The Influence of Treadmill Training on the Bioelectrical Activity of the Lower Limb Muscles in Patients with Intermittent Claudication
Abstract
:1. Introduction
2. Methods
2.1. Subjects
2.2. Training Program
2.3. Methodological Approach
2.4. Surface Electromyography
2.5. Maximal Voluntary Contraction (MVC)
2.6. Muscle Bioelectrical Activity (sEMG) Assessment during Gait
- -
- Average mean amplitude of sEMG signal—expressed as %MVC (a decrease in the average mean amplitude after training means that the muscle responds better to the walking effort, requires less active units to perform a similar effort and this is a symptom of lower muscle fatigue);
- -
- Mean amplitude range of the sEMG signal between minimal and maximal value measured at evaluated intervals—expressed as %MVC (a smaller mean amplitude range after training means that the muscle used less motor units to perform a similar effort and this is a symptom of lower muscle fatigue).
- -
- Whole measurement (entire treadmill test);
- -
- Pain-free part of the treadmill test (from the beginning to onset of claudication pain);
- -
- Painful part of the treadmill test (from the onset of claudication pain to the end of the test).
- -
- Group 1—up to 600 s (up to 10 min);
- -
- Group 2—600–1000 s (from 10 to 16.5 min);
- -
- Group 3—over 1000 s (over 16.5 min).
2.7. Statistical Analysis
3. Results
3.1. Post-Training Changes in Bioelectrical Activity of the Muscles for the Entire Treadmill Test (Total Measurement)
3.2. Post-Training Changes in Bioelectrical Activity of the Muscles during the Pain-Free Interval of the Treadmill Test
3.3. Post-Training Changes in Bioelectrical Activity of the Muscles during the Painful Interval of the Treadmill Test
3.4. Differences in Muscle Bioelectrical Activity between Pain-Free and Painful Intervals of the Treadmill Test
3.5. Changes in Muscle Bioelectrical Activity among Patients with Different Walking Time
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gardner, A.W.; Forrester, L.; Smith, G.V. Altered gait profile in subjects with peripheral arterial disease. Vasc. Med. 2001, 6, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Regensteiner, J.G.; Meyer, T.J.; Krupski, W.C.; Cranford, L.S.; Hiatt, W.R. Hospital versus home-based exercise rehabilitation for patients with peripheral arterial occlusive disease. Angiology 1997, 48, 291–300. [Google Scholar] [CrossRef] [PubMed]
- Scherer, S.A.; Bainbridge, J.S.; Hiatt, W.R.; Regensteiner, J.G. Gait characteristics of patients with claudication. Arch. Phys. Med. Rehabil. 1998, 79, 529–531. [Google Scholar] [CrossRef]
- Schieber, M.N.; Pipinos, I.I.; Johanning, J.M.; Casale, G.P.; Williams, M.A.; DeSpiegelaere, H.K.; Senderling, B.; Myers, S.A. Supervised walking exercise therapy improves gait biomechanics in patients with peripheral artery disease. J. Vasc. Surg. 2020, 71, 575–583. [Google Scholar] [CrossRef] [PubMed]
- Myers, S.A.; Johanning, J.M.; Stergiou, N.; Celis, R.I.; Robinson, L.; Pipinos, I.I. Gait variability is altered in patients with peripheral arterial disease. J. Vasc. Surg. 2009, 49, 924–931. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.J.; Pipinos, I.; Johanning, J.; Radovic, M.; Huisinga, J.M.; Myers, S.A.; Stergiou, N. Bilateral claudication results in alterations in the gait biomechanics at the hip and ankle joints. J. Biomech. 2008, 41, 2506–2514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schieber, M.N.; Hasenkamp, R.M.; Pipinos, I.I.; Johanning, J.M.; Stergiou, N.; DeSpiegelaere, H.K.; Chien, J.H.; Myers, S.A. Muscle strength and control characteristics are altered by peripheral artery disease. J. Vasc. Surg. 2017, 66, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Wurdeman, S.R.; Koutakis, P.; Myers, S.A.; Johanning, J.M.; Pipinos, I.I.; Stergiou, N. Patients with peripheral arterial disease exhibit reduced joint powers compared to velocity-matched controls. Gait Posture 2012, 36, 506–509. [Google Scholar] [CrossRef] [Green Version]
