) | 38 (37.2%) | 25 (50%) | 0.206 |
| Stroke n (%) | 3 (6%) | 5 (4.9%) | 7 (14%) | 0.12 |
| PAD n (%) | 4 (8%) | 9 (8.8%) | 6 (12%) | 0.759 |
| Obesity * n (%) | 20 (40%) | 41 (40.1%) | 18 (36%) | 0.817 |
| Prior PCI n (%) | 5 (10%) | 11 (10.7%) | 4 (8%) | 0.864 |
| Infarct Size I (<14.79 g) Tertile (n = 50) | Infarct Size II (14.79–38.89 g) Tertile (n = 102) | Infarct Size III (>38.89 g) Tertile (n = 50) | p-Value | |
|---|---|---|---|---|
| IRA | ||||
| LAD | 21 (42%) | 43 (42.1%) | 27 (54%) | |
| CX | 10 (20%) | 16 (15.6%) | 8 (16%) | 0.528 |
| RCA | 19 (38%) | 40 (39.2%) | 13 (26%) | |
| LM | 0 (0%) | 3 (6%) | 2 (4%) | |
| Killip class ≥2 | 10 (10%) | 19 (18.6%) | 18 (36%) | 0.048 |
| Stent implantation | 48 (96%) | 97 (95%) | 48 (96%) | 0.811 |
| Multivessel PCI | 17 (34%) | 36 (35.2%) | 18 (36%) | 0.496 |
| Pre-PCI TIMI flow 0–1 | 45 (90%) | 89 (87.2%) | 46 (92%) | 0.117 |
| Post-PCI TIMI flow 3 | 46 (92%) | 95 (93.1%) | 40 (80%) | 0.120 |
| Infarct Size I (<14.79 g) Tertile (n = 50) | Infarct Size II (14.79–38.89 g) Tertile (n = 102) | Infarct Size III (>38.89 g) Tertile (n = 50) | p-Value | |
|---|---|---|---|---|
| hs-CRP (mg/L) | 6.60 ± 9.45 | 14.90 ± 33.87 | 14.67 ± 36.61 | 0.822 |
| hs-CRP day 5 (mg/L) | 17.01 ± 17.28 | 22.65 ± 16.73 | 27.31 ± 27.12 | 0.350 |
| IL-6 (pg/mL) | 9.17 ± 17.86 | 12.86 ± 18.61 | 13 ± 9.59 | 0.002 |
| E-selectin (ng/mL) | 64.31 ± 11.71 | 61.58 ± 11.29 | 63.08 ± 10.94 | 0.938 |
| P-selectin | 99.07 ± 96.78 | 96.44 ± 66.56 | 115.7 ± 101.5 | 0.518 |
| V-CAM (ng/mL) | 383.96 ± 504.5 | 447.67 ± 620.77 | 354.54 ± 579.12 | 0.792 |
| I-CAM (ng/mL) | 108.47 ± 217.09 | 175.50 ± 419.39 | 89.40 ± 154.75 | 0.648 |
| MMP-9 (ng/mL) | 451.6 ± 595.0 | 433.5 ± 566.3 | 324.8 ± 477.3 | 0.799 |
| NT-proBNP (pg/mL) | 270 ± 269.8 | 682 ± 624.1 | 1693 ± 2165 | 0.033 |
| Apolipoprotein B (g/L) | 0.99 ± 0.41 | 1.07 ± 0.37 | 3.59 ± 13.07 | 0.104 |
| HDL chol (mg/dL) | 57.38 ± 48.47 | 48.37 ± 39.38 | 55.48 ± 46.42 | 0.491 |
| LDL chol (mg/dL) | 103.3 ± 68.64 | 111.8 ± 68.49 | 129.6 ± 69.24 | 0.319 |
| Triglycerides (mg/dL) | 166.2 ± 111.5 | 169.7 ± 101.3 | 156.4 ± 89.4 | 0.117 |
| Infarct Size I (<14.79 g) Tertile (n = 50) | Infarct Size II (14.79–38.89 g) Tertile (n = 102) | Infarct Size III (>38.89 g) Tertile (n = 50) | p-Value | |
|---|---|---|---|---|
| LV myocardium volume (mL) | 129.5 ± 32.89 | 159.2 ± 43.65 | 206.1 ± 47.85 | <0.0001 |
| LV myocardium mass (g) | 127.1 ± 43.89 | 164.5 ± 49.58 | 216.4 ± 50.24 | <0.0001 |
| LV infarct size volume (mL) LV infarct size mass (g) | 10.22 ± 2.90 10.37 ± 2.90 | 14.90 ± 33.87 25.50 ± 6.54 | 52.03 ± 17.16 54.65 ± 18.01 | <0.0001 <0.0001 |
| LV infarct size percentage (%) | 8.01 ± 3.22 | 16.22 ± 4.98 | 26.35 ± 9.34 | <0.0001 |
| High transmural extent (mL) | 3.27 ± 3.39 | 14.65 ± 8.49 | 38.78 ± 24.86 | <0.0001 |
| High transmural extent (g) | 7.07 ± 15.84 | 15.35 ± 8.89 | 39.83 ± 26.65 | <0.0001 |
| LVEF (%) | 62.44 ± 9.59 | 53.17 ± 10.90 | 43.79 ± 10.22 | <0.0001 |
| EDVI (mL/m2) ESVI (mL/m2) | 128.2 ± 46.28 55.11 ± 40.89 | 137.8 ± 36.05 71.37 ± 31.53 | 183.3 ± 47.46 103.5 ± 35.85 | <0.0001 <0.0001 |
| SVI (mL/m2) | 73.11 ± 13.52 | 70.98 ± 18.50 | 83.14 ± 24.10 | 0.069 |
| Cardiac index (L/min/m2) | 5.10 ± 1.64 | 6.0 ± 8.19 | 5.56 ± 1.83 | 0.287 |
| LVMI (g/m2) | 125 ± 35.98 | 123.3 ± 42.18 | 148.9 ± 40.05 | 0.019 |
| IL-6 | hs-CRP Day 1 | hs-CRP Day 5 | MMP-9 | I-CAM | V-CAM | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | |
| LV myocardium volume (mL) | 0.130 | 0.332 | −0.042 | 0.692 | 0.161 | 0.130 | 0.130 | 0.291 | 0.118 | 0.457 | −0.096 | 0.457 |
| LV myocardium mass (g) | 0.130 | 0.322 | −0.042 | 0.692 | 0.161 | 0.130 | 0.130 | 0.291 | 0.118 | 0.457 | −0.096 | 0.457 |
| LV infarct size volume (mL) LV infarct size mass (g) | 0.319 0.324 | 0.013 0.011 | −0.103 −0.103 | 0.333 0.334 | 0.182 0.182 | 0.047 0.046 | 0.319 0.324 | 0.045 0.044 | 0.222 0.023 | 0.539 0.539 | 0.079 0.079 | 0.539 0.539 |
| LV infarct size percentage (%) | 0.303 | 0.018 | −0.172 | 0.106 | 0.117 | 0.271 | 0.303 | 0.081 | 0.194 | 0.268 | 0.142 | 0.268 |
| High transmural extent (mL) | 0.300 | 0.019 | −0.033 | 0.753 | 0.225 | 0.033 | 0.300 | 0.162 | 0.156 | 0.478 | 0.091 | 0.478 |
| High transmural extent (g) | 0.300 | 0.019 | −0.035 | 0.744 | 0.223 | 0.035 | 0.300 | 0.154 | 0.159 | 0.481 | 0.091 | 0.481 |
| LVEF (%) | −0.305 | 0.020 | −0.152 | 0.166 | −0.234 | 0.031 | −0.305 | 0.312 | −0.116 | 0.029 | −0.283 | 0.029 |
| EDVI (mL/m2) ESVI (mL/m2) | 0.132 0.236 | 0.327 0.076 | 0.073 0.133 | 0.507 0.230 | 0.027 0.160 | 0.807 0.807 | 0.132 0.236 | 0.385 0.274 | 0.100 0.125 | 0.755 0.222 | −0.041 0.161 | 0.481 0.755 |
| SVI (mL/m2) | −0.186 | 0.166 | −0.059 | 0.595 | −0.112 | 0.310 | −0.186 | 0.850 | −0.021 | 0.006 | −0.353 | 0.006 |
| Cardiac index (L/min/m2) | −0.046 | 0.733 | 0.120 | 0.277 | −0.010 | 0.924 | −0.046 | 0.977 | −0.003 | 0.029 | −0.283 | 0.029 |
| LVMI (g/m2) | 0.023 | 0.863 | 0.151 | 0.172 | −0.089 | 0.422 | 0.023 | 0.266 | 0.128 | 0.088 | −0.223 | 0.088 |
| Alive n = 176 | Deceased n = 26 | p-Value | |
|---|---|---|---|
| Demographics and Comorbidities | |||
| Age (years ± SD) | 61.16 ± 11.36 | 67.73 ± 9.38 | 0.033 |
| Male gender n (%) | 101 (58.04%) | 18 (69.23%) | 0.832 |
| Hypertension n (%) | 101 (58.04%) | 17 (65.38%) | 0.677 |
| Diabetes mellitus n (%) | 18 (10.22%) | 8 (30.76%) | 0.008 |
| Documented CKD n (%) | 9 (5.11%) | 3 (11.53%) | 0.188 |
| Current smoker n (%) | 72 (40.9%) | 11 (42.3%) | 1.000 |
| Dyslipidaemia n (%) | 67 (38.08%) | 13 (50%) | 0.285 |
| Stroke n (%) | 9 (5.11%) | 6 (23.07%) | 0.005 |
| PAD n (%) | 14 (7.95%) | 5 (19.23%) | 0.077 |
| Obesity n (%) | 68 (38.63%) | 11 (42.3%) | 0.830 |
| Prior PCI n (%) | 17 (9.65%) | 3 (11.53%) | 0.727 |
| PCI characteristics | |||
| Killip class ≥2 | 34 (19.31%) | 13 (50%) | 0.0004 |
| Multivessel PCI | 57 (32.38%) | 14 (53.84%) | 0.046 |
| Pre-PCI TIMI flow 0–1 | 156 (88.63%) | 24 (92.3%) | 0.745 |
| Post-PCI TIMI flow 3 | 162 (92.04%) | 19 (73.07%) | 0.008 |
| Serum inflammatory biomarkers | |||
| hs-CRP day 1 (mg/L) | 16.97 ± 48.31 | 20.69 ± 22.10 | 0.057 |
| hs-CRP day 5 (mg/L) | 14.74 ± 19.09 | 47.26 ± 48.55 | 0.020 |
| IL-6 (pg/mL) | 7.512 ± 4.67 | 17.55 ± 16.53 | 0.029 |
| E-selectin (ng/mL) | 71.25 ± 30.59 | 70.26 ± 20.44 | 0.806 |
| P-selectin (ng/mL) | 90.54 ± 70.82 | 96.54 ± 54.94 | 0.372 |
| V-CAM (ng/mL) | 444.51 ± 52.84 | 466.11 ± 71.74 | 0.928 |
| I-CAM (ng/mL) | 172.4 ± 85.97 | 133.7 ± 91.85 | 0.704 |
| MMP-9 (ng/mL) | 398.0 ± 41.54 | 274.0 ± 68.52 | 0.129 |
| CMR parameters | |||
| LV infarct size mass (g) | 28.17 ± 18.42 | 40.89 ± 21.31 | 0.006 |
| LV infarct size percentage (%) | 16.56 ± 8.70 | 20.07 ± 9.998 | 0.128 |
| High transmural extent (g) | 17.14 ± 18.60 | 27.98 ± 20.71 | 0.006 |
| LVEF (%) | 53.24 ± 11.92 | 43.62 ± 13.71 | 0.006 |
| EDVI (mL/m2) | 147.1 ± 46.35 | 196.5 ± 48.78 | 0.020 |
| ESV I(mL/m2) | 75.42 ± 37.28 | 122.0 ± 45.15 | 0.015 |
| SV (mL/m2) | 75.13 ± 20.70 | 74.67 ± 12.06 | 0.957 |
| Cardiac index (L/min/m2) | 5.03 ± 1.45 | 4.96 ± 1.08 | 0.984 |
| LVMI (g/m2) | 129.3 ± 40.12 | 166.7 ± 53.29 | 0.033 |
| Multivariable Analysis for IS | |||
| Variable | Adjusted OR | 95% CI for Adjusted OR | p-Value |
| hs-CRP day 5 | 0.5590 | 0.02916 to 3.370 | 0.5 |
| IL-6 | 2.059 | 1.010 to 4.197 | 0.04 |
| EDVI | 1.02 | 1.00–1.05 | 0.1 |
| ESVI | 1.04 | 1.00–1.09 | 0.1 |
| LVMI | 0.98 | 0.95–1.00 | 0.2 |
| LVEF (%) | 1.00 | 0.90–1.11 | 0.3 |
| Age | 1.00 | 0.977–1.04 | 0.4 |
| Diabetes | 1.71 | 0.72–4.15 | 0.2 |
| Killip class > 2 | 1.49 | 0.26–8.26 | 0.6 |
| Multivessel PCI | 2.50 | 0.93–7.14 | 0.07 |
| Post-PCI TIMI flow 3 | 2.28 | 1.06–8.39 | 0.7 |
| Multivariable Analysis For 2-year mortality | |||
| Variable | Adjusted OR | 95% CI for adjusted OR | p-Value |
| hs-CRP day 5 | 13.75 | 1.8–23.7 | 0.004 |
| IL-6 | 1.84 | 0.54–3.81 | 0.1 |
| Infarct size mass (g) | 0.97 | 0.80–1.17 | 0.7 |
| High transmural extent (g) | 1.04 | 0.93–1.18 | 0.4 |
| EDVI | 1.14 | 0.99–1.57 | 0.3 |
| ESVI | 0.84 | 0.50–1.06 | 0.4 |
| LVMI | 1.06 | 1.00–1.17 | 0.07 |
| LVEF (%) | 0.77 | 0.31–1.30 | 0.5 |
| Age | 1.05 | 1.00–1.12 | 0.05 |
| Stroke | 2.58 | 0.33–14.51 | 0.3 |
| Diabetes | 1.41 | 0.31–5.33 | 0.6 |
| Killip Class > 2 | 1.61 | 0.07–13.39 | 0.6 |
| Multivessel PCI | 1.00 | 0.17–4.27 | 0.9 |
| Post-PCI TIMI flow 3 | 1.58 | 0.49–9.36 | 0.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mester, A.; Rat, N.; Benedek, T.; Opincariu, D.; Hodas, R.; Chitu, M.; Benedek, I. Acute-Phase Inflammatory Reaction Predicts CMR Myocardial Scar Pattern and 2-Year Mortality in STEMI Patients Undergoing Primary PCI. J. Clin. Med. 2022, 11, 1222. https://doi.org/10.3390/jcm11051222
Mester A, Rat N, Benedek T, Opincariu D, Hodas R, Chitu M, Benedek I. Acute-Phase Inflammatory Reaction Predicts CMR Myocardial Scar Pattern and 2-Year Mortality in STEMI Patients Undergoing Primary PCI. Journal of Clinical Medicine. 2022; 11(5):1222. https://doi.org/10.3390/jcm11051222
Chicago/Turabian StyleMester, Andras, Nora Rat, Theodora Benedek, Diana Opincariu, Roxana Hodas, Monica Chitu, and Imre Benedek. 2022. "Acute-Phase Inflammatory Reaction Predicts CMR Myocardial Scar Pattern and 2-Year Mortality in STEMI Patients Undergoing Primary PCI" Journal of Clinical Medicine 11, no. 5: 1222. https://doi.org/10.3390/jcm11051222
APA StyleMester, A., Rat, N., Benedek, T., Opincariu, D., Hodas, R., Chitu, M., & Benedek, I. (2022). Acute-Phase Inflammatory Reaction Predicts CMR Myocardial Scar Pattern and 2-Year Mortality in STEMI Patients Undergoing Primary PCI. Journal of Clinical Medicine, 11(5), 1222. https://doi.org/10.3390/jcm11051222







