Associations among Ratio of Free Triiodothyronine to Free Thyroxine, Chronic Kidney Disease, and Subclinical Hypothyroidism
Abstract
:1. Introduction
2. Materials and Methods
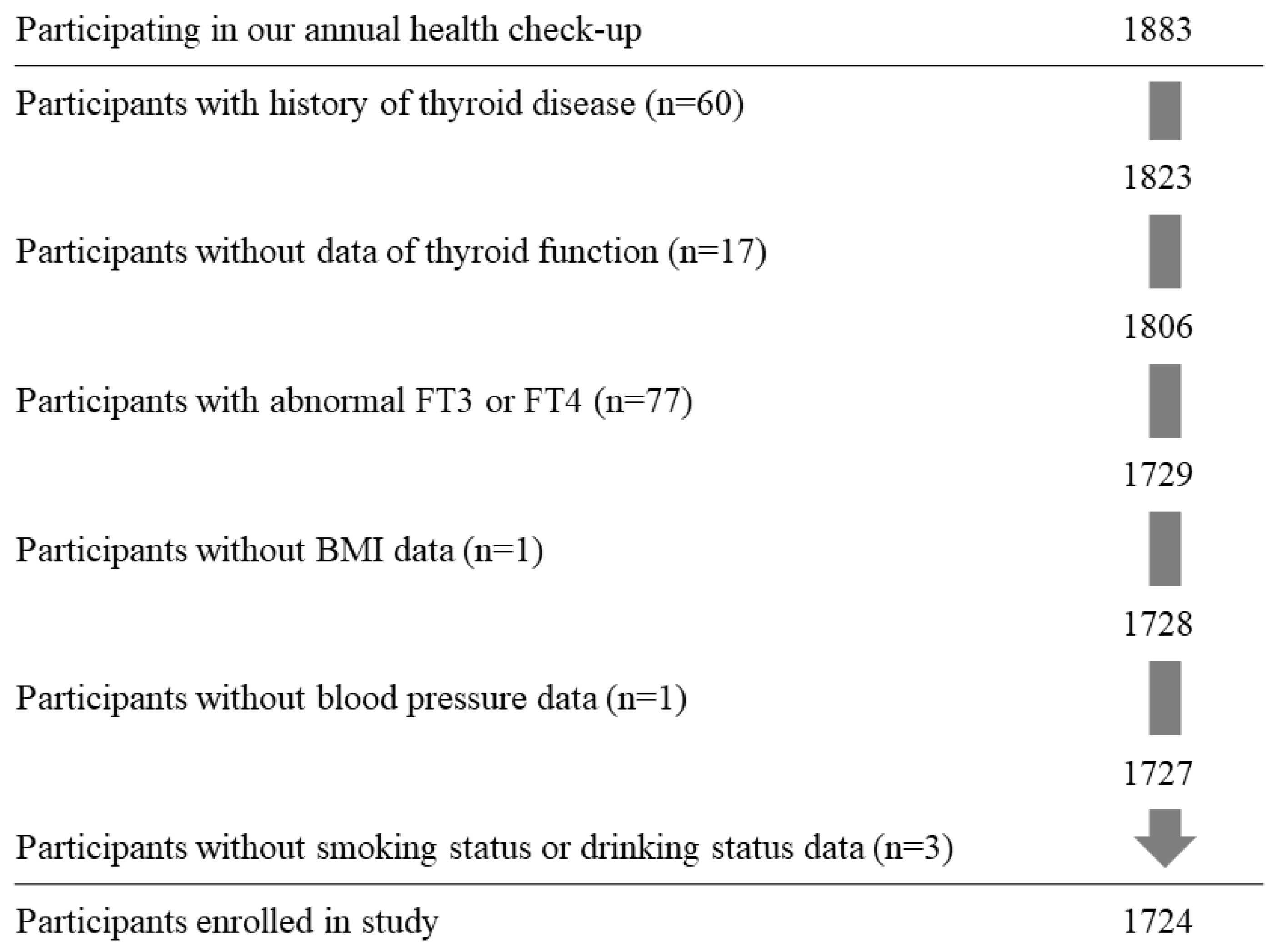
2.1. Study Population
2.2. Data Collection and Laboratory Measurements
2.3. Statistical Analysis
3. Results
3.1. Characteristics of the Study Population by FT3/FT4 Tertile
3.2. Association between SCH and CKD
3.3. Associations between FT3/FT4 and CKD and between FT3/FT4 and SCH
3.4. Association between FT3/FT4 and SCH with or without CKD
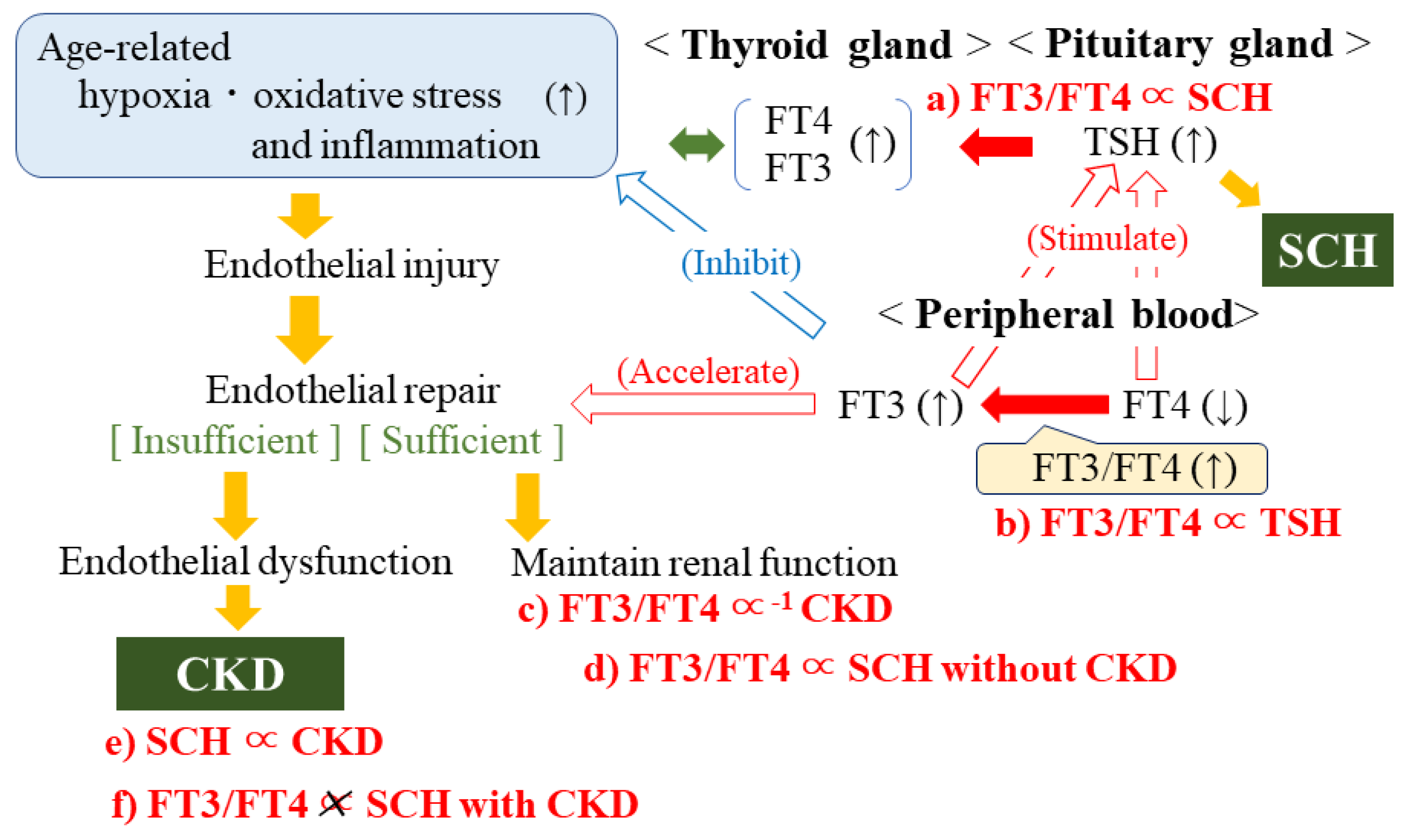
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Braverman, L.E. Werner & Ingbar’s The Thyroid: A Fundamental and Clinical Text, 11th ed.; Lipppincott Williams & Wilkins (LWW): Philadelphia, PA, USA, 2020. [Google Scholar]
- Stanbury, J.B.; Morris, M.L. Deiodination of diiodotyrosine by cell-free systems. J. Biol. Chem. 1958, 233, 106–108. [Google Scholar] [CrossRef]
- Stanbury, J.B.; Morris, M.L. The metabolism of 3:3′-diiodothyronine in man. J. Clin. Endocrinol. Metab. 1957, 17, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Bassols, J.; Prats-Puig, A.; Soriano-Rodríguez, P.; García-González, M.M.; Reid, J.; Martínez-Pascual, M.; Mateos-Comerón, F.; de Zegher, F.; Ibáñez, L.; López-Bermejo, A. Lower free thyroxin associates with a less favorable metabolic phenotype in healthy pregnant women. J. Clin. Endocrinol. Metab. 2011, 96, 3717–3723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuya, F.; Ishii, T.; Tamura, S.; Takahashi, K.; Kobayashi, H.; Ichijo, M.; Takizawa, S.; Kaneshige, M.; Suzuki-Inoue, K.; Kitamura, K. The ligand-bound thyroid hormone receptor in macrophages ameliorates kidney injury via inhibition of nuclear factor-κB activities. Sci. Rep. 2017, 7, 43960. [Google Scholar] [CrossRef] [Green Version]
- Patil, V.P.; Shilpasree, A.S.; Patil, V.S.; Pravinchandra, K.R.; Ingleshwar, D.G.; Vani, A.C. Evaluation of renal function in subclinical hypothyroidism. J. Lab. Physicians 2018, 10, 50–55. [Google Scholar] [CrossRef]
- Rhee, C.M.; Kalantar-Zadeh, K.; Streja, E.; Carrero, J.J.; Ma, J.Z.; Lu, J.L.; Kovesdy, C.P. The relationship between thyroid function and estimated glomerular filtration rate in patients with chronic kidney disease. Nephrol. Dial. Transplant. 2015, 30, 282–287. [Google Scholar] [CrossRef]
- Lo, J.C.; Chertow, G.M.; Go, A.S.; Hsu, C.Y. Increased prevalence of subclinical and clinical hypothyroidism in persons with chronic kidney disease. Kidney Int. 2005, 67, 1047–1052. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Kawashiri, S.Y.; Noguchi, Y.; Nagata, Y.; Maeda, T.; Hayashida, N. Association between thyroid cysts and hypertension by atherosclerosis status: A cross-sectional study. Sci. Rep. 2021, 11, 13922. [Google Scholar] [CrossRef]
- Lu, M.; Yang, C.B.; Gao, L.; Zhao, J.J. Mechanism of subclinical hypothyroidism accelerating endothelial dysfunction (Review). Exp. Ther. Med. 2015, 9, 3–10. [Google Scholar] [CrossRef] [Green Version]
- Endemann, D.H.; Schiffrin, E.L. Endothelial dysfunction. J. Am. Soc. Nephrol. 2004, 15, 1983–1992. [Google Scholar] [CrossRef]
- Shakoor, S.K.; Aldibbiat, A.; Ingoe, L.E.; Campbell, S.C.; Sibal, L.; Shaw, J.; Home, P.D.; Razvi, S.; Weaver, J.U. Endothelial progenitor cells in subclinical hypothyroidism: The effect of thyroid hormone replacement therapy. J. Clin. Endocrinol. Metab. 2010, 95, 319–322. [Google Scholar] [CrossRef] [Green Version]
- Daub, K.; Langer, H.; Seizer, P.; Stellos, K.; May, A.E.; Goyal, P.; Bigalke, B.; Schönberger, T.; Geisler, T.; Siegel-Axel, D.; et al. Platelets induce differentiation of human CD34+ progenitor cells into foam cells and endothelial cells. FASEB J. 2006, 20, 2559–2561. [Google Scholar] [CrossRef] [Green Version]