- Scott-Pandorf, M.M.; Stergiou, N.; Johanning, J.M.; Robinson, L.; Lynch, T.G.; Pipinos, I.I. Peripheral arterial disease affects ground reaction forces during walking. J. Vasc. Surg. 2007, 46, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Koutakis, P.; Pipinos, I.I.; Myers, S.A.; Stergiou, N.; Lynch, T.G.; Johanning, J.M. Joint torques and powers are reduced during ambulation for both limbs in patients with unilateral claudication. J. Vasc. Surg. 2010, 51, 80–88. [Google Scholar] [CrossRef] [Green Version]
- Gommans, L.N.; Smid, A.T.; Scheltinga, M.R.; Brooijmans, F.A.; van Disseldorp, E.M.; van der Linden, F.T.; Meijer, K.; Teijink, J.A. Altered joint kinematics and increased electromyographic muscle activity during walking in patients with intermittent claudication. J. Vasc. Surg. 2016, 63, 664–672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gommans, L.N.; Smid, A.T.; Scheltinga, M.R.M.; Cancrinus, E.; Brooijmans, F.A.; Meijer, K.; Teijink, J.A. Prolonged stance phase during walking in intermittent claudication. J. Vasc. Surg. 2017, 66, 515–522. [Google Scholar] [CrossRef]
- Norgren, L.; Hiatt, W.R.; Dormandy, J.A.; Nehler, M.R.; Harris, K.A.; Fowkes, F.G.; TASC II Working Group. Inter-Society Consensus for the Management of Peripheral Arterial Disease (TASC II). J. Vasc. Surg. 2007, 45, S5–S67. [Google Scholar] [CrossRef] [Green Version]
- Wang, E.; Helgerud, J.; Loe, H.; Indseth, K.; Kaehler, N.; Hoff, J. Maximal strength training improves walking performance in peripheral arterial disease patients. Scand. J. Med. Sci. Sports 2010, 20, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Søgaard, K.; Blangsted, A.K.; Jørgensen, L.V.; Madeleine, P.; Sjøgaard, G. Evidence of long term muscle fatigue following prolonged intermittent contractions based on mechano- and electromyograms. J. Electromyogr. Kinesiol. 2003, 13, 441–450. [Google Scholar] [CrossRef]
