Minimally Invasive Partial vs. Total Adrenalectomy for the Treatment of Unilateral Primary Aldosteronism: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Material and Methods
- Population: patients affected by uPHA with indication to surgical treatment;
- Intervention: minimally-invasive partial adrenalectomy (mi-PA);
- Comparator: minimally-invasive total adrenalectomy (mi-TA);
- Outcomes: perioperative and functional results.
2.1. Search Strategy
2.2. Selection of Eligible Studies and Data Extraction
2.3. Data Quality Assessment
- -
- Baseline characteristics: age, BMI, gender, ASA score, HTN duration, number of antihypertensive medications, preoperative systolic blood pressure (SBP), diastolic blood pressure (DBP), serum Aldosterone (sA), serum Renin Activity (sRA), serum Aldosterone Renin Activity Ratio (ARR), and serum Potassium (sP);
- -
- Perioperative outcomes: surgical approach (retro/transperitoneal), minimally-invasive technique (laparoscopic/robot-assisted), tumor size, operating time (OR), estimated blood loss (EBL), length of hospital stay (LOS), intraoperative transfusions rate, postoperative complications, and histopathological diagnosis;
- -
- Functional outcomes: clinical and biochemical success according to standardized PASO criteria [27] (complete, partial, absent), postoperative SBP, DBP, postoperative hypokalemia, sA, sRA, sP, and recurrence rate.
2.4. Data Analysis
3. Results
3.1. Study Selection
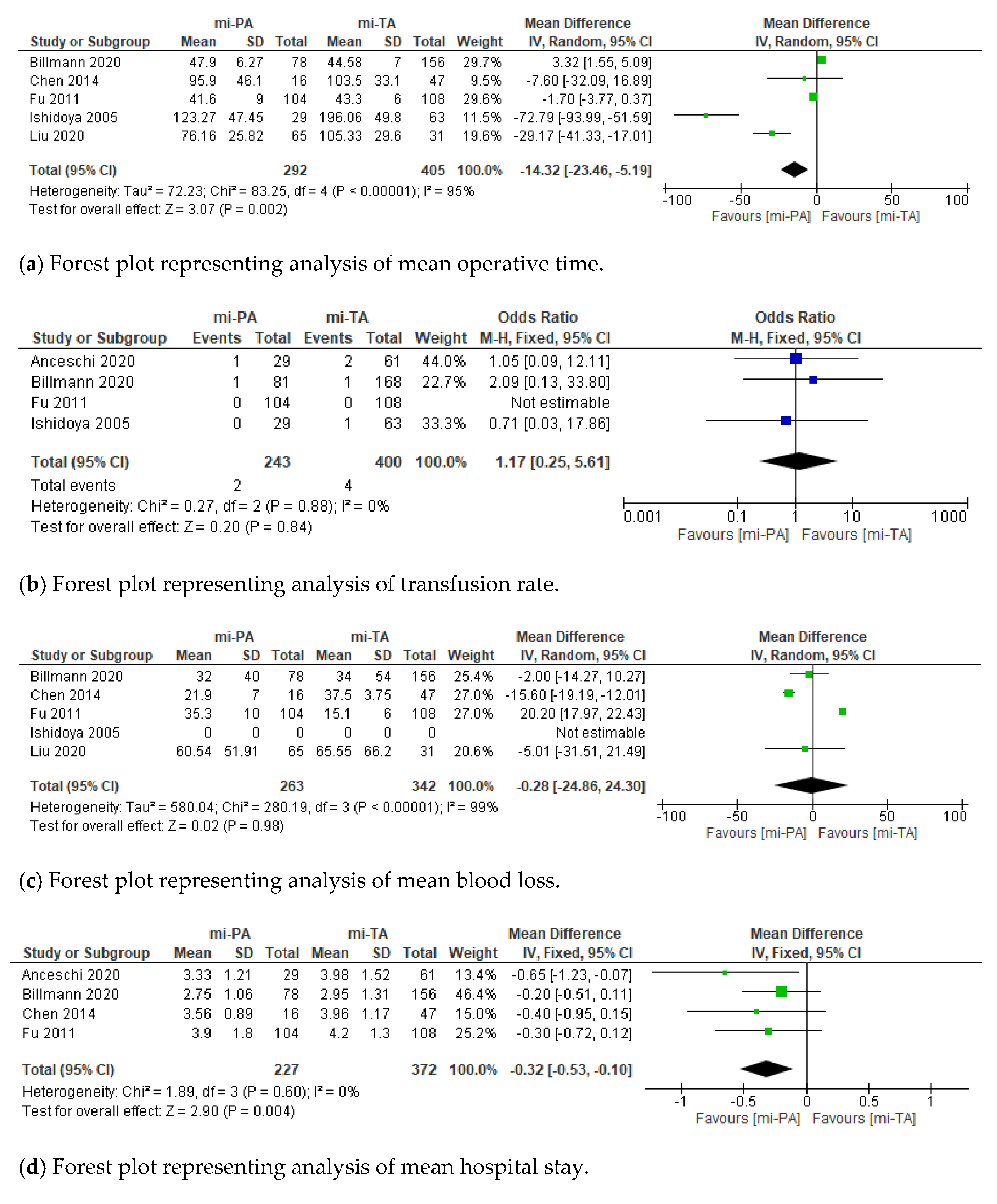
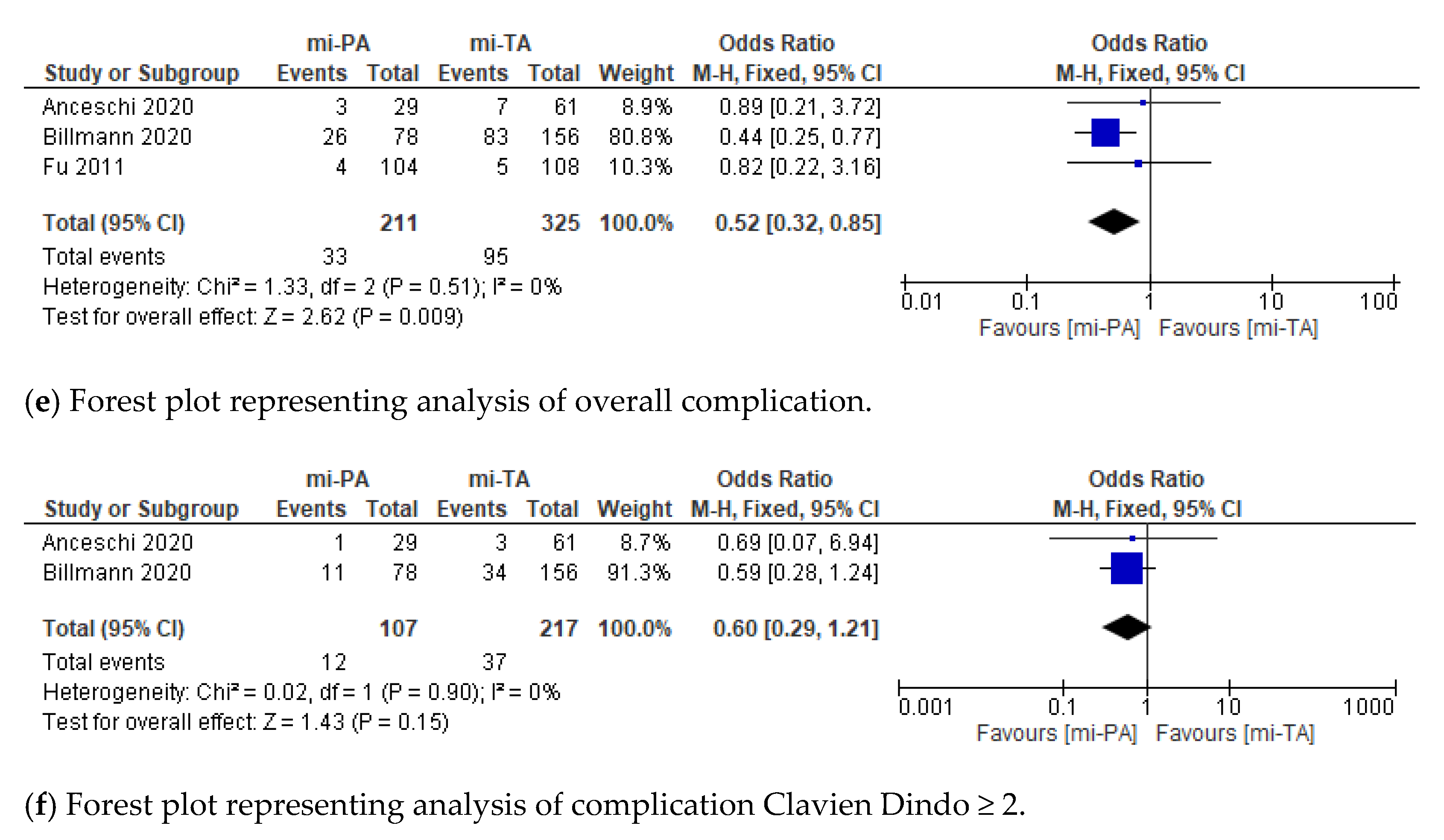
3.2. Perioperative Outcomes
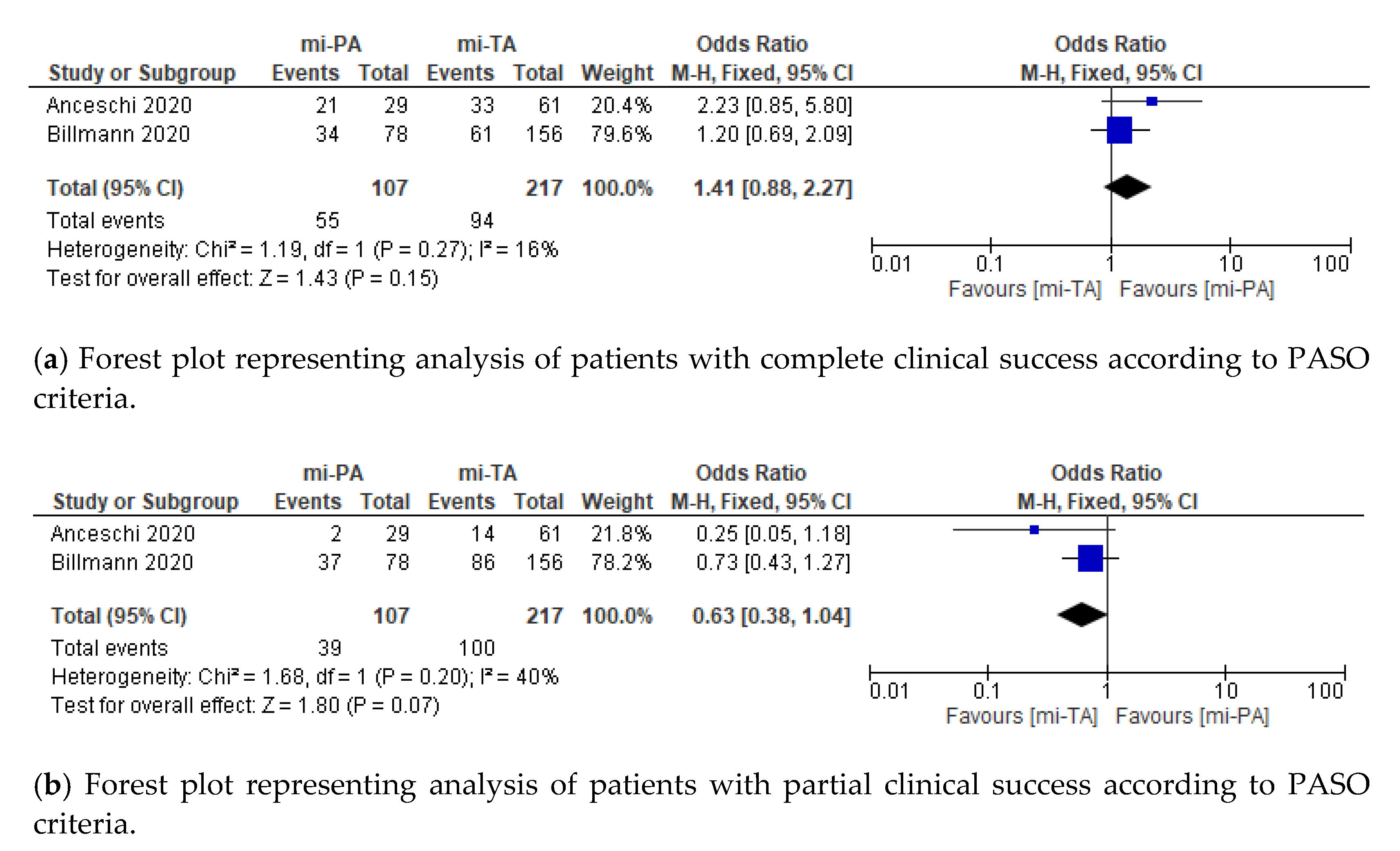
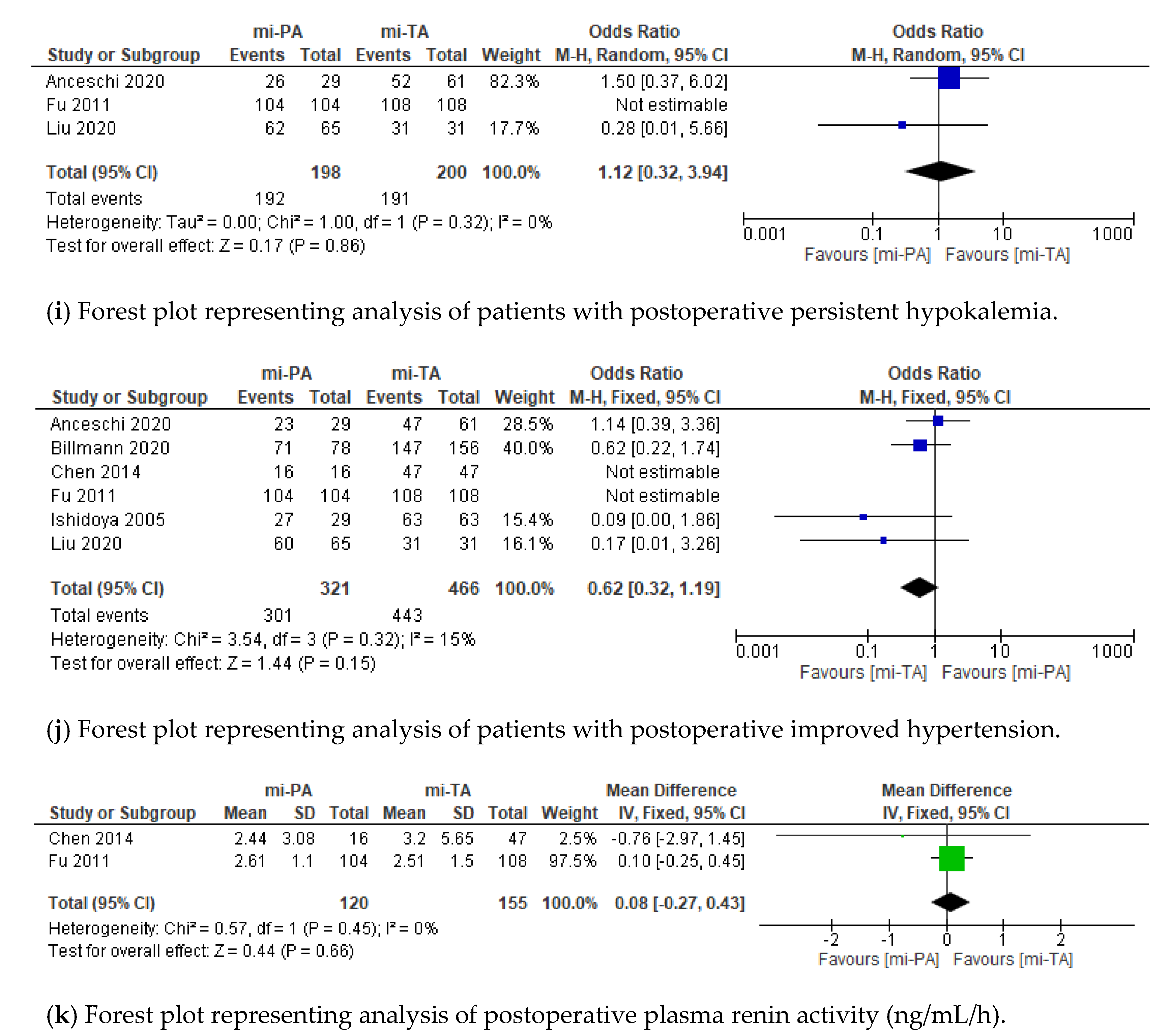
3.3. Functional Outcomes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fuchs, F.D.; Whelton, P.K. High Blood Pressure and Cardiovascular Disease. Hypertension 2020, 75, 285–292. [Google Scholar] [CrossRef]
- Rossi, G.P. Primary Aldosteronism: JACC State-of-the-Art Review. J. Am. Coll. Cardiol. 2019, 74, 2799–2811. [Google Scholar] [CrossRef]
- Rossi, G.P.; Bernini, G.; Caliumi, C.; Desideri, G.; Fabris, B.; Ferri, C.; Ganzaroli, C.; Giacchetti, G.; Letizia, C.; Maccario, M.; et al. A Prospective Study of the Prevalence of Primary Aldosteronism in 1,125 Hypertensive Patients. J. Am. Coll. Cardiol. 2006, 48, 2293–2300. [Google Scholar] [CrossRef] [PubMed]
- Mulatero, P.; Stowasser, M.; Loh, K.C.; Fardella, C.E.; Gordon, R.D.; Mosso, L.; Gomez-Sanchez, C.E.; Veglio, F.; Young, W.F. Increased diagnosis of primary aldosteronism, including surgically correctable forms, in centers from five continents. J. Clin. Endocrinol. Metab. 2004, 89, 1045–1050. [Google Scholar] [CrossRef] [Green Version]
- Lim, P.O.; Rodgers, P.; Cardale, K.; Watson, A.D.; MacDonald, T.M. Potentially high prevalence of primary aldosteronism in a primary-care population. Lancet 1999, 353, 40. [Google Scholar] [CrossRef]