- Stellos, K.; Langer, H.; Gnerlich, S.; Panagiota, V.; Paul, A.; Schönberger, T.; Ninci, E.; Menzel, D.; Mueller, I.; Bigalke, B.; et al. Junctional adhesion molecule a expressed on human CD34+ cells promotes adhesion on vascular wall and differentiation into endothelial progenitor cells. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1127–1136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ministry of Health, Labour and Welfare. Home Page. Available online: https://www.mhlw.go.jp/english/ (accessed on 20 December 2021).
- Shimizu, Y.; Kawashiri, S.Y.; Noguchi, Y.; Nagata, Y.; Maeda, T.; Hayashida, N. HbA1c is inversely associated with thyroid cysts in a euthyroid population: A cross-sectional study. PLoS ONE 2021, 16, e0253841. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, Y.; Nabeshima-Kimura, Y.; Kawashiri, S.Y.; Noguchi, Y.; Nagata, Y.; Maeda, T.; Hayashida, N. Anti-thyroid peroxidase antibody and thyroid cysts among the general Japanese population: A cross-sectional study. Environ. Health Prev. Med. 2020, 25, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuo, S.; Imai, E.; Horio, M.; Yasuda, Y.; Tomita, K.; Nitta, K.; Yamagata, K.; Tomino, Y.; Yokoyama, H.; Hishida, A.; et al. Revised equations for estimated GFR from serum creatinine in Japan. Am. J. Kidney Dis. 2009, 53, 982–992. [Google Scholar] [CrossRef] [PubMed]
- Jostel, A.; Ryder, W.D.; Shalet, S.M. The use of thyroid function tests in the diagnosis of hypopituitarism: Definition and evaluation of the TSH Index. Clin. Endocrinol. 2009, 71, 529–534. [Google Scholar] [CrossRef]
- Yuasa, R.; Ohashi, Y.; Saito, A.; Tsuboi, K.; Shishido, S.; Sakai, K. Prevalence of hypothyroidism in Japanese chronic kidney disease patients. Ren. Fail. 2020, 42, 572–579. [Google Scholar] [CrossRef]