- Rudroff, T.; Staudenmann, D.; Enoka, R.M. Electromyographic measures of muscle activation and changes in muscle architecture of human elbow flexors during fatiguing contractions. J. Appl. Physiol. 2008, 104, 1720–1726. [Google Scholar] [CrossRef] [Green Version]
- Fallentin, N.; Jørgensen, K.; Simonsen, E.B. Motor unit recruitment during prolonged isometric contractions. Eur. J. Appl. Physiol. Occup. Physiol. 1993, 67, 335–341. [Google Scholar] [CrossRef]
- Evangelopoulou, E.; Jones, R.K.; Jameel, M.; Boyd, P.; Nester, C. Effects of intermittent claudication due to arterial disease on pain-free gait. Clin. Biomech. 2021, 83, 105309. [Google Scholar] [CrossRef] [PubMed]
- Mika, P.; Mika, A.; Oleksy, Ł.; Konik, A. Changes of sEMG activity in proximal and distal leg muscles in patients with intermittent claudication over 12-week treadmill training—The pilot study. Atheroscler. Suppl. 2010, 11, 183. [Google Scholar] [CrossRef]
- Mika, P.; Konik, A.; Januszek, R.; Petriczek, T.; Mika, A.; Nowobilski, R.; Nizankowski, R.; Szczeklik, A. Comparison of two treadmill training programs on walking ability and endothelial function in intermittent claudication. Int. J. Cardiol. 2013, 168, 838–842. [Google Scholar] [CrossRef]
- Hermens, H.J.; Freriks, B.; Disselhorst-Klug, C.; Rau, G. Development of recommendations for SEMG sensors and sensor placement procedures. J. Electromyogr. Kinesiol. 2000, 10, 361–374. [Google Scholar] [CrossRef]
- Merletti, R.; Parker, P. Electromyography: Physiology, Engineering, and Non-Invasive Applications; Wiley-IEEE Press: Hoboken, NJ, USA, 2004. [Google Scholar]
- Hermens, H.J.; Freriks, B.; Merletti, R. SENIAM 8: European recommendations for surface electromyography. Roessingh Res. Dev. 1999, 8, 13–54. [Google Scholar]
- Szymczak, M.; Krupa, P.; Oszkinis, G.; Majchrzycki, M. Gait pattern in patients with peripheral artery disease. BMC Geriatr. 2018, 18, 52. [Google Scholar] [CrossRef] [PubMed]
- Gardner, A.W.; Ritti-Dias, R.M.; Stoner, J.A.; Montgomery, P.S.; Scott, K.J.; Blevins, S.M. Walking economy before and after the onset of claudication pain in patients with peripheral arterial disease. J. Vasc. Surg. 2010, 51, 628–633. [Google Scholar] [CrossRef] [Green Version]