- Meng, Z.; Dai, Z.; Huang, K.; Xu, C.; Zhang, Y.-G.; Zheng, H.; Liu, T.-Z. Long-Term Mortality for Patients of Primary Aldosteronism Compared with Essential Hypertension: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2020, 11, 121. [Google Scholar] [CrossRef]
- Funder, J.W.; Carey, R.M.; Mantero, F.; Murad, M.H.; Reincke, M.; Shibata, H.; Stowasser, M.; Young, W.F. The management of primary aldosteronism: Case detection, diagnosis, and treatment: An endocrine society clinical practice guideline. J. Clin. Endocrinol. Metab. 2016, 101, 1889–1916. [Google Scholar] [CrossRef] [PubMed]
- Williams, T.A.; Reincke, M. Diagnosis and management of primary aldosteronism: The endocrine society guideline 2016 revisited. Eur. J. Endocrinol. 2018, 179, R19–R29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ball, M.W.; Hemal, A.K.; Allaf, M.E. International Consultation on Urological Diseases and European Association of Urology International Consultation on Minimally Invasive Surgery in Urology: Laparoscopic and robotic adrenalectomy. BJU Int. 2017, 119, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Anceschi, U.; Tuderti, G.; Fiori, C.; Zappalà, O.; Ferriero, M.C.; Brassetti, A.; Carrara, A.; Tirone, G.; De Concilio, B.; Celia, A.; et al. Minimally Invasive Partial versus Total Adrenalectomy for the Treatment of Primary Aldosteronism: Results of a Multicenter Series According to the PASO Criteria. Eur. Urol. Focus 2021, 7, 1418–1423. [Google Scholar] [CrossRef]
- Liu, J.H.; Wei, X.D.; Fu, C.C.; Li, Q.X.; Hou, J.Q.; Lv, J.X.; Huang, Y. Long-Term Results of Laparoscopic Partial Versus Total Adrenalectomy for Aldosterone Producing Adenoma. Urol. J. 2020, 17, 4981. [Google Scholar] [CrossRef]
- Jeschke, K.; Janetschek, G.; Peschel, R.; Schellander, L.; Bartsch, G.; Henning, K. Laparoscopic partial adrenalectomy in patients with aldosterone-producing adenomas: Indications, technique, and results. Urology 2003, 61, 69–72. [Google Scholar] [CrossRef]
- Nakada, T.; Kubota, Y.; Sasagawa, I.; Yagisawa, T.; Watanabe, M.; Ishigooka, M. Therapeutic Outcome of Primary Aldosteronism: Adrenalectomy versus Enucleation of Aldosterone-Producing Adenoma. J. Urol. 1995, 153, 1775–1780. [Google Scholar] [CrossRef]
- Walz, M.K.; Gwosdz, R.; Levin, S.L.; Alesina, P.F.; Suttorp, A.-C.; Metz, K.A.; Wenger, F.A.; Petersenn, S.; Mann, K.; Schmid, K.W. Retroperitoneoscopic adrenalectomy in Conn’s syndrome caused by adrenal adenomas or nodular hyperplasia. World J. Surg. 2008, 32, 847–853. [Google Scholar] [CrossRef]