- Meza, C.A.; La Favor, J.D.; Kim, D.H.; Hickner, R.C. Endothelial Dysfunction: Is There a Hyperglycemia-Induced Imbalance of NOX and NOS? Int. J. Mol. Sci. 2019, 20, 3775. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, Y.; Kawashiri, S.Y.; Noguchi, Y.; Nakamichi, S.; Nagata, Y.; Maeda, T.; Hayashida, N. Effect of Subclinical Hypothyroidism on the Association between Hemoglobin A1c and Reduced Renal Function: A Prospective Study. Diagnostics 2022, 12, 462. [Google Scholar] [CrossRef]
- Yang, S.; Lai, S.; Wang, Z.; Liu, A.; Wang, W.; Guan, H. Thyroid Feedback Quantile-based Index correlates strongly to renal function in euthyroid individuals. Ann. Med. 2021, 53, 1945–1955. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.L.C.; Gruppen, E.G.; van Tienhoven-Wind, L.; Eisenga, M.F.; de Vries, H.; Gansevoort, R.T.; Bakker, S.J.L.; Dullaart, R.P.F. Glomerular filtration rate is associated with free triiodothyronine in euthyroid subjects: Comparison between various equations to estimate renal function and creatinine clearance. Eur. J. Intern. Med. 2018, 48, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Schultheiss, U.T.; Steinbrenner, I.; Nauck, M.; Schneider, M.P.; Kotsis, F.; Baid-Agrawal, S.; Schaeffner, E.; Eckardt, K.U.; Köttgen, A.; Sekula, P. GCKD investigators. Thyroid function, renal events and mortality in chronic kidney disease patients: The German Chronic Kidney Disease study. Clin. Kidney J. 2020, 14, 959–968. [Google Scholar] [CrossRef]
- Kim, B. Thyroid hormone as a determinant of energy expenditure and the basal metabolic rate. Thyroid 2008, 18, 141–144. [Google Scholar] [CrossRef] [PubMed]
- McNab, B.K. What determines the basal rate of metabolism? J. Exp. Biol. 2019, 222 Pt 15, jeb205591. [Google Scholar] [CrossRef] [Green Version]
- Zemirli, N.; Morel, E.; Molino, D. Mitochondrial Dynamics in Basal and Stressful Conditions. Int. J. Mol. Sci. 2018, 19, 564. [Google Scholar] [CrossRef] [Green Version]
| FT3/FT4 Levels (Tertile) | p | |||
|---|---|---|---|---|
