Efficacy of Arthroscopic Shavers for the Retrieval and Processing of Connective Tissue Progenitor Cells from Subacromial Bursal Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Selection
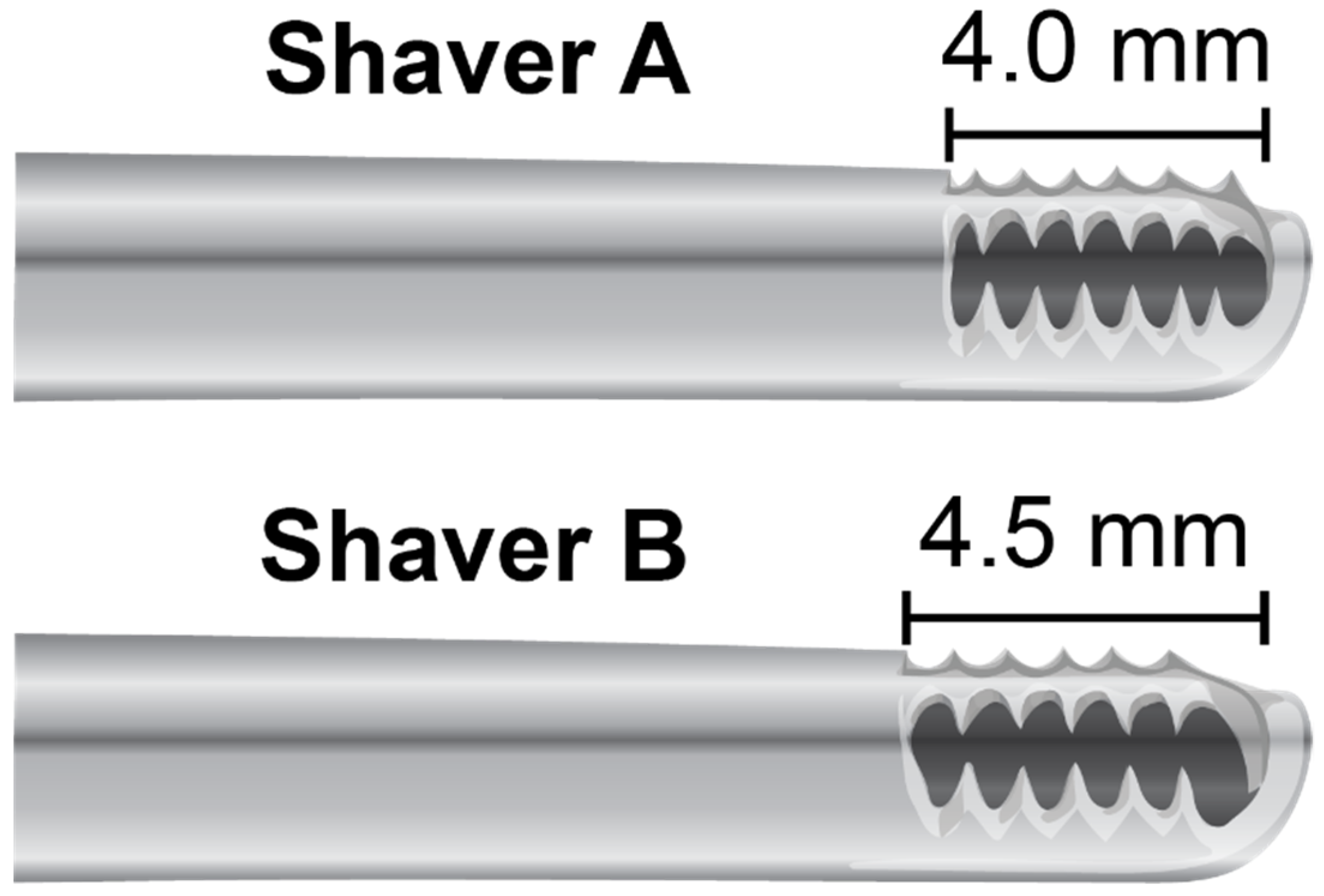
2.2. Harvesting of Subacromial Bursa Tissue
2.3. Colony-Forming Units
2.4. Cellular Concentration
2.5. Cellular Proliferation
2.6. FACS Analysis
2.7. Live/Dead Assay
2.8. Cytokine and Growth Factor Analysis
2.9. Statistics
3. Results
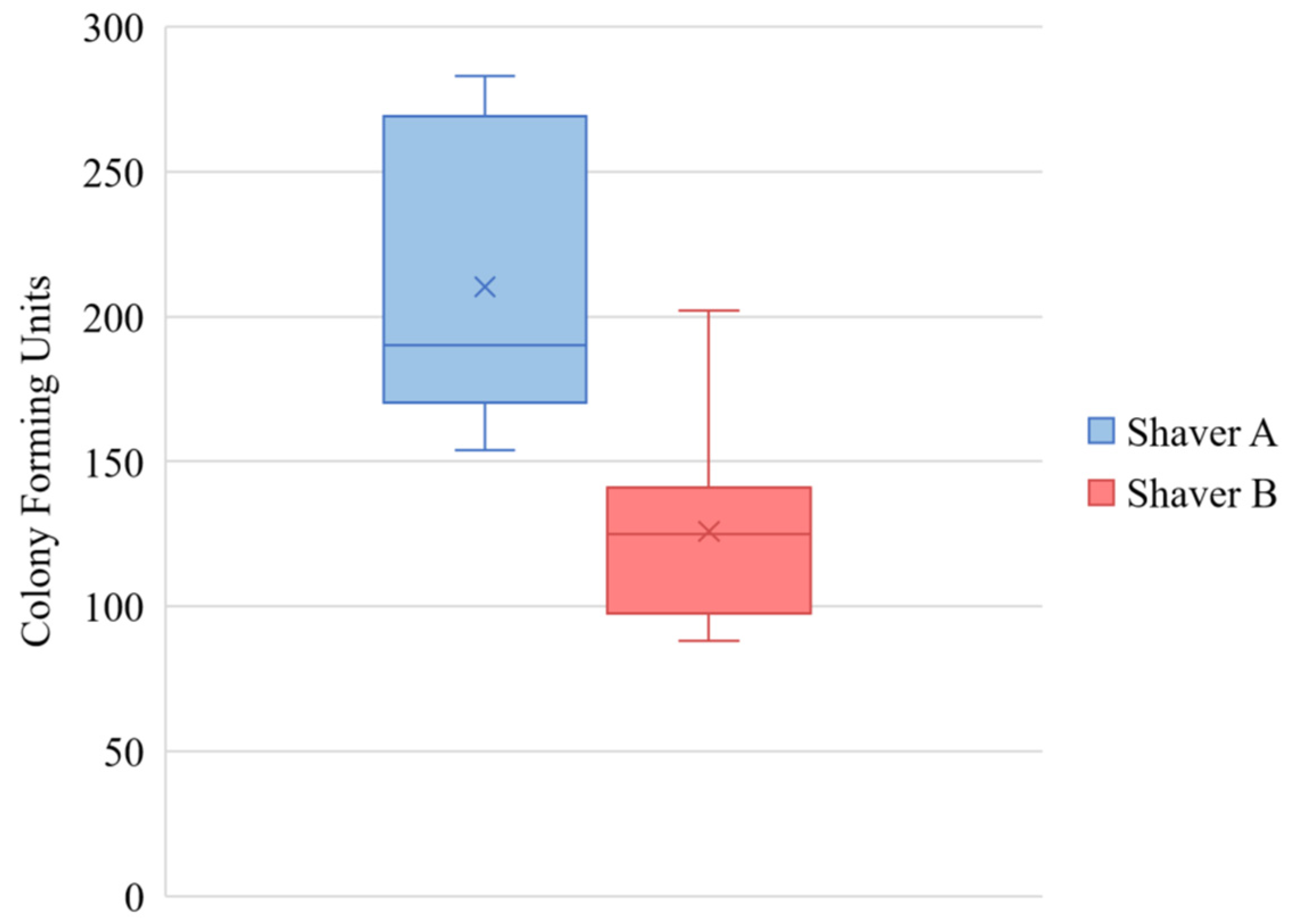
3.1. Colony Forming Units
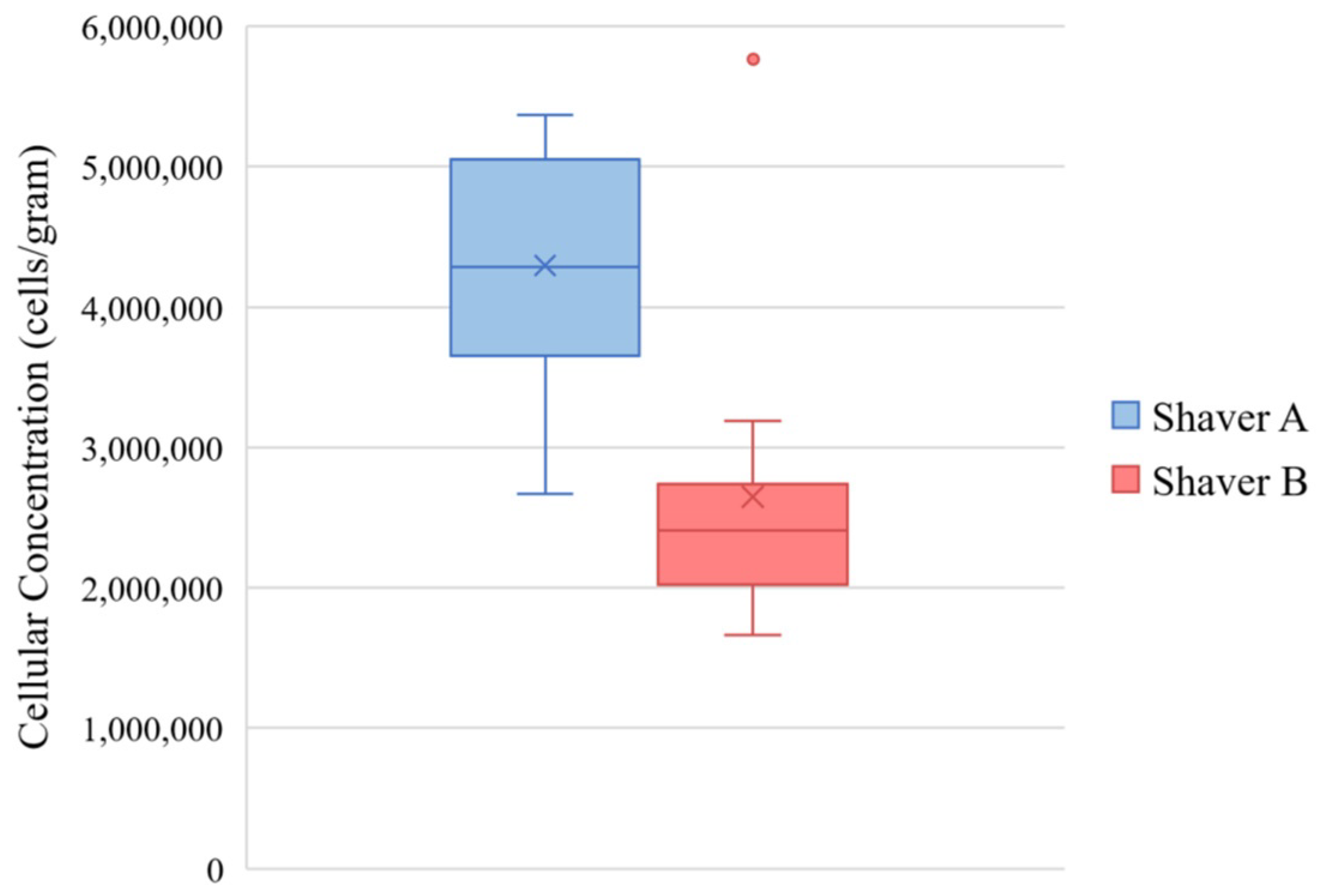
3.2. Cellular Concentration
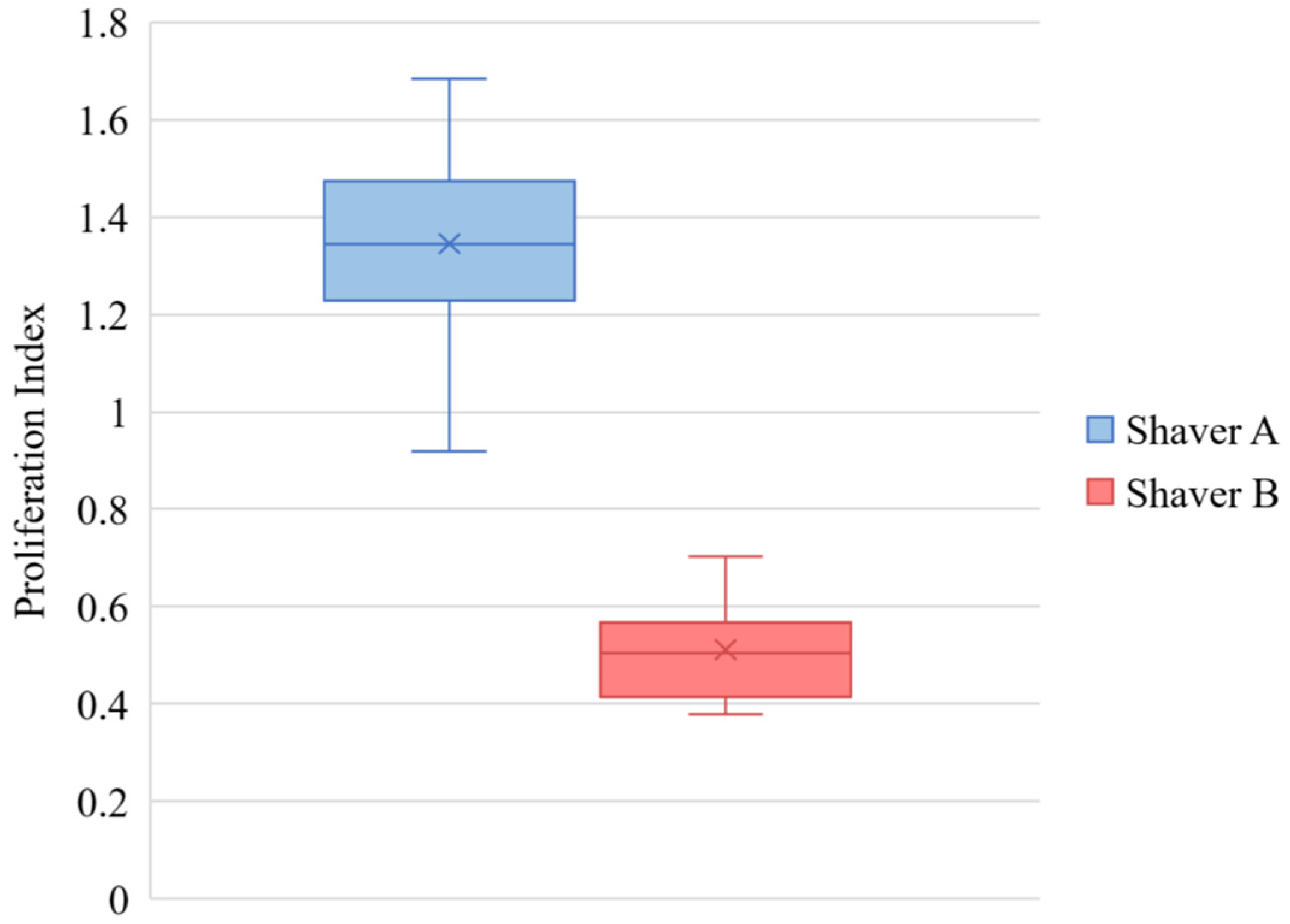
3.3. Cellular Proliferation
3.4. FACS Analysis
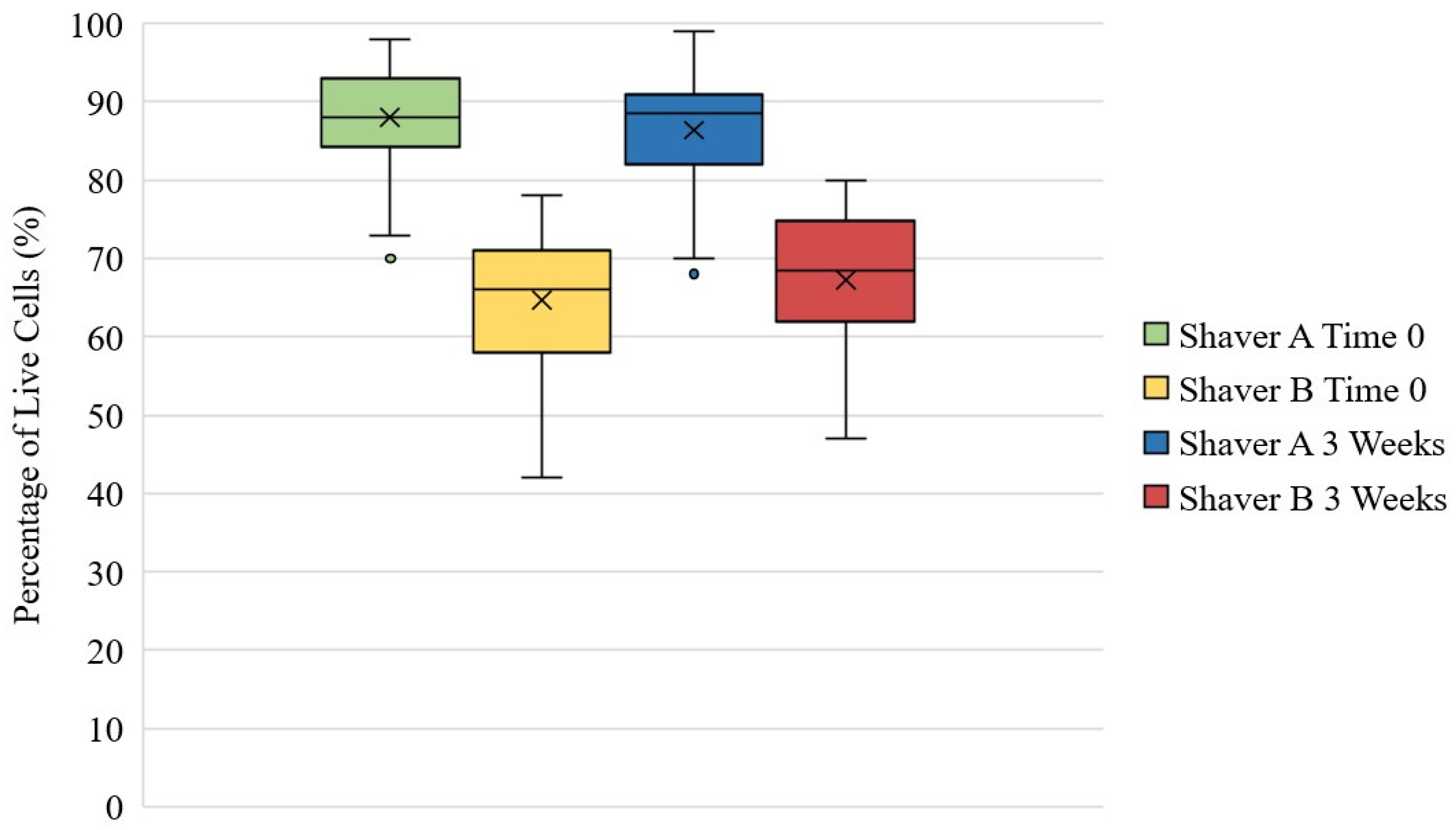
3.5. Live/Dead Assay
3.6. Cytokine and Growth Factor Analysis
3.7. Post Hoc Power Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Morikawa, D.; Muench, L.N.; Baldino, J.B.; Kia, C.; Johnson, J.; Otto, A.; Pauzenberger, L.; Dyrna, F.; McCarthy, M.B.R.; Mazzocca, A.D. Comparison of Preparation Techniques for Isolating Subacromial Bursa-Derived Cells as a Potential Augment for Rotator Cuff Repair. Arthrosc. J. Arthrosc. Relat. Surg. 2020, 36, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, D.; Johnson, J.D.; Kia, C.; McCarthy, M.B.R.; Macken, C.; Bellas, N.; Baldino, J.B.; Cote, M.P.; Mazzocca, A.D. Examining