EHO-85: A Multifunctional Amorphous Hydrogel for Wound Healing Containing Olea europaea Leaf Extract: Effects on Wound Microenvironment and Preclinical Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Amorphous Hydrogel Containing Olea Europaea Leaf Extract (EHO-85)
2.2. Moistening Ability of EHO-85 Compared to Other Amorphous Hydrogels
2.3. Rat Wound Model for the Study of the Effect of EHO-85 on Lipid Peroxidation and pH in the Wound Bed
2.4. Evaluation of the Antioxidant (Scavenger) Capacity of Hydrogel Containing EHO-85 in the Wound Bed of the Rat Wound Model, Assessed As Lipid Peroxidation and Reduced/Oxidized Glutathione Ratio
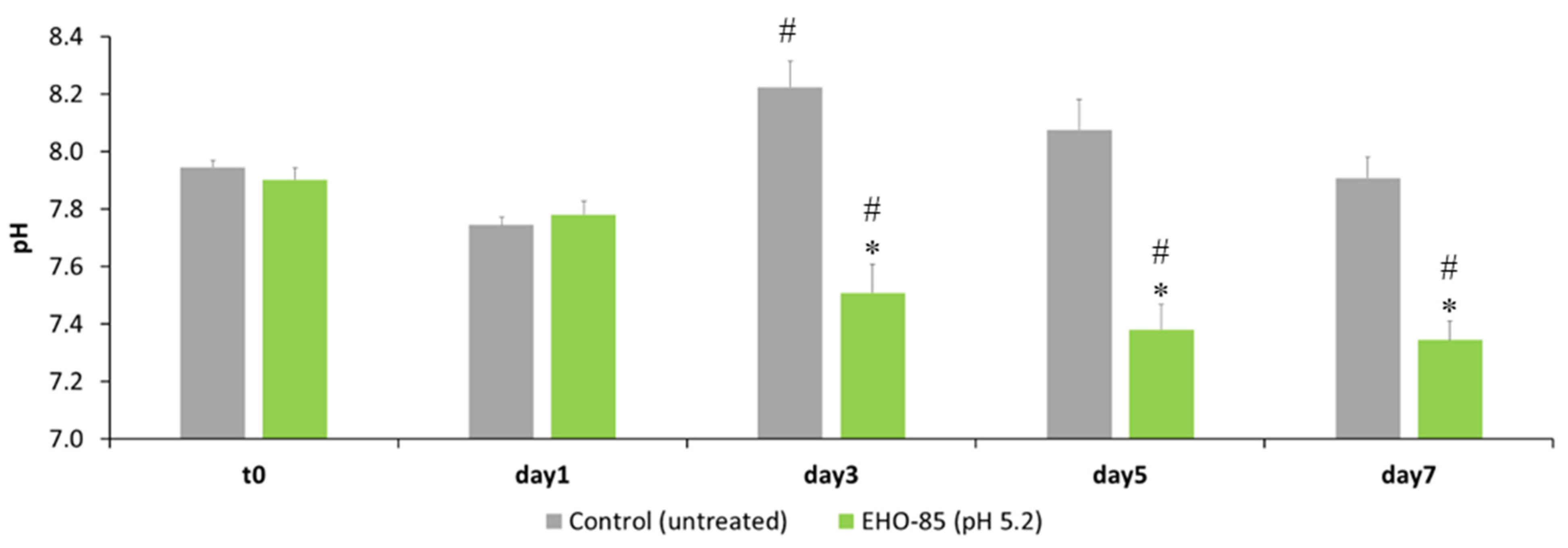
2.5. Effect of EHO-85 on Wound pH in an Animal Model
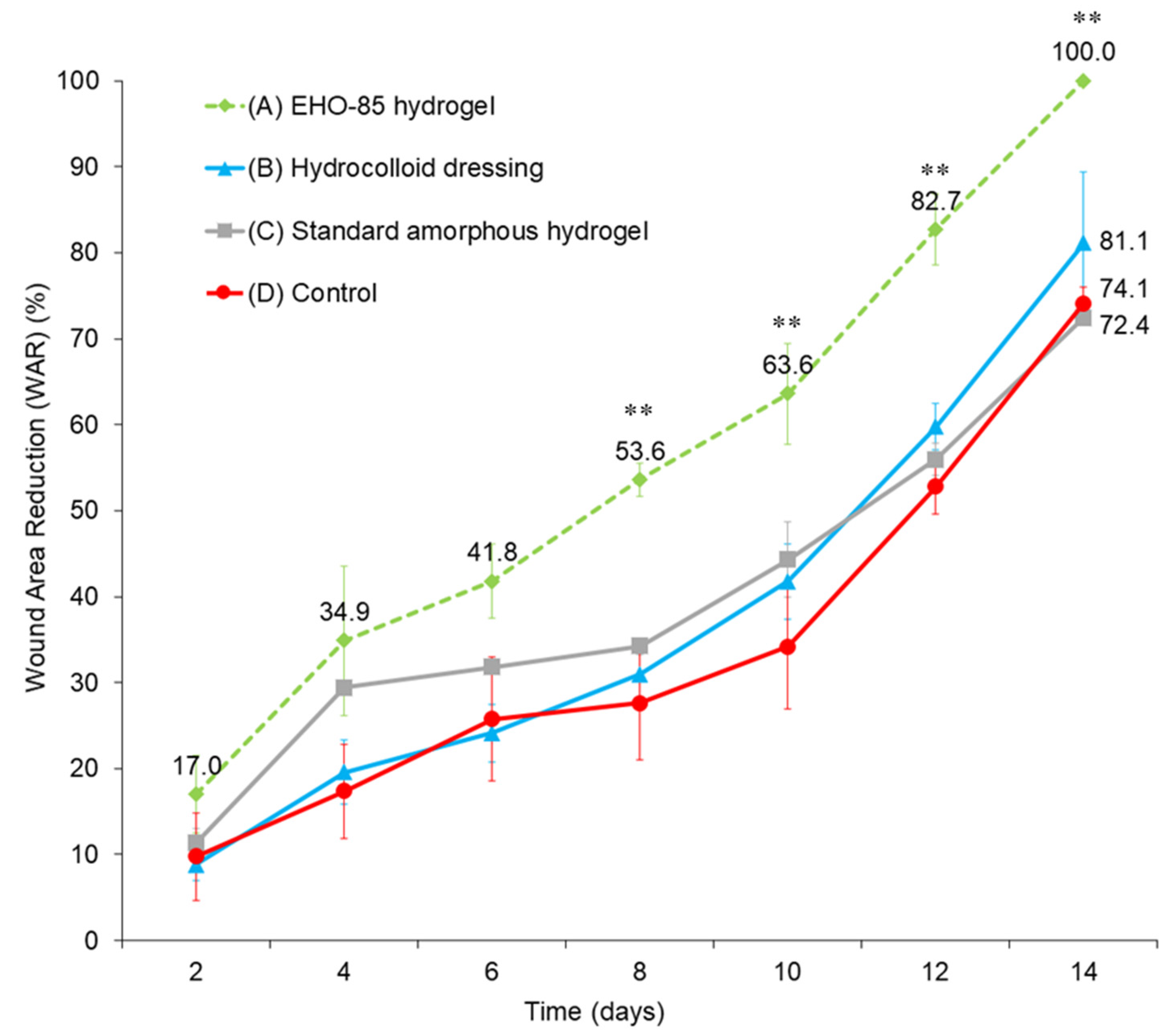
2.6. Effect of EHO-85 on Wound Healing in a Murine Model of Impaired Wound Healing (BKS. Cg-m +/+ Leprdb)
2.7. Statistical Analysis
3. Results
3.1. Moistening Capacity of the EHO-85 Hydrogel Compared to Other Amorphous Hydrogels
3.2. Evaluation of the Antioxidant (Scavenger) Capacity of the EHO-85 Hydrogel in the Wound Bed of Experimental Animals, Assessed As Lipid Peroxidation and the GSH/GSSG Ratio
3.3. Effect of EHO-85 on Wound Bed pH in an Animal Model
3.4. Effect of EHO-85 Amorphous Hydrogel with Antioxidant Properties on Wound Healing in a Murine Model of Impaired Wound Healing (BKS. Cg-m +/+ Leprdb)
4. Discussion
4.1. Moist Wound Healing (MWH): Moisturizing Effect on Wound Healing
4.2. Oxidative Stress Reduction and Wound Healing
4.3. pH Regulation and Wound Healing
4.4. Wound Healing Promotion In Vivo
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [Green Version]
- Guest, J.F.; Vowden, K.; Vowden, P. The health economic burden that acute and chronic wounds impose on an average clinical commissioning group/ health board in the UK. J. Wound Care 2017, 26, 292–303. [Google Scholar] [CrossRef] [Green Version]
- Olsson, M.; Järbrink, K.; Divakar, U.; Bajpai, R.; Upton, Z.; Schmidtchen, A.; Car, J. The humanistic and economic burden of chronic wounds: A systematic review. Wound Repair Regen. 2019, 27, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Q.; Gibb, M.; Graves, N.; Finlayson, K.; Pacella, R.E. Cost-effectiveness analysis of guideline-based optimal care for venous leg ulcers in Australia. BMC Health Serv. Res. 2018, 18, 421. [Google Scholar] [CrossRef]
- Gray, T.A.; Rhodes, S.; Atkinson, R.A.; Rothwell, K.; Wilson, P.; Dumville, J.C.; Cullum, N.A. Opportunities for better value wound care: A multiservice, cross-sectional survey of complex wounds and their care in a UK community population. BMJ Open 2018, 8, e019440. [Google Scholar] [CrossRef] [Green Version]
- Hamdan, S.; Pastar, I.; Drakulich, S.; Dikici, E.; Tomic-Canic, M.; Deo, S.; Daunert, S. Nanotechnology-Driven Therapeutic Interventions in Wound Healing: Potential Uses and Applications. ACS Cent. Sci. 2017, 3, 163–175. [Google Scholar] [CrossRef]
- Goossens, A.; Cleenewerck, M.B. New wound dressings: Classification, tolerance. Eur. J. Dermatol. 2010, 20, 24–26. [Google Scholar] [CrossRef]
- Baron, J.M.; Glatz, M.; Proksch, E. Optimal Support of Wound Healing: New Insights. Dermatology 2020, 236, 593–600. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Kopeček, J.; Yang, J. Hydrogels as smart biomaterials. Polym. Int. 2007, 56, 1078–1098. [Google Scholar] [CrossRef]
- Vaneau, M.; Chaby, G.; Guillot, B.; Martel, P.; Senet, P.; Téot, L.; Chosidow, O. Consensus panel recommendations for chronic and acute wound dressings. Arch. Dermatol. 2007, 143, 1291–1294. [Google Scholar] [CrossRef]
- Field, C.K.; Kerstein, M.D. Overview of wound healing in a moist environment. Am. J. Surg. 1994, 167, 2S–6S. [Google Scholar] [CrossRef]
- Moody, A. Use of a hydrogel dressing for management of a painful leg ulcer. Br. J. Community Nurs. 2006, 11, S12–S17. [Google Scholar] [CrossRef] [Green Version]
- Van Vlierberghe, S.; Dubruel, P.; Schacht, E. Biopolymer-based hydrogels as scaffolds for tissue engineering applications: A review. Biomacromolecules 2011, 12, 1387–1408. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Wang, J.; Li, R.; Li, T.; Chang, M.; Yan, F.; Wang, Y. Encapsulation of Curcumin Nanoparticles with MMP9-Responsive and Thermos-Sensitive Hydrogel Improves Diabetic Wound Healing. ACS Appl. Mater. Interfaces 2018, 10, 16315–16326. [Google Scholar] [CrossRef]
- Xu, Z.; Han, S.; Gu, Z.; Wu, J. Advances and Impact of Antioxidant Hydrogel in Chronic Wound Healing. Adv. Healthc. Mater. 2020, 9, e1901502. [Google Scholar] [CrossRef]
- Winter, G.D. Formation of the scab and the rate of epithelization of superficial wounds in the skin of the young domestic pig. Nature 1962, 193, 293–294. [Google Scholar] [CrossRef]
- Okan, D.; Woo, K.; Ayello, E.A.; Sibbald, G. The role of moisture balance in wound healing. Adv. Skin Wound Care 2007, 20, 39–53. [Google Scholar] [CrossRef]
- Rollman, O.; Jensen, U.B.; Östman, A.; Bolund, L.; Gústafsdóttir, S.M.; Jensen, T.G. Platelet derived growth factor (PDGF) responsive epidermis formed from human keratinocytes transduced with the PDGFβ receptor gene. J. Invest. Dermatol. 2003, 120, 742–749. [Google Scholar] [CrossRef] [Green Version]
- Cho, C.Y.; Lo, J.S. Dressing the part. Dermatol. Clin. 1998, 16, 25–47. [Google Scholar] [CrossRef]
- John Chen, W.Y.; Rogers, A.A.; Lydon, M.J. Characterization of biologic properties of wound fluid collected during early stages of wound healing. J. Invest. Dermatol. 1992, 99, 550–558. [Google Scholar] [CrossRef] [Green Version]
- Roy, S.; Khanna, S.; Nallu, K.; Hunt, T.K.; Sen, C.K. Dermal wound healing is subject to redox control. Mol. Ther. 2006, 13, 211–220. [Google Scholar] [CrossRef] [PubMed]
- Wagener, F.A.D.T.G.; Carels, C.E.; Lundvig, D.M.S. Targeting the redox balance in inflammatory skin conditions. Int. J. Mol. Sci. 2013, 14, 9126–9167. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, M.; Werner, S. Oxidative stress in normal and impaired wound repair. Pharmacol. Res. 2008, 58, 165–171. [Google Scholar] [CrossRef] [PubMed]
- Dunnill, C.; Patton, T.; Brennan, J.; Barrett, J.; Dryden, M.; Cooke, J.; Leaper, D.; Georgopoulos, N.T. Reactive oxygen species (ROS) and wound healing: The functional role of ROS and emerging ROS-modulating technologies for augmentation of the healing process. Int. Wound J. 2017, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Benavente-García, O.; Castillo, J.; Lorente, J.; Ortuño, A.; Del Rio, J.A. Antioxidant activity of phenolics extracted from Olea europaea L. leaves. Food Chem. 2000, 68, 457–462. [Google Scholar] [CrossRef]
- Kontogianni, V.G.; Gerothanassis, I.P. Phenolic compounds and antioxidant activity of olive leaf extracts. Nat. Prod. Res. 2012, 26, 186–189. [Google Scholar] [CrossRef]
- De Mello Andrade, J.M.; Fasolo, D. Polyphenol Antioxidants from Natural Sources and Contribution to Health Promotion. In Polyphenols in Human Health and Disease; Academic Press: Amsterdam, The Netherlands, 2013; Volume 1, pp. 253–265. ISBN 9780123984562. [Google Scholar]
- Mehraein, F.; Sarbishegi, M.; Aslani, A. Evaluation of effect of oleuropein on skin wound healing in aged male BALB/c mice. Cell J. 2014, 16, 25–30. [Google Scholar]
- Mehraein, F.; Sarbishegi, M.; Aslani, A. Therapeutic effects of oleuropein on wounded skin in young male Balb/c mice. Wounds 2014, 26, 83–88. [Google Scholar]
- Koca, U.; Süntar, I.; Akkol, E.K.; Yilmazer, D.; Alper, M. Wound repair potential of Olea europaea L. leaf extracts revealed by in vivo experimental models and comparative evaluation of the extracts’ antioxidant activity. J. Med. Food 2011, 14, 140–146. [Google Scholar] [CrossRef]
- Al-Basher, G.; Al-Otibi, F. Biological activity of olive leaf extract and regulation of tissue transglutaminase expression in diabetic wound healing. Int. J. Pharmacol. 2018, 14, 963–972. [Google Scholar] [CrossRef]
- Jones, E.M.; Cochrane, C.A.; Percival, S.L. The Effect of pH on the Extracellular Matrix and Biofilms. Adv. Wound Care 2015, 4, 431–439. [Google Scholar] [CrossRef] [PubMed]