- Kaye, D.R.; Storey, B.B.; Pacak, K.; Pinto, P.A.; Linehan, W.M.; Bratslavsky, G. Partial adrenalectomy: Underused first line therapy for small adrenal tumors. J. Urol. 2010, 184, 18–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simone, G.; Anceschi, U.; Tuderti, G.; Misuraca, L.; Celia, A.; De Concilio, B.; Costantini, M.; Stigliano, A.; Minisola, F.; Ferriero, M.; et al. Robot-assisted Partial Adrenalectomy for the Treatment of Conn’s Syndrome: Surgical Technique, and Perioperative and Functional Outcomes. Eur. Urol. 2019, 75, 811–816. [Google Scholar] [CrossRef]
- Anceschi, U.; Simone, G. Reply to Franco Gaboardi, Guglielmo Mantica, and Nazareno Suardi’s Letter to the Editor re: Giuseppe Simone, Umberto Anceschi, Gabriele Tuderti, et al. Robot-assisted Partial Adrenalectomy for the Treatment of Conn’s Syndrome: Surgical Technique, and Perioperative and Functional Outcomes. Eur Urol 2019;75:811-6. Eur. Urol. 2019, 76, e144–e145. [Google Scholar] [CrossRef]
- Nagaraja, V.; Eslick, G.D.; Edirimanne, S. Recurrence and functional outcomes of partial adrenalectomy: A systematic review and meta-analysis. Int. J. Surg. 2015, 16, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Muth, A.; Ragnarsson, O.; Johannsson, G.; Wängberg, B. Systematic review of surgery and outcomes in patients with primary aldosteronism. Br. J. Surg. 2015, 102, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Li, K.-P.; Duan, X.; Yang, X.-S.; Huang, J.; Wu, T. Partial versus total adrenalectomy for the treatment of unilateral aldosterone-producing adenoma: A systematic review and meta-analysis. Updates Surg. 2021, 73, 2301–2313. [Google Scholar] [CrossRef]
- Liberati, A.; Altman, D.G.; Tetzlaff, J.; Mulrow, C.; Gøtzsche, P.C.; Ioannidis, J.P.A.; Clarke, M.; Devereaux, P.J.; Kleijnen, J.; Moher, D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: Explanation and elaboration. Ann. Intern. Med. 2009, 151. [Google Scholar] [CrossRef] [Green Version]
- Eriksen, M.B.; Frandsen, T.F. The impact of patient, intervention, comparison, outcome (Pico) as a search strategy tool on literature search quality: A systematic review. J. Med. Libr. Assoc. 2018, 106, 420–431. [Google Scholar] [CrossRef]
- Frandsen, T.F.; Bruun Nielsen, M.F.; Lindhardt, C.L.; Eriksen, M.B. Using the full PICO model as a search tool for systematic reviews resulted in lower recall for some PICO elements. J. Clin. Epidemiol. 2020, 127, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higgins, J.P.; Savović, J.; Page, M.J.; Elbers, R.G.; Sterne, J.A. Assessing risk of bias in a randomized trial. In Cochrane Handbook for Systematic Reviews of Interventions; Wiley: Hoboken, NJ, USA, 2019; pp. 205–228. [Google Scholar]
- Phillips, B.; Ball, C.; Sackett, D. Levels of Evidence Andgrades of Recommendation; Oxford Centre for Evidence-based Medicine: Oxford, UK, 2009. [Google Scholar]
- Williams, T.A.; Lenders, J.W.M.; Mulatero, P.; Burrello, J.; Rottenkolber, M.; Adolf, C.; Satoh, F.; Amar, L.; Quinkler, M.; Deinum, J.; et al. Outcomes after adrenalectomy for unilateral primary aldosteronism: An international consensus on outcome measures and analysis of remission rates in an international cohort. Lancet Diabetes Endocrinol. 2017, 5, 689–699. [Google Scholar] [CrossRef] [Green Version]