the Potency of Subacromial Bursal Cells as a Potential Augmentation for Rotator Cuff Healing: An In Vitro Study. Arthrosc. J. Arthrosc. Relat. Surg. 2019, 35, 2978–2988. [Google Scholar] [CrossRef] [PubMed]
- Barth, J.; Fotiadis, E.; Barthelemy, R.; Genna, S.; Saffarini, M. Ultrasonic evaluation of the repair integrity can predict functional outcomes after arthroscopic double-row rotator cuff repair. Knee Surg. Sport Traumatol. Arthrosc. 2015, 23, 376–385. [Google Scholar] [CrossRef]
- Galatz, L.M.; Ball, C.M.; Teefey, S.A.; Middleton, W.D.; Yamaguchi, K. The Outcome and Repair Integrity of Completely Arthroscopically Repaired Large and Massive Rotator Cuff Tears. JBJS 2004, 86, 219–224. [Google Scholar] [CrossRef]
- Le, B.T.N.; Wu, X.L.; Lam, P.H.; Murrell, G.A.C. Factors Predicting Rotator Cuff Retears: An Analysis of 1000 Consecutive Rotator Cuff Repairs. Am. J. Sports Med. 2014, 42, 1134–1142. [Google Scholar] [CrossRef]
- Zumstein, M.A.; Lädermann, A.; Raniga, S.; Schär, M.O. The biology of rotator cuff healing. Orthop. Traumatol. Surg. Res. 2017, 103, S1–S10. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, P.; Flouzat Lachaniette, C.H.; Delambre, J.; Zilber, S.; Duffiet, P.; Chevallier, N.; Rouard, H. Biologic augmentation of rotator cuff repair with mesenchymal stem cells during arthroscopy improves healing and prevents further tears: A case-controlled study. Int. Orthop. 2014, 38, 1811–1818. [Google Scholar] [CrossRef] [PubMed]
- Imam, M.A.; Holton, J.; Ernstbrunner, L.; Pepke, W.; Grubhofer, F.; Narvani, A.; Snow, M. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. Int. Orthop. 2017, 41, 2213–2220. [Google Scholar] [CrossRef]
- Ellera Gomes, J.L.; da Silva, R.C.; Silla, L.M.R.; Abreu, M.R.; Pellanda, R. Conventional rotator cuff repair complemented by the aid of mononuclear autologous stem cells. Knee Surg. Sport Traumatol. Arthrosc. 2012, 20, 373–377. [Google Scholar] [CrossRef] [Green Version]
- Imam, M.A.; Holton, J.; Horriat, S.; Negida, A.S.; Grubhofer, F.; Gupta, R.; Narvani, A.; Snow, M. A systematic review of the concept and clinical applications of bone marrow aspirate concentrate in tendon pathology. SICOT-J 2017, 3, 58. [Google Scholar] [CrossRef] [Green Version]
- Uhthoff, H.K.; Sarkar, K. Surgical repair of rotator cuff ruptures. The importance of the subacromial bursa. J. Bone Jt. Surg. 1991, 73, 399–401. [Google Scholar] [CrossRef] [Green Version]
- Steinert, A.F.; Kunz, M.; Prager, P.; Göbel, S.; Klein-Hitpass, L.; Ebert, R.; Nöth, U.; Jakob, F.; Gohlke, F. Characterization of bursa subacromialis-derived mesenchymal stem cells. Stem Cell Res. Ther. 2015, 6, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, N.; Armstrong, A.D.; Li, F.; Ouyang, H.; Niyibizi, C. Multipotent Mesenchymal Stem Cells from Human Subacromial Bursa: Potential for Cell Based Tendon Tissue Engineering. Tissue Eng. Part A 2013, 20, 239–249. [Google Scholar] [CrossRef] [PubMed]
- Utsunomiya, H.; Uchida, S.; Sekiya, I.; Sakai, A.; Moridera, K.; Nakamura, T. Isolation and Characterization of Human Mesenchymal Stem Cells Derived From Shoulder Tissues Involved in Rotator Cuff Tears. Am. J. Sports Med. 2013, 41, 657–668. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.I.; Kim, M.; Park, D.Y.; Min, B.H.; Yun, H.W.; Chung, J.Y.; Min, K.J. Motorized Shaver Harvest Results in Similar Cell Yield and Characteristics Compared With Rongeur Biopsy During Arthroscopic Synovium-Derived Mesenchymal Stem Cell Harvest. Arthrosc. J. Arthrosc. Relat. Surg. 2021, 37, 2873–2882. [Google Scholar] [CrossRef]
- Ferro, T.; Santhagunam, A.; Madeira, C.; Salgueiro, J.B.; da Silva, C.L.; Cabral, J.M.S. Successful isolation and ex vivo expansion of human mesenchymal stem/stromal cells obtained from different synovial tissue-derived (biopsy) samples. J. Cell. Physiol. 2019, 234, 3973–3984. [Google Scholar] [CrossRef]
- Liang, P.; Zhao, G.; Gu, X.; Chen, Z.; Xu, S.; Lai, W.; Liu, W. Assessment of arthroscopic shavers: A comparison test of resection performance and quality. J. Orthop. Surg. Res. 2020, 15, 62. [Google Scholar] [CrossRef] [Green Version]
- Beitzel, K.; McCarthy, M.B.R.; Cote, M.P.; Durant, T.J.S.; Chowaniec, D.M.; Solovyova, O.; Russell, R.P.; Arciero, R.A.; Mazzocca, A.D. Comparison of mesenchymal stem cells (osteoprogenitors) harvested from proximal humerus and distal femur during arthroscopic surgery. Arthrosc. J. Arthrosc. Relat. Surg. 2013, 29, 301–308. [Google Scholar] [CrossRef]
- Ahn, H.-J.; Lee, W.-J.; Kwack, K.; Do Kwon, Y. FGF2 stimulates the proliferation of human mesenchymal stem cells through the transient activation of JNK signaling. FEBS Lett. 2009, 583, 2922–2926. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Lourenco, E.S.; Mourão, C.F.D.A.B.; Leite, P.E.C.; Granjeiro, J.M.; Calasans-Maia, M.D.; Alves, G.G. The in vitro release of cytokines and growth factors from fibrin membranes produced through horizontal centrifugation. J. Biomed. Mater. Res. Part A 2018, 106, 1373–1380. [Google Scholar] [CrossRef] [PubMed]
- Abrams, G.D.; Luria, A.; Carr, R.A.; Rhodes, C.; Robinson, W.H.; Sokolove, J. Association of synovial inflammation and inflammatory mediators with glenohumeral rotator cuff pathology. J. Shoulder Elb. Surg. 2016, 25, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Riley, G.P.; Curry, V.; DeGroot, J.; van El, B.; Verzijl, N.; Hazleman, B.L.; Bank, R.A. Matrix metalloproteinase activities and their relationship with collagen remodelling in tendon pathology. Matrix Biol. 2002, 21, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Ferrara, N.; Gerber, H.-P.; LeCouter, J. The biology of VEGF and its receptors. Nat. Med. 2003, 9, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Galliera, E.; Corsi, M.M.; Banfi, G. Platelet rich plasma therapy: Inflammatory molecules involved in tissue healing. J. Biol. Regul. Homeost. Agents 2012, 26, 35S–42S. [Google Scholar] [PubMed]
- Singh, S.; Tavakkolizadeh, A.; Arya, A.; Compson, J. Arthroscopic powered instruments: A review of shavers and burrs. Orthop. Trauma 2009, 23, 357–361. [Google Scholar] [CrossRef]


| Patient Number | Age | Gender | Surgery |
|---|---|---|---|
| 1 | 46 | Male | Rotator Cuff Repair |
| 2 | 39 | Male | Rotator Cuff Repair |
| 3 | 54 | Male | Revision Rotator Cuff Repair |