| Low | Middle | High | ||
| No. of participants | 573 | 577 | 574 | |
| Men, % | 37.3 | 36.4 | 37.3 | 0.933 |
| Age | 60.6 ± 9.4 | 60.2 ± 9.1 | 60.7 ± 9.0 | 0.552 |
| FT3, pg/mL | 3.0 ± 0.3 | 3.2 ± 0.3 | 3.3 ± 0.3 | <0.001 |
| FT4, ng/dL | 1.4 ± 0.1 | 1.2 ± 0.1 | 1.1 ± 0.1 | <0.001 |
| TSH, μIU/mL | 1.50 [1.05, 2.22] *1 | 1.57 [1.12, 2.24] *1 | 1.64 [1.11, 2.39] *1 | 0.046 *2 |
| Jostel’s TSH index | 2.80 ± 0.64 | 2.61 ± 0.60 | 2.45 ± 0.60 | <0.001 |
| BMI, kg/m2 | 22.7 ± 3.5 | 22.7 ± 3.3 | 22.9 ± 3.3 | 0.489 |
| Daily drinker, % | 42.9 | 39.0 | 39.0 | 0.295 |
| Non-drinker, % | 53.1 | 58.4 | 57.3 | 0.155 |
| Current smoker, % | 12.9 | 13.9 | 14.3 | 0.787 |
| Former smoker, % | 22.2 | 20.8 | 19.7 | 0.586 |
| Systolic blood pressure, mmHg | 125 ± 16 | 125 ± 16 | 125 ± 18 | 0.789 |
| TG, mg/dL | 92 [65, 131] *1 | 84 [63, 123] *1 | 85 [63, 119] *1 | 0.026 *2 |
| HDLc, mg/dL | 61 ± 17 | 60 ± 14 | 60 ± 14 | 0.221 |
| HbA1c, % | 5.7 ± 0.7 | 5.6 ± 0.6 | 5.6 ± 0.6 | 0.058 |
| Subclinical Hypothyroidism (SCH) | p | ||
|---|---|---|---|
| (–) | (+) | ||
| No. at risk | 1626 | 98 | |
| Chronic kidney disease (CKD) | |||
| No. of case (%) | 272 (16.7) | 30 (30.6) | |
| Model 1 | Ref | 2.10 (1.31, 3.35) | 0.002 |
| Model 4 | Ref | 2.23 (1.38, 3.59) | 0.001 |
| FT3/FT4 Levels (Tertile) | p | 1 SD Increment of FT3/FT4 | |||
|---|---|---|---|---|---|
| Low | Middle | High | |||
| No. at risk | 573 | 577 | 574 | ||
| Subclinical hypothyroidism (SCH) | |||||
| No. of case (%) | 24 (4.2) | 33 (5.7) | 41 (7.1) | ||
| Model 1 | Ref | 1.40 (0.82, 2.40) | 1.76 (1.05, 2.95) | 0.032 | 2.33 (1.31, 4.13) |
| Model 4 | Ref | 1.52 (0.87, 2.64) | 1.92 (1.12, 3.26) | 0.018 | 2.40 (1.34, 4.29) |
| Chronic kidney disease (CKD) | |||||
| No. of case (%) | 125 (21.8) | 88 (15.3) | 89 (15.5) | ||
| Model 1 | Ref | 0.66 (0.48, 0.89) | 0.64 (0.47, 0.87) | 0.004 | 0.58 (0.40, 0.83) |
| Model 2 | Ref | 0.64 (0.47, 0.87) | 0.60 (0.44, 0.82) | 0.001 | 0.52 (0.36, 0.75) |
| Model 3 | Ref | 0.64 (0.46, 0.87) | 0.58 (0.42, 0.80) | <0.001 | 0.51 (0.35, 0.74) |
| FT3/FT4 Levels (Tertile) | p | 1 SD Increment of FT3/FT4 | |||
|---|---|---|---|---|---|
| Low | Middle | High | |||
| No. at risk | 573 | 577 | 574 | ||
| Subclinical hypothyroidism (SCH) without chronic kidney disease (CKD) | |||||
| No. of case (%) | 12 (2.1) | 26 (4.5) | 30 (5.2) | ||
| Model 1 | Ref | 2.20 (1.10, 4.41) | 2.58 (1.31, 5.09) | 0.007 | 3.17 (1.60, 6.30) |
| Model 4 | Ref | 2.54 (1.24, 5.20) | 2.98 (1.47, 6.05) | 0.003 | 3.44 (1.72, 6.91) |
| Subclinical hypothyroidism (SCH) with chronic kidney disease (CKD) | |||||
| No. of case (%) | 12 (2.1) | 7 (1.2) | 11 (1.9) | ||
| Model 1 | Ref | 0.60 (0.23, 1.53) | 0.92 (0.40, 2.12) | 0.839 | 1.16 (0.43, 3.17) |
| Model 4 | Ref | 0.59 (0.23, 1.54) | 0.91 (0.39, 2.14) | 0.833 | 1.11 (0.40, 3.06) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimizu, Y.; Kawashiri, S.-Y.; Noguchi, Y.; Nakamichi, S.; Nagata, Y.; Hayashida, N.; Maeda, T. Associations among Ratio of Free Triiodothyronine to Free Thyroxine, Chronic Kidney Disease, and Subclinical Hypothyroidism. J. Clin. Med. 2022, 11, 1269. https://doi.org/10.3390/jcm11051269
Shimizu Y, Kawashiri S-Y, Noguchi Y, Nakamichi S, Nagata Y, Hayashida N, Maeda T. Associations among Ratio of Free Triiodothyronine to Free Thyroxine, Chronic Kidney Disease, and Subclinical Hypothyroidism. Journal of Clinical Medicine. 2022; 11(5):1269. https://doi.org/10.3390/jcm11051269
Chicago/Turabian StyleShimizu, Yuji, Shin-Ya Kawashiri, Yuko Noguchi, Seiko Nakamichi, Yasuhiro Nagata, Naomi Hayashida, and Takahiro Maeda. 2022. "Associations among Ratio of Free Triiodothyronine to Free Thyroxine, Chronic Kidney Disease, and Subclinical Hypothyroidism" Journal of Clinical Medicine 11, no. 5: 1269. https://doi.org/10.3390/jcm11051269