- Guilleron, C.; Abraham, P.; Beaune, B.; Pouliquen, C.; Henni, S.; Durand, S. Location of ischemia and ischemic pain intensity affect spatiotemporal parameters and leg muscles activity during walking in patients with intermittent claudication. Sci. Rep. 2021, 11, 6809. [Google Scholar] [CrossRef]
- Askew, C.D.; Green, S.; Walker, P.J.; Kerr, G.K.; Green, A.A.; Williams, A.D.; Febbraio, M.A. Skeletal muscle phenotype is associated with exercise tolerance in patients with peripheral arterial disease. J. Vasc. Surg. 2005, 41, 802–807. [Google Scholar] [CrossRef] [Green Version]
- Gardner, A.W.; Montgomery, P.S.; Ritti-Dias, R.M.; Forrester, L. The effect of claudication pain on temporal and spatial gait measures during self-paced ambulation. Vasc. Med. 2010, 15, 21–26. [Google Scholar] [CrossRef] [Green Version]
- McCully, K.; Leiper, C.; Sanders, T.; Griffin, E. The effects of peripheral vascular disease on gait. J. Gerontol. A Biol. Sci. Med. Sci. 1999, 54, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Marconi, C.; Ferretti, G.; Anchisi, S.; Catalano, M.; Scandale, G.; Antico, A.; Iob, G.; Peinetti, F.; Cerretelli, P. Energetics of walking in patients with peripheral arterial disease: A proposed functional evaluation protocol. Clin. Sci. 2003, 105, 105–111. [Google Scholar] [CrossRef] [Green Version]
- Pipinos, I.I.; Sharov, V.G.; Shepard, A.D.; Anagnostopoulos, P.V.; Katsamouris, A.; Todor, A.; Filis, K.A.; Sabbah, H.N. Abnormal mitochondrial respiration in skeletal muscle in patients with peripheral arterial disease. J. Vasc. Surg. 2003, 38, 827–832. [Google Scholar] [CrossRef] [Green Version]
- Pipinos, I.I.; Judge, A.R.; Selsby, J.T.; Zhu, Z.; Swanson, S.A.; Nella, A.A.; Dodd, S.L. The myopathy of peripheral arterial occlusive disease: Part 1. Functional and histomorphological changes and evidence for mitochondrial dysfunction. Vasc. Endovasc. Surg. 2007, 41, 481–489. [Google Scholar] [CrossRef]
- Koutakis, P.; Myers, S.A.; Cluff, K.; Ha, D.M.; Haynatzki, G.; McComb, R.D.; Uchida, K.; Miserlis, D.; Papoutsi, E.; Johanning, J.M.; et al. Abnormal myofiber morphology and limb dysfunction in claudication. J. Surg. Res. 2015, 196, 172–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- England, J.D.; Regensteiner, J.G.; Ringel, S.P.; Carry, M.R.; Hiatt, W.R. Muscle denervation in peripheral arterial disease. Neurology 1992, 42, 994–999. [Google Scholar] [CrossRef] [PubMed]
- Konik, A.; Mika, P.; Nowobilski, R.; Andrzejak, A.; Szczeklik, A. Mechanisms responsible for reducing intermittent claudication as a result of treadmill training. Acta Angiol. 2010, 16, 49–66. [Google Scholar]
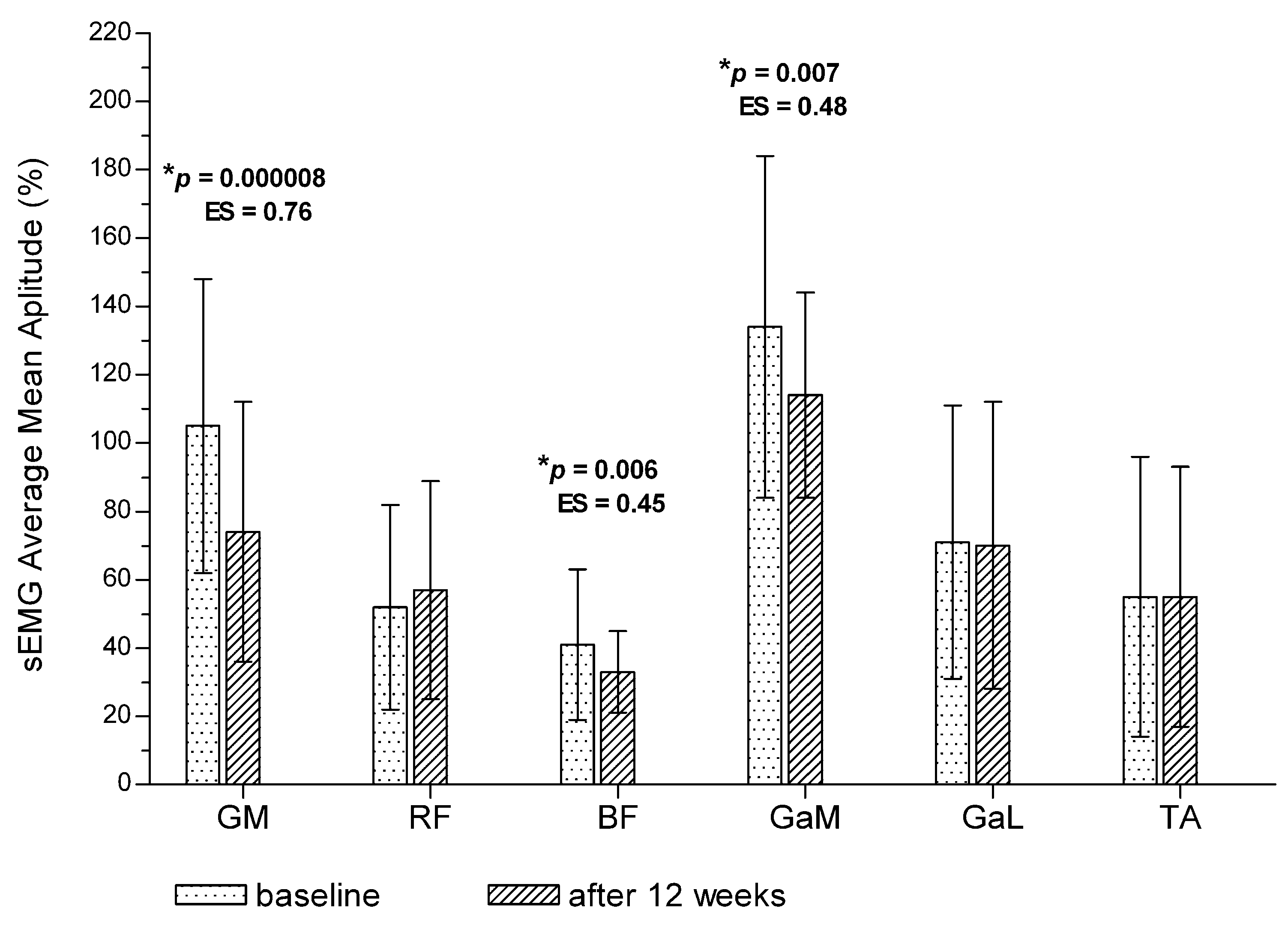

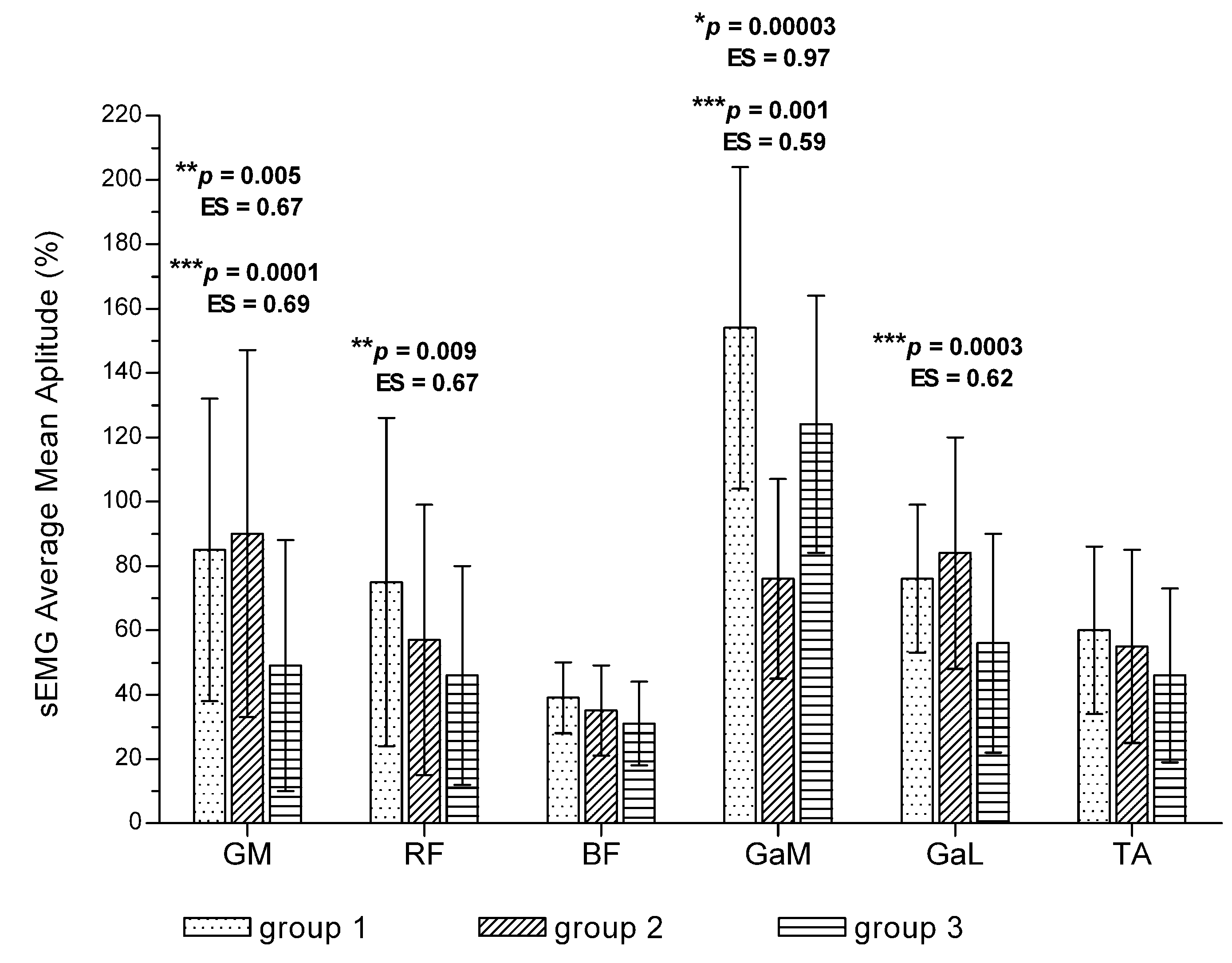
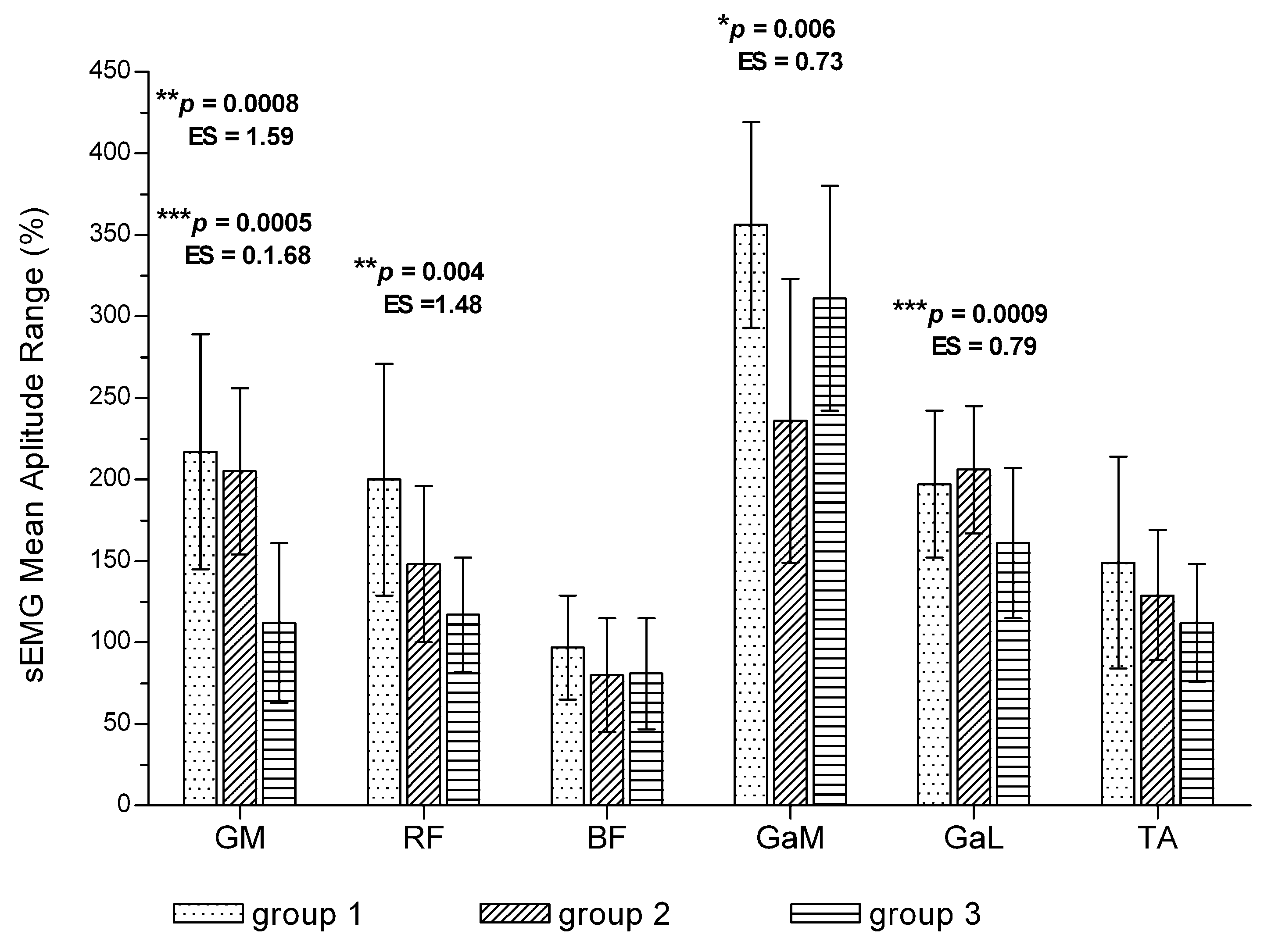
| Outcome Measure | Pain-Free Interval | Change | p # | ES # | Painful Interval | Change | p # | ES # | p * | ES * | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gluteus Medius (%) | Baseline | 95 ± 43 | −22 | 0.06 | 0.52 | 116 ± 45 | −43 | 0.0008 | 0.94 | 0.29 | 0.47 |
| Post-training | 73 ± 40 | 73 ± 46 | 0.17 | 0.03 | |||||||
| Rectus Femoris (%) | Baseline | 50 ± 30 | 2 | 0.81 | 0.05 | 53 ± 35 | 6 | 0.38 | 0.16 | 0.78 | 0.09 |
| Post-training | 52 ± 38 | 59 ± 37 | 0.51 | 0.18 | |||||||
| Biceps Femoris (%) | Baseline | 37 ± 21 | −8 | 0.11 | 0.4 | 47 ± 21 | −16 | 0.005 | 0.83 | 0.42 | 0.47 |
| Post-training | 29 ± 18 | 31 ± 17 | 0.12 | 0.11 | |||||||
| Gastrocnemius Medialis (%) | Baseline | 134 ± 50 | −21 | 0.19 | 0.46 | 144 ± 53 | −37 | 0.007 | 0.8 | 0.86 | 0.19 |
| Post-training | 113 ± 40 | 107 ± 38 | 0.81 | 0.15 | |||||||
| Gastrocnemius Lateralis (%) | Baseline | 61 ± 36 | 5 | 0.54 | 0.14 | 74 ± 33 | 1 | 0.67 | 0.02 | 0.15 | 0.37 |
| Post-training | 66 ± 31 | 75 ± 38 | 0.37 | 0.25 | |||||||
| Tibialis Anterior (%) | Baseline | 47 ± 29 | 2 | 0.64 | 0.07 | 60 ± 24 | −3 | 0.75 | 0.13 | 0.17 | 0.48 |
| Post-training | 49 ± 28 | 57 ± 21 | 0.26 | 0.32 |
| Outcome Measure | Pain-Free Interval | p # | ES # | Painful Interval | p # | ES # | p * | ES * | |
|---|---|---|---|---|---|---|---|---|---|
| Gluteus Medius (%) | Baseline | 246 ± 73 | 0.12 | 0.69 | 257 ± 55 | 0.008 | 1.74 | 0.63 | 0.17 |
| Post-training | 194 ± 77 | 170 ± 44 | 0.37 | 0.38 | |||||
| Rectus Femoris (%) | Baseline | 149 ± 52 | 0.34 | 0.09 | 137 ± 35 | 0.32 | 0.11 | 0.72 | 0.27 |
| Post-training | 154 ± 55 | 141 ± 32 | 0.23 | 0.28 | |||||
| Biceps Femoris (%) | Baseline | 111 ± 69 | 0.07 | 0.46 | 123 ± 54 | 0.003 | 0.79 | 0.92 | 0.19 |
| Post-training | 86 ± 32 | 86 ± 37 | 0.96 | 0.03 | |||||
| Gastrocnemius Medialis (%) | Baseline | 319 ± 81 | 0.69 | 0.07 | 305 ± 90 | 0.57 | 0.19 | 0.73 | 0.16 |
| Post-training | 313 ± 83 | 288 ± 85 | 0.3 | 0.29 | |||||
| Gastrocnemius Lateralis (%) | Baseline | 183 ± 56 | 0.38 | 0.25 | 181 ± 50 | 0.45 | 0.09 | 0.9 | 0.03 |
| Post-training | 197 ± 53 | 176 ± 55 | 0.55 | 0.38 | |||||
| Tibialis Anterior (%) | Baseline | 111 ± 49 | 0.12 | 0.43 | 104 ± 43 | 0.01 | 0.81 | 0.59 | 0.15 |
| Post-training | 134 ± 57 | 144 ± 55 | 0.92 | 0.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mika, A.; Mika, P.; Oleksy, Ł.; Kulik, A. The Influence of Treadmill Training on the Bioelectrical Activity of the Lower Limb Muscles in Patients with Intermittent Claudication. J. Clin. Med. 2022, 11, 1302. https://doi.org/10.3390/jcm11051302
Mika A, Mika P, Oleksy Ł, Kulik A. The Influence of Treadmill Training on the Bioelectrical Activity of the Lower Limb Muscles in Patients with Intermittent Claudication. Journal of Clinical Medicine. 2022; 11(5):1302. https://doi.org/10.3390/jcm11051302
Chicago/Turabian StyleMika, Anna, Piotr Mika, Łukasz Oleksy, and Anita Kulik. 2022. "The Influence of Treadmill Training on the Bioelectrical Activity of the Lower Limb Muscles in Patients with Intermittent Claudication" Journal of Clinical Medicine 11, no. 5: 1302. https://doi.org/10.3390/jcm11051302







