Antioxidant Effects of Statins by Modulating Nrf2 and Nrf2/HO-1 Signaling in Different Diseases
Abstract
1. Introduction
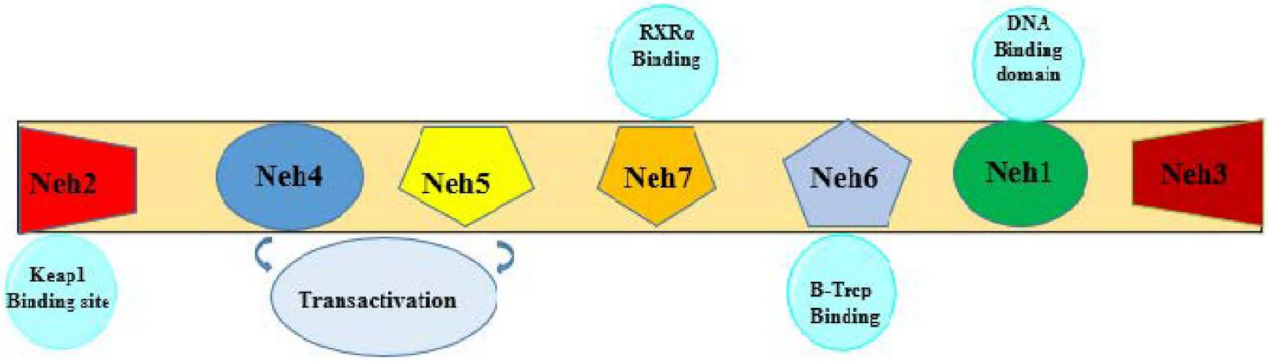
2. Antioxidant Effects of Statins by Influencing Nrf2/HO-1 Pathway
3. The Effects of Statins on Different Organs and Diseases
3.1. Effects on the Heart and Cardiovascular Diseases
3.2. Effects on Lung Diseases
3.3. Effects on Kidney Disease
3.4. Effects on Liver Disease
3.5. Effects on Eye Diseases
3.6. Effects on Cancer
3.7. Effect on Neurodegenerative Disorders
3.8. Effect on Diabetes
4. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Feingold, K.R. Cholesterol Lowering Drugs. 2020. Available online: https://www.ncbi.nlm.nih.gov/sites/books/NBK395573/ (accessed on 21 February 2022).
- Reiner, Ž. Statins in the primary prevention of cardiovascular disease. Nat. Rev. Cardiol. 2013, 10, 453–464. [Google Scholar] [CrossRef]
- Pinal-Fernandez, I.; Casal-Dominguez, M.; Mammen, A.L. Statins: Pros and cons. Med. Clínica 2018, 150, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Nurmohamed, N.S.; Navar, A.M.; Kastelein, J.J.P. New and Emerging Therapies for Reduction of LDL-Cholesterol and Apolipoprotein B: JACC Focus Seminar 1/4. J. Am. Coll. Cardiol. 2021, 77, 1564–1575. [Google Scholar] [CrossRef]
- Ruscica, M.; Ferri, N.; Santos, R.D.; Sirtori, C.R.; Corsini, A. Lipid Lowering Drugs: Present Status and Future Developments. Curr. Atheroscler. Rep. 2021, 23, 17. [Google Scholar] [CrossRef]
- Sahebkar, A.; Watts, G.F. New therapies targeting apoB metabolism for high-risk patients with inherited dyslipidaemias: What can the clinician expect? Cardiovasc. Drugs Ther. 2013, 27, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Watts, G.F. New LDL-cholesterol lowering therapies: Pharmacology, clinical trials, and relevance to acute coronary syndromes. Clin. Ther. 2013, 35, 1082–1098. [Google Scholar] [CrossRef] [PubMed]
- Almuti, K.; Rimawi, R.; Spevack, D.; Ostfeld, R.J. Effects of statins beyond lipid lowering: Potential for clinical benefits. Int. J. Cardiol. 2006, 109, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Gorabi, A.M.; Kiaie, N.; Pirro, M.; Bianconi, V.; Jamialahmadi, T.; Sahebkar, A. Effects of statins on the biological features of mesenchymal stem cells and therapeutic implications. Heart Fail. Rev. 2020, 26, 1259–1272. [Google Scholar] [CrossRef]
- Chruściel, P.; Sahebkar, A.; Rembek-Wieliczko, M.; Serban, M.-C.; Ursoniu, S.; Mikhailidis, D.P.; Jones, S.R.; Mosteoru, S.; Blaha, M.J.; Martin, S.S. Impact of statin therapy on plasma adiponectin concentrations: A systematic review and meta-analysis of 43 randomized controlled trial arms. Atherosclerosis 2016, 253, 194–208. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, M.-C.; Mikhailidis, D.P.; Toth, P.P.; Muntner, P.; Ursoniu, S.; Mosterou, S.; Glasser, S.; Martin, S.S.; Jones, S.R. Head-to-head comparison of statins versus fibrates in reducing plasma fibrinogen concentrations: A systematic review and meta-analysis. Pharmacol. Res. 2016, 103, 236–252. [Google Scholar] [CrossRef]