- Tsukada, K. The pH changes of pressure ulcers related headling process of wound. Wounds 1992, 4, 16–20. [Google Scholar]
- Wilson, I.A.; Henry, M.; Quill, R.; Byrne, P. The pH of varicose ulcer surfaces and its relationship to healing. Vasa 1979, 8, 339–342. [Google Scholar]
- Roberts, G.; Hammad, L.; Collins, C.; Shearman, C.; Mani, R. Some effects of sustained compression on ulcerated tissues. Angiology 2002, 53, 451–456. [Google Scholar] [CrossRef]
- Honnegowda, T.M.; Kumar, P.; Padmanabha Udupa, E.G.; Sharan, A.; Singh, R.; Prasad, H.; Rao, P. Effects of limited access dressing in chronic wounds: A biochemical and histological study. Indian J. Plast. Surg. 2015, 48, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Nagoba, B.S.; Suryawanshi, N.M.; Wadher, B.; Selkar, S. Acidic environment and wound healing: A review. Wounds 2015, 27, 5–11. [Google Scholar]
- Casado-Diaz, A.; Moreno-Rojas, J.M.; Verdú-Soriano, J.; Lázaro-Martínez, J.L.; Rodríguez-Mañas, L.; Tunez, I.; La Torre, M.; Pérez, M.B.; Priego-Capote, F.; Pereira-Caro, G. Evaluation of Antioxidant and Wound-Healing Properties of EHO-85, a Novel Multifunctional Amorphous Hydrogel Containing Olea europaea Leaf Extract. Pharmaceutics 2022, 14, 349. [Google Scholar] [CrossRef]
- Fresno Contreras, M.J.; Ramírez Diéguez, A.; Jiménez Soriano, M.M. Viscosity and temperature relationship in ethanol/water mixtures gelified with Carbopol® UltrezTM 10. Farmaco 2001, 56, 443–445. [Google Scholar] [CrossRef]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological characterization of topical carbomer gels neutralized to different pH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef] [Green Version]
- Gregory, S.R. Physical Properties of Glycerine. In Glycerin: A key Cosmetic Ingredient; Jungermann, E., Sonntag, N.O.V., Eds.; CRC Press: New York, NY, USA, 2018; Volume 1, pp. 113–156. ISBN 9780203753071. [Google Scholar]
- Albèr, C.; Buraczewska-Norin, I.; Kocherbitov, V.; Saleem, S.; Lodén, M.; Engblom, J. Effects of water activity and low molecular weight humectants on skin permeability and hydration dynamics—A double-blind, randomized and controlled study. Int. J. Cosmet. Sci. 2014, 36, 412–418. [Google Scholar] [CrossRef] [PubMed]
- Péterszegi, G.; Isnard, N.; Robert, A.M.; Robert, L. Studies on skin aging. Preparation and properties of fucose-rich oligo- and polysaccharides. Effect on fibroblast proliferation and survival. Biomed. Pharmacother. 2003, 57, 187–194. [Google Scholar] [CrossRef]
- Anjum, A.; Sim, C.H.; Ng, S.F. Hydrogels Containing Antibiofilm and Antimicrobial Agents Beneficial for Biofilm-Associated Wound Infection: Formulation Characterizations and In vitro Study. AAPS PharmSciTech 2018, 19, 1219–1230. [Google Scholar] [CrossRef] [PubMed]
- BS EN 13726-1:2002 Test Methods for Primary Wound Dressings Aspects of Absorbency—European Standards. Available online: https://www.en-standard.eu/bs-en-13726-1-2002-test-methods-for-primary-wound-dressings-aspects-of-absorbency/ (accessed on 13 February 2022).
- Michaels, J.; Churgin, S.S.; Blechman, K.M.; Greives, M.R.; Aarabi, S.; Galiano, R.D.; Gurtner, G.C. db/db mice exhibit severe wound-healing impairments compared with other murine diabetic strains in a silicone-splinted excisional wound model. Wound Repair Regen. 2007, 15, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Galiano, R.D.; Michaels, J.; Dobryansky, M.; Levine, J.P.; Gurtner, G.C. Quantitative and reproducible murine model of excisional wound healing. Wound Repair Regen. 2004, 12, 485–492. [Google Scholar] [CrossRef] [PubMed]
- Singer, A.J.; Tassiopoulos, A.; Kirsner, R.S. Evaluation and Management of Lower-Extremity Ulcers. N. Engl. J. Med. 2017, 377, 1559–1567. [Google Scholar] [CrossRef] [PubMed]
- Comino-Sanz, I.M.; López-Franco, M.D.; Castro, B.; Pancorbo-Hidalgo, P.L. The role of antioxidants on wound healing: A review of the current evidence. J. Clin. Med. 2021, 10, 3558. [Google Scholar] [CrossRef] [PubMed]
- Boateng, J.; Catanzano, O. Advanced Therapeutic Dressings for Effective Wound Healing—A Review. J. Pharm. Sci. 2015, 104, 3653–3680. [Google Scholar] [CrossRef] [Green Version]
- Junker, J.P.E.; Kamel, R.A.; Caterson, E.J.; Eriksson, E. Clinical Impact Upon Wound Healing and Inflammation in Moist, Wet, and Dry Environments. Adv. Wound Care 2013, 2, 348–356. [Google Scholar] [CrossRef] [Green Version]
- Gwon, H.J.; Lim, Y.M.; Nho, Y.C.; Baik, S.H. Humectants effect on aqueous fluids absorption of γ-irradiated PVA hydrogel followed by freeze thawing. Radiat. Phys. Chem. 2010, 79, 650–653. [Google Scholar] [CrossRef]
- Cheng, A.Y.; García, A.J. Engineering the matrix microenvironment for cell delivery and engraftment for tissue repair. Curr. Opin. Biotechnol. 2013, 24, 864–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Opt Veld, R.C.; Walboomers, X.F.; Jansen, J.A.; Wagener, F.A.D.T.G. Design Considerations for Hydrogel Wound Dressings: Strategic and Molecular Advances. Tissue Eng.-Part B Rev. 2020, 26, 230–248. [Google Scholar] [CrossRef] [PubMed]
- Kanta, J. The role of hydrogen peroxide and other reactive oxygen species in wound healing. Acta Med. (Hradec Kral.) 2011, 54, 97–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, B.; Palomares, T.; Azcoitia, I.; Bastida, F.; del Olmo, M.; Soldevilla, J.J.; Alonso-Varona, A. Development and preclinical evaluation of a new galactomannan-based dressing with antioxidant properties for wound healing. Histol. Histopathol. 2015, 30, 1499–1512. [Google Scholar] [CrossRef]