- Higgins, J.P.T.; Thompson, S.G.; Deeks, J.J.; Altman, D.G. Measuring inconsistency in meta-analyses. Br. Med. J. 2003, 327, 557–560. [Google Scholar] [CrossRef] [Green Version]
- McGrath, S.; Zhao, X.F.; Steele, R.; Thombs, B.D.; Benedetti, A.; Levis, B.; Riehm, K.E.; Saadat, N.; Levis, A.W.; Azar, M.; et al. Estimating the sample mean and standard deviation from commonly reported quantiles in meta-analysis. Stat. Methods Med. Res. 2020, 29, 2520–2537. [Google Scholar] [CrossRef] [Green Version]
- Liao, C.-H.; Chung, S.-D.; Lai, M.-K.; Yu, H.-J.; Chueh, S.-C. Laparoscopic simultaneous bilateral partial and total adrenalectomy: A longer follow-up. BJU Int. 2009, 104, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liang, Y.; Lin, W.; Fu, G.-Q.; Ma, Z.-W. Surgical management of large adrenal tumors: Impact of different laparoscopic approaches and resection methods on perioperative and long-term outcomes. BMC Urol. 2018, 18, 31. [Google Scholar] [CrossRef] [Green Version]
- Balci, M.; Tuncel, A.; Aslan, Y.; Aykanat, C.; Berker, D.; Guzel, O. Laparoscopic Partial versus Total Adrenalectomy in Nonhereditary Unilateral Adrenal Masses. Urol. Int. 2020, 104, 75–80. [Google Scholar] [CrossRef] [PubMed]
- Simforoosh, N.; Soltani, M.H.; Shemshaki, H.; Bonakdar Hashemi, M.; Dadpour, M.; Kashi, A.H. Symptom Resolution and Recurrence Outcomes after Partial versus Total Laparoscopic Adrenalectomy: 13 years of Experience with Medium-Long Term Follow up. Urol. J. 2020, 18, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Fu, B.; Zhang, X.; Wang, G.; Lang, B.; Ma, X.; Li, H.; Wang, B.; Shi, T.; Ai, X.; Zhou, H.; et al. Long-term results of a prospective, randomized trial comparing retroperitoneoscopic partial versus total adrenalectomy for aldosterone producing adenoma. J. Urol. 2011, 185, 1578–1582. [Google Scholar] [CrossRef]
- Chen, S.-F.; Chueh, S.-C.; Wang, S.-M.; Wu, V.-C.; Pu, Y.-S.; Wu, K.-D.; Huang, K.-H. Clinical outcomes in patients undergoing laparoscopic adrenalectomy for unilateral aldosterone producing adenoma: Partial versus total adrenalectomy. J. Endourol. 2014, 28, 1103–1106. [Google Scholar] [CrossRef]
- Billmann, F.; Billeter, A.; Thomusch, O.; Keck, T.; El Shishtawi, S.; Langan, E.A.; Strobel, O.; Müller-Stich, B.P. Minimally invasive partial versus total adrenalectomy for unilateral primary hyperaldosteronism-a retrospective, multicenter matched-pair analysis using the new international consensus on outcome measures. Surgery 2021, 169, 1361–1370. [Google Scholar] [CrossRef]
- Ishidoya, S.; Ito, A.; Sakai, K.; Satoh, M.; Chiba, Y.; Sato, F.; Arai, Y. Laparoscopic partial versus total adrenalectomy for aldosterone producing adenoma. J. Urol. 2005, 174, 40–43. [Google Scholar] [CrossRef]
- Proye, C.A.G.; Mulliez, E.A.R.; Carnaille, B.M.L.; Lecomte-Houcke, M.; Decoulx, M.; Wemeau, J.L.; Lefebvre, J.; Racadot, A.; Ernst, O.; Huglo, D.; et al. Essential hypertension: First reason for persistent hypertension after unilateral adrenalectomy for primary aldosteronism? Surgery 1998, 124, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Rossi, G.P.; Bolognesi, M.; Rizzoni, D.; Seccia, T.M.; Piva, A.; Porteri, E.; Tiberio, G.A.M.; Giulini, S.M.; Agabiti-Rosei, E.; Pessina, A.C. Vascular remodeling and duration of hypertension predict outcome of adrenalectomy in primary aldosteronism patients. Hypertension 2008, 51, 1366–1371. [Google Scholar] [CrossRef] [Green Version]
- Steichen, O.; Zinzindohoué, F.; Plouin, P.F.; Amar, L. Outcomes of adrenalectomy in patients with unilateral primary aldosteronism: A review. Horm. Metab. Res. 2012, 44, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Vorselaars, W.M.C.M.; van Beek, D.J.; Postma, E.L.; Spiering, W.; Borel Rinkes, I.H.M.; Valk, G.D.; Vriens, M.R.; Zarnegar, R.; Drake, F.T.; Duh, Q.Y.; et al. Clinical outcomes after surgery for primary aldosteronism: Evaluation of the PASO-investigators’ consensus criteria within a worldwide cohort of patients. Surgery 2019, 166, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Sellgren, F.; Koman, A.; Nordenström, E.; Hellman, P.; Hennings, J.; Muth, A. Outcomes after Surgery for Unilateral Dominant Primary Aldosteronism in Sweden. World J. Surg. 2020, 44, 561–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, B.S.; Turcu, A.F.; Nanba, A.T.; Hughes, D.T.; Cohen, M.S.; Gauger, P.G.; Auchus, R.J. Refining the Definitions of Biochemical and Clinical Cure for Primary Aldosteronism Using the Primary Aldosteronism Surgical Outcome (PASO) Classification System. World J. Surg. 2018, 42, 453–463. [Google Scholar] [CrossRef] [PubMed]
- Ettehad, D.; Emdin, C.A.; Kiran, A.; Anderson, S.G.; Callender, T.; Emberson, J.; Chalmers, J.; Rodgers, A.; Rahimi, K. Blood pressure lowering for prevention of cardiovascular disease and death: A systematic review and meta-analysis. Lancet 2016, 387, 957–967. [Google Scholar] [CrossRef] [Green Version]
- Brunström, M.; Carlberg, B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels a systematic review and meta-analysis. JAMA Intern. Med. 2018, 178, 28–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Reincke, M.; Williams, T.A. Treatment of Unilateral PA by Adrenalectomy: Potential Reasons for Incomplete Biochemical Cure. Exp. Clin. Endocrinol. Diabetes 2019, 127, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Ferriere, A.; Kerlan, V.; Tabarin, A. La chirurgie d’épargne surrénalienne: Du cortex à la médulla. Ann. Endocrinol. 2017, 78, S11–S20. [Google Scholar] [CrossRef]
- Brandao, L.F.; Autorino, R.; Laydner, H.; Haber, G.P.; Ouzaid, I.; De Sio, M.; Perdonà, S.; Stein, R.J.; Porpiglia, F.; Kaouk, J.H. Robotic versus laparoscopic adrenalectomy: A systematic review and meta-analysis. Eur. Urol. 2014, 65, 1154–1161. [Google Scholar] [CrossRef] [PubMed]
- Merseburger, A.S.; Herrmann, T.R.W.; Shariat, S.F.; Kyriazis, I.; Nagele, U.; Traxer, O.; Liatsikos, E.N. EAU guidelines on robotic and single-site surgery in Urology. Eur. Urol. 2013, 64, 277–291. [Google Scholar] [CrossRef] [PubMed]
- Cacciamani, G.E.; Shakir, A.; Tafuri, A.; Gill, K.; Han, J.; Ahmadi, N.; Hueber, P.A.; Gallucci, M.; Simone, G.; Campi, R.; et al. Best practices in near-infrared fluorescence imaging with indocyanine green (NIRF/ICG)-guided robotic urologic surgery: A systematic review-based expert consensus. World J. Urol. 2020, 38, 883–896. [Google Scholar] [CrossRef] [PubMed]
- Manny, T.B.; Pompeo, A.S.; Hemal, A.K. Robotic partial adrenalectomy using indocyanine green dye with near-infrared imaging: The initial clinical experience. Urology 2013, 82, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Colvin, J.; Zaidi, N.; Berber, E. The utility of indocyanine green fluorescence imaging during robotic adrenalectomy. J. Surg. Oncol. 2016, 114, 153–156. [Google Scholar] [CrossRef]
- Young, W.F.; Stanson, A.W.; Thompson, G.B.; Grant, C.S.; Farley, D.R.; Van Heerden, J.A. Role for adrenal venous sampling in primary aldosteronism. Surgery 2004, 136, 1227–1235. [Google Scholar] [CrossRef] [PubMed]


| Author | Year | Study Design | Institution | Patients | Time | Surgical Procedure mi-PA vs. mi-TA | Surgical Technique | Follow-Up (mo) mi-PA vs. mi-TA | Inclusion Criteria | Exclusion Criteria | Diagnostic Test | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anceschi et al. | 2020 | Retrospective | Multiple | 90 | 2011–2020 | 29 vs. 61 | Robotic (24) Lap (85) | 41 vs. 46 | (a) Primary Hyperaldosteronism; (b) Single and unilateral adrenal mass | (a) Follow-up < 18 mo (b) Bilateral adenomas (c) Malignant histology | (a) CT and/or MRI (b) AVS ° | Perioperative and Functional outcomes * |
| Billmann et al. | 2020 | Retrospective matched-cohorts | Multiple | 249 | 2008–2018 | 81 vs. 168 | Lap | 22.8 vs. 24.8 | (a) Unilateral Primary Aldosteronism (b) adrenal mass<6cm | (a) Follow-up < 60 mo (b) malignant histology (c) no data on extent of adrenal resection | (a) CT and/or MRI (b) AVS ° (c) Biochemical test | Perioperative and Functional outcomes * |
| Liu et al. | 2020 | Retrospective | Single | 96 | 2012–2017 | 65 vs. 31 | Lap | 32.3 vs. 40.8 | (a) APA | (a) IHA (b) Bilateral adenoma (c) UAH | (a) CT scan (b) Biochemical test | Perioperative and Functional outcome |
| Chen et al. | 2014 | Prospective | Single | 63 | 2008–2011 | 16 vs. 47 | Lap | 12 | (a) Primary Aldosteronism (b) Unilateral adrenal mass | NA | (a) CT scan (b) AVS ° or Scintigraphy (c) Biochemical test | Perioperative and Functional outcomes * |
| Fu et al. | 2011 | RCT | Single | 212 | 2000–2004 | 104 vs. 108 | Lap | 96 | (a) APA | (a) Previous ipsilateral adrenal surgery; (b) Doubtful BAH | (a) CT and/or MRI (b) AVS ° (c) Biochemical test | Perioperative and Functional outcome |
| Ishidoya et al. | 2005 | Retrospective | Single | 92 | 1995–2004 | 29 vs. 63 | Lap | 60.3 vs. 29.3 | (a) APA | (a) IHA | (a) CT scan (b) AVS ° (c) Biochemical test | Parioperative outcome |
| APA = aldosterone producing adenoma, IHA = idiopathic aldosteronism, UAH = unilateral adrenal hypeplasia, mo = months. * Use of PASO (Primary Aldosteronism Surgical Outcome study) criteria for the assessment of Clinical and/or Biochemical success. ° Adrenal venous sampling (AVS) was not performed in all patients and no clear indication were reported in most studies. | ||||||||||||
| Author | Patients | Age | Gender (M:F) | Tumor Size (cm) | Side (R:L) | ASA (≤2:>2) or Mean (SD) | BMI | Surgical Approach (Rt:Tr) | HYT Duration (years) | ant-HYT Drugs (n°) | SBP (mmHg) | DBO (mmHg) | sP (mmol/L) | sA (ng/dL) | sRA (ng/mL/h) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Anceschi et al., 2020 | PA (29) | median (IQR) 57 (43.5–67.5) | 13:16 | median (IQR) 2.7 (1.8–2.85) | 7:22 | 23;6 | NA | 2:27 | NA | NA | NA | NA | NA | NA | NA |
| TA (61) | median (IQR) 54 (44.5–63) | 23:38 | median (IQR) 4.2 (2.35–6) | 38:23 | 50;11 | NA | 20:41 | NA | NA | NA | NA | NA | NA | NA | |
| Billmann et al., 2020 | PA (81) | mean (SD) 45.9 (14) | 46:35 | mean (SD) 1.8 (1.6) | 36:45 | 80;1 | mean (SD) 27 (4) | NA | median (range) 8 (2–12) | median (range) 3 (2–5) | NA | NA | NA | NA | NA |
| TA (168) | mean (SD) 53.1 (15) | 69:99 | mean (SD) 3.4 (2.3) | 81:87 | 158;8 | mean (SD) 25 (5) | NA | median (range) 8 (2–13) | median (range) 3 (2–5) | NA | NA | NA | NA | NA | |
| Liu et al., 2020 | PA (65) | mean (SD) 48.27 (11.47) | 22:43 | mean (SD) 1.86 (0.65) | 27:38 | NA | NA | 65:0 | NA | NA | mean (SD) 166.52 (24.08) | mean (SD) 101 (14.12) | mean (SD) 2.55 (0.72) | mean (SD) 26.65 (6.89) | NA |
| TA (31) | mean (SD) 56.42 (16.42) | 12:19 | mean (SD) 1.96 (0.96) | 13:18 | NA | NA | 31:0 | NA | NA | mean (SD) 174 (36.06) | mean (SD) 100.75 (21.44) | mean (SD) 2.37 (0.63) | mean (SD) 27.29 (7.60) | NA | |
| Chen et al., 2014 | PA (16) | mean (SD) 48.5 (10.9) | 7:9 | mean (SD) 2.02 (0.91) | 11:5 | NA | mean (SD) 23.3 (2.61) | 0:16 | NA | NA | mean (SD) 150.4 (26.9) | mean (SD) 92.1 (14) | mean (SD) 3.52 (0.63) | mean (SD) 57.3 (24.8) | mean (SD) 0.43 (0.82) |
| TA (47) | mean (SD) 48.7 (11.3) | 21:26 | mean (SD) 2.10 (0.83) | 25:22 | NA | mean (SD) 23.3 (2.60) | 0:47 | NA | NA | mean (SD) 155.3 (23.5) | mean (SD) 93.7 (15.7) | mean (SD) 3.36 (0.76) | mean (SD) 54.8 (37.4) | mean (SD) 0.75 (1.89) | |
| Fu et al., 2011 | PA (104) | mean (SD) 43 (5.8) | 45:59 | mean (SD) 1.9 (0.2) | 58:46 | 2.3 (1.7) | mean (SD) 26.2 (4.2) | 104:0 | mean (SD) 5.9 (1.2) | mean (SD) 2.2 (0.8) | mean (SD) 175.9 (23) | mean (SD) 101.8 (13) | mean (SD) 3 (3,4) | mean (SD) 34.5 (16) | mean (SD) 0.27 (0.4) |
| TA (108) | mean (SD) 41 (7.8) | 48:60 | mean (SD) 1.8 (0.4) | 55:53 | 2.6 (1.3) | mean (SD) 25.7 (3.5) | 108:0 | mean (SD) 6.3 (1.3) | mean (SD) 2.1 (0.8) | Mean (SD) 179.5 (24) | Mean (SD) 108.3 (14) | Mean (SD) 3.1 (0.5) | mean (SD) 32.2 (15) | mean (SD) 0.24 (0.6) | |
| Ishidoya et al., 2005 | PA (29) | median (range) 49.3 (30–68) | 15:14 | median (range) 1.55 (0.6–2.4) | 17:12 | NA | NA | 23:6 | NA | NA | NA | NA | NA | NA | NA |
| TA (63) | median (range) 50.2 (29–75) | 31:32 | median (range) 1.6 (0.3–4) | 25:37 | NA | NA | 0:63 | NA | NA | NA | NA | NA | NA | NA | |
| NA = not avialble; BMI = body mass index; HYT = hypertension; SBP = systolic blood pressure; DBO = diastolic blood pressure; sP = serum potassium, sA = serum aldosterone; sRA = serum Renin Activity. | |||||||||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Flammia, R.S.; Anceschi, U.; Tufano, A.; Bologna, E.; Proietti, F.; Bove, A.M.; Misuraca, L.; Mastroianni, R.; Tirone, G.; Carrara, A.; et al. Minimally Invasive Partial vs. Total Adrenalectomy for the Treatment of Unilateral Primary Aldosteronism: A Systematic Review and Meta-Analysis. J. Clin. Med. 2022, 11, 1263. https://doi.org/10.3390/jcm11051263
Flammia RS, Anceschi U, Tufano A, Bologna E, Proietti F, Bove AM, Misuraca L, Mastroianni R, Tirone G, Carrara A, et al. Minimally Invasive Partial vs. Total Adrenalectomy for the Treatment of Unilateral Primary Aldosteronism: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine. 2022; 11(5):1263. https://doi.org/10.3390/jcm11051263
Chicago/Turabian StyleFlammia, Rocco Simone, Umberto Anceschi, Antonio Tufano, Eugenio Bologna, Flavia Proietti, Alfredo Maria Bove, Leonardo Misuraca, Riccardo Mastroianni, Giuseppe Tirone, Alessandro Carrara, and et al. 2022. "Minimally Invasive Partial vs. Total Adrenalectomy for the Treatment of Unilateral Primary Aldosteronism: A Systematic Review and Meta-Analysis" Journal of Clinical Medicine 11, no. 5: 1263. https://doi.org/10.3390/jcm11051263
APA StyleFlammia, R. S., Anceschi, U., Tufano, A., Bologna, E., Proietti, F., Bove, A. M., Misuraca, L., Mastroianni, R., Tirone, G., Carrara, A., Luciani, L., Cai, T., Leonardo, C., & Simone, G. (2022). Minimally Invasive Partial vs. Total Adrenalectomy for the Treatment of Unilateral Primary Aldosteronism: A Systematic Review and Meta-Analysis. Journal of Clinical Medicine, 11(5), 1263. https://doi.org/10.3390/jcm11051263