- Bianconi, V.; Sahebkar, A.; Banach, M.; Pirro, M. Statins, haemostatic factors and thrombotic risk. Curr. Opin. Cardiol. 2017, 32, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Pirro, M.; Simental-Mendía, L.E.; Bianconi, V.; Watts, G.F.; Banach, M.; Sahebkar, A. Effect of statin therapy on arterial wall inflammation based on 18F-FDG PET/CT: A systematic review and meta-analysis of interventional studies. J. Clin. Med. 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Sohrevardi, S.M.; Nasab, F.S.; Mirjalili, M.R.; Bagherniya, M.; Tafti, A.D.; Jarrahzadeh, M.H.; Azarpazhooh, M.R.; Saeidmanesh, M.; Banach, M.; Jamialahmadi, T.; et al. Effect of atorvastatin on delirium status of patients in the intensive care unit: A randomized controlled trial. Arch. Med. Sci. 2021, 17, 1423. [Google Scholar] [CrossRef] [PubMed]
- Vahedian-Azimi, A.; Mohammadi, S.M.; Beni, F.H.; Banach, M.; Guest, P.C.; Jamialahmadi, T.; Sahebkar, A. Improved COVID-19 ICU admission and mortality outcomes following treatment with statins: A systematic review and meta-analysis. Arch. Med. Sci. 2021, 17, 579–595. [Google Scholar] [CrossRef] [PubMed]
- Ikezaki, H.; Lim, E.; Cupples, L.A.; Liu, C.T.; Asztalos, B.F.; Schaefer, E.J. Small Dense Low-Density Lipoprotein Cholesterol Is the Most Atherogenic Lipoprotein Parameter in the Prospective Framingham Offspring Study. J. Am. Heart Assoc. 2021, 10, e019140. [Google Scholar] [CrossRef]
- Witztum, J.L.; Steinberg, D. The oxidative modification hypothesis of atherosclerosis: Does it hold for humans? Trends Cardiovasc. Med. 2001, 11, 93–102. [Google Scholar] [CrossRef]
- Fattman, C.L.; Schaefer, L.M.; Oury, T.D. Extracellular superoxide dismutase in biology and medicine. Free. Radic. Biol. Med. 2003, 35, 236–256. [Google Scholar] [CrossRef]
- ’t Hoen, P.A.; Van der Lans, C.A.; Van Eck, M.; Bijsterbosch, M.K.; Van Berkel, T.J.; Twisk, J. Aorta of ApoE-deficient mice responds to atherogenic stimuli by a prelesional increase and subsequent decrease in the expression of antioxidant enzymes. Circ. Res. 2003, 93, 262–269. [Google Scholar] [CrossRef]
- Aviram, M.; Rosenblat, M.; Billecke, S.; Erogul, J.; Sorenson, R.; Bisgaier, C.L.; Newton, R.S.; La Du, B. Human serum paraoxonase (PON 1) is inactivated by oxidized low density lipoprotein and preserved by antioxidants. Free. Radic. Biol. Med. 1999, 26, 892–904. [Google Scholar] [CrossRef]
- Girona, J.; La Ville, A.E.; Solà, R.; Plana, N.; Masana, L.s. Simvastatin decreases aldehyde production derived from lipoprotein oxidation. Am. J. Cardiol. 1999, 83, 846–851. [Google Scholar] [CrossRef]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2–Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Ariens, E.; Simonis, A. General principles of nutritional toxicology. Nutr. Toxicol. 1982, 1, 17–80. [Google Scholar]
- Godwill, E.A.; Jane, I.C.; Scholastica, I.U.; Marcellus, U.; Eugene, A.L.; Gloria, O.A. Determination of some soft drink constituents and contamination by some heavy metals in Nigeria. Toxicol. Rep. 2015, 2, 384–390. [Google Scholar] [CrossRef]
- Geng, Y.-J.; Wu, Q.; Muszynski, M.; Hansson, G.r.K.; Libby, P. Apoptosis of vascular smooth muscle cells induced by In Vitro stimulation with Interferon-γ, tumor necrosis Factor–α, and Interleukin-1β. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Araujo, J.A.; Meng, L.; Tward, A.D.; Hancock, W.W.; Zhai, Y.; Lee, A.; Ishikawa, K.; Iyer, S.; Buelow, R.; Busuttil, R.W. Systemic rather than local heme oxygenase-1 overexpression improves cardiac allograft outcomes in a new transgenic mouse. J. Immunol. 2003, 171, 1572–1580. [Google Scholar] [CrossRef] [PubMed]