| 4 | 57 | Female | Rotator Cuff Repair |
| 5 | 55 | Female | Rotator Cuff Repair |
| 6 | 51 | Male | Rotator Cuff Repair |
| 7 | 49 | Female | Rotator Cuff Repair |
| 8 | 61 | Male | Revision Rotator Cuff Repair |
| 9 | 67 | Female | Rotator Cuff Repair |
| 10 | 62 | Male | Rotator Cuff Repair |
| Shaver A Time 0 | Shaver B Time 0 | p-Value | Shaver A Time 96 h | Shaver B Time 96 h | p-Value | |
|---|---|---|---|---|---|---|
| IL-1β | 0.20 ± 0.28 | 0.23 ± 0.36 | 0.610 | 0.14 ± 0.16 | 0.27 ± 0.19 | 0.001 |
| IL-1Ra | 57.31 ± 165.99 | 31.16 ± 68.15 | 0.048 | 152.43 ± 151.82 | 147.40 ± 113.84 | 0.785 |
| IL-6 | 2.02 ± 5.44 | 3.46 ± 7.42 | 0.256 | 20.61 ± 32.88 | 70.05 ± 122.88 | <0.001 |
| TNF-α | ND | 0.09 ± 0.39 | N/A | ND | 0.13 ± 0.49 | N/A |
| MMP-1 | 44.85 ± 63.49 | 26.22 ± 53.48 | 0.037 | 3741.00 ± 2842.63 | 5500.11 ± 2867.97 | <0.001 |
| MMP-3 | 527.21 ± 318.48 | 765.32 ± 473.88 | 0.001 | 1130.87 ± 408.29 | 1871.05 ± 1268.86 | <0.001 |
| MMP-13 | ND | 18.89 ± 31.77 | N/A | 178.85 ± 89.70 | 401.23 ± 169.52 | <0.001 |
| VEGF | 47.84 ± 129.54 | 8.96 ± 11.43 | 0.009 | 345.54 ± 182.43 | 169.66 ± 160.51 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wellington, I.J.; Hawthorne, B.C.; Messina, J.C.; LeVasseur, M.R.; McCarthy, M.B.; Cote, M.P.; Mazzocca, A.D. Efficacy of Arthroscopic Shavers for the Retrieval and Processing of Connective Tissue Progenitor Cells from Subacromial Bursal Tissue. J. Clin. Med. 2022, 11, 1272. https://doi.org/10.3390/jcm11051272
Wellington IJ, Hawthorne BC, Messina JC, LeVasseur MR, McCarthy MB, Cote MP, Mazzocca AD. Efficacy of Arthroscopic Shavers for the Retrieval and Processing of Connective Tissue Progenitor Cells from Subacromial Bursal Tissue. Journal of Clinical Medicine. 2022; 11(5):1272. https://doi.org/10.3390/jcm11051272
Chicago/Turabian StyleWellington, Ian J., Benjamin C. Hawthorne, James C. Messina, Matthew R. LeVasseur, Mary Beth McCarthy, Mark P. Cote, and Augustus D. Mazzocca. 2022. "Efficacy of Arthroscopic Shavers for the Retrieval and Processing of Connective Tissue Progenitor Cells from Subacromial Bursal Tissue" Journal of Clinical Medicine 11, no. 5: 1272. https://doi.org/10.3390/jcm11051272
APA StyleWellington, I. J., Hawthorne, B. C., Messina, J. C., LeVasseur, M. R., McCarthy, M. B., Cote, M. P., & Mazzocca, A. D. (2022). Efficacy of Arthroscopic Shavers for the Retrieval and Processing of Connective Tissue Progenitor Cells from Subacromial Bursal Tissue. Journal of Clinical Medicine, 11(5), 1272. https://doi.org/10.3390/jcm11051272