- Lee, O.-H.; Lee, B.-Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef]
- Vidičević, S.; Tošić, J.; Stanojević, Ž.; Isaković, A.; Mitić, D.; Ristić, D.; Dekanski, D. Standardized Olea europaea L. leaf extract exhibits protective activity in carbon tetrachloride-induced acute liver injury in rats: The insight into potential mechanisms. Arch. Physiol. Biochem. 2020, 126, 399–407. [Google Scholar] [CrossRef]
- Kumral, A.; Giriş, M.; Soluk-Tekkeşin, M.; Olgaç, V.; Doğru-Abbasoğlu, S.; Türkoğlu, Ü.; Uysal, M. Effect of olive leaf extract treatment on doxorubicin-induced cardiac, hepatic and renal toxicity in rats. Pathophysiology 2015, 22, 117–123. [Google Scholar] [CrossRef]
- Tasić-Kostov, M.; Arsić, I.; Pavlović, D.; Stojanović, S.; Najman, S.; Naumović, S.; Tadić, V. Towards a modern approach to traditional use: In vitro and in vivo evaluation of Alchemilla vulgaris L. gel wound healing potential. J. Ethnopharmacol. 2019, 238, 111789. [Google Scholar] [CrossRef]
- Affonso, R.C.L.; Voytena, A.P.L.; Fanan, S.; Pitz, H.; Coelho, D.S.; Horstmann, A.L.; Pereira, A.; Uarrota, V.G.; Hillmann, M.C.; Varela, L.A.C.; et al. Phytochemical Composition, Antioxidant Activity, and the Effect of the Aqueous Extract of Coffee (Coffea arabica L.) Bean Residual Press Cake on the Skin Wound Healing. Oxid. Med. Cell. Longev. 2016, 2016, 1923754. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Garg, P.; Goyal, R.; Kaur, G.; Li, X.; Negi, P.; Valis, M.; Kuca, K.; Kulshrestha, S. A novel herbal hydrogel formulation of moringa oleifera for wound healing. Plants 2021, 10, 25. [Google Scholar] [CrossRef]
- Lengheden, A.; Jansson, L. PH effects on experimental wound healing of human fibroblasts in vitro. Eur. J. Oral Sci. 1995, 103, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Greener, B.; Hughes, A.A.; Bannister, N.P.; Douglass, J. Proteases and pH in chronic wounds. J. Wound Care 2005, 14, 59–61. [Google Scholar] [CrossRef]
- Leveen, H.H.; Falk, G.; Borek, B.; Diaz, C.; Lynfield, Y.; Wynkoop, B.J.; Mabunda, G.A.; Rubricius, J.L.; Christoudias, G.C. Chemical acidification of wounds. An adjuvant to healing and the unfavorable action of alkalinity and ammonia. Ann. Surg. 1973, 178, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Hunt, T.K.; Hopf, H.W. Wound healing and wound infection: What surgeons and anesthesiologists can do. Surg. Clin. N. Am. 1997, 77, 587–606. [Google Scholar] [CrossRef]
- Thomas, L.V.; Wimpenny, J.W.T.; Davis, J.G. Effect of three preservatives on the growth of Bacillus cereus, Vero cytotoxigenic Escherichia coli and Staphylococcus aureus, on plates with gradients of pH and sodium chloride concentration. Int. J. Food Microbiol. 1993, 17, 289–301. [Google Scholar] [CrossRef]
- Runeman, B.; Faergemann, J.; Larkö, O. Experimental Candida albicans lesions in healthy humans: Dependence on skin pH. Acta Derm. Venereol. 2000, 80, 421–424. [Google Scholar] [CrossRef] [Green Version]
- Glibbery, A.; Mani, R. pH in leg ulcers. Int. J. Microcirc. Clin. Exp. 1992, 11, S109. [Google Scholar]
| Fluid Affinity (Donation) (%) Loss in Gel Weight | Type |
|---|---|
| 0–5 | A |
| >5–10 | B |
| >10–15 | C |
| >15–20 | D |
| >20–25 | E |
| Hydrogel | (%) Average Decrease of Gel Weight | Type |
|---|---|---|
| Askina® Gel | 19.0 ± 2.0 | D |
| Normlgel® | 13.0 ± 3.0 | C |
| Purilon® Gel | 12.5 ± 0.3 | C |
| Intrasite® Gel | 6.0 ± 2.0 | B |
| Nu-Gel® | 9.0 ± 3.0 | B |
| EHO-85 | 15.2 ± 0.9 | D |
| µM (MDA + 4HDA)/mg Protein | |||
|---|---|---|---|
| Treatment | 48 h | 96 h | |
| EHO-85 without 0.1% OELE | 0.0097 ± 0.0005 | 0.0134 ± 0.0008 | p < 0.001 |
| EHO-85 hydrogel | 0.0052 ± 0.0003 | 0.0073 ± 0.0004 | p < 0.001 |
| p < 0.001 | p < 0.001 | ||
| GSH/GSSG | |||
|---|---|---|---|
| Treatment | 48 h | 96 h | |
| EHO-85 without 0.1% OELE | 0.382 ± 0.093 | 0.743 ± 0.050 | p < 0.05 |
| EHO-85 | 0.346 ± 0.034 | 1.195 ± 0.174 | p < 0.05 |
| ns | p < 0.05 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado-Díaz, A.; La Torre, M.; Priego-Capote, F.; Verdú-Soriano, J.; Lázaro-Martínez, J.L.; Rodríguez-Mañas, L.; Berenguer Pérez, M.; Tunez, I. EHO-85: A Multifunctional Amorphous Hydrogel for Wound Healing Containing Olea europaea Leaf Extract: Effects on Wound Microenvironment and Preclinical Evaluation. J. Clin. Med. 2022, 11, 1229. https://doi.org/10.3390/jcm11051229
Casado-Díaz A, La Torre M, Priego-Capote F, Verdú-Soriano J, Lázaro-Martínez JL, Rodríguez-Mañas L, Berenguer Pérez M, Tunez I. EHO-85: A Multifunctional Amorphous Hydrogel for Wound Healing Containing Olea europaea Leaf Extract: Effects on Wound Microenvironment and Preclinical Evaluation. Journal of Clinical Medicine. 2022; 11(5):1229. https://doi.org/10.3390/jcm11051229
Chicago/Turabian StyleCasado-Díaz, Antonio, Manuel La Torre, Feliciano Priego-Capote, José Verdú-Soriano, José Luis Lázaro-Martínez, Leocadio Rodríguez-Mañas, Miriam Berenguer Pérez, and Isaac Tunez. 2022. "EHO-85: A Multifunctional Amorphous Hydrogel for Wound Healing Containing Olea europaea Leaf Extract: Effects on Wound Microenvironment and Preclinical Evaluation" Journal of Clinical Medicine 11, no. 5: 1229. https://doi.org/10.3390/jcm11051229