- Otterbein, L.E.; Kolls, J.K.; Mantell, L.L.; Cook, J.L.; Alam, J.; Choi, A.M. Exogenous administration of heme oxygenase-1 by gene transfer provides protection against hyperoxia-induced lung injury. J. Clin. Investig. 1999, 103, 1047–1054. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Ding, M.; Zhu, P.; Huang, H.; Zhuang, Q.; Shen, J.; Cai, Y.; Zhao, M.; He, Q. New Insights into the Nrf-2/HO-1 Signaling Axis and Its Application in Pediatric Respiratory Diseases. Oxidative Med. Cell. Longev. 2019, 2019, 3214196. [Google Scholar] [CrossRef]
- Son, Y.; Lee, J.H.; Chung, H.-T.; Pae, H.-O. Therapeutic Roles of Heme Oxygenase-1 in Metabolic Diseases: Curcumin and Resveratrol Analogues as Possible Inducers of Heme Oxygenase-1. Oxidative Med. Cell. Longev. 2013, 2013, 639541. [Google Scholar] [CrossRef]
- Ndisang, J.F. Synergistic Interaction Between Heme Oxygenase (HO) and Nuclear-Factor E2- Related Factor-2 (Nrf2) against Oxidative Stress in Cardiovascular Related Diseases. Curr. Pharm. Des. 2017, 23, 1465–1470. [Google Scholar] [CrossRef]
- Loboda, A.; Damulewicz, M.; Pyza, E.; Jozkowicz, A.; Dulak, J. Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: An evolutionarily conserved mechanism. Cell. Mol. Life Sci.: CMLS 2016, 73, 3221–3247. [Google Scholar] [CrossRef] [PubMed]
- Shishehbor, M.H.; Brennan, M.-L.; Aviles, R.J.; Fu, X.; Penn, M.S.; Sprecher, D.L.; Hazen, S.L. Statins promote potent systemic antioxidant effects through specific inflammatory pathways. Circulation 2003, 108, 426–431. [Google Scholar] [CrossRef]
- Bahrami, A.; Parsamanesh, N.; Atkin, S.L.; Banach, M.; Sahebkar, A. Effect of statins on toll-like receptors: A new insight to pleiotropic effects. Pharmacol. Res. 2018, 135, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Khalifeh, M.; Penson, P.E.; Banach, M.; Sahebkar, A. Statins as anti-pyroptotic agents. Arch. Med. Sci. 2021, 17, 1414–1417. [Google Scholar] [CrossRef]
- Shakour, N.; Ruscica, M.; Hadizadeh, F.; Cirtori, C.; Banach, M.; Jamialahmadi, T.; Sahebkar, A. Statins and C-reactive protein: In silico evidence on direct interaction. Arch. Med. Sci. 2020, 16, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Davignon, J.; Jacob, R.F.; Mason, R.P. The antioxidant effects of statins. Coron. Artery Dis. 2004, 15, 251–258. [Google Scholar] [CrossRef]
- Habeos, I.G.; Ziros, P.G.; Chartoumpekis, D.; Psyrogiannis, A.; Kyriazopoulou, V.; Papavassiliou, A.G. Simvastatin activates Keap1/Nrf2 signaling in rat liver. J. Mol. Med. 2008, 86, 1279. [Google Scholar] [CrossRef]
- Chartoumpekis, D.; Ziros, P.G.; Psyrogiannis, A.; Kyriazopoulou, V.; Papavassiliou, A.G.; Habeos, I.G. Simvastatin lowers reactive oxygen species level by Nrf2 activation via PI3K/Akt pathway. Biochem. Biophys. Res. Commun. 2010, 396, 463–466. [Google Scholar] [CrossRef]
- Nowak, W.N.; Taha, H.; Markiewicz, J.; Kachamakova-Trojanowska, N.; Stępniewski, J.; Klóska, D.; Florczyk-Soluch, U.; Niżankowski, R.; Frołow, M.; Walter, Z. Atorvastatin and conditioned media from atorvastatin-treated human hematopoietic stem/progenitor-derived cells show proangiogenic activity in vitro but not in vivo. Mediat. Inflamm. 2019, 2019, 1868170. [Google Scholar] [CrossRef]
- Mouawad, C.A.; Mrad, M.F.; Al-Hariri, M.; Soussi, H.; Hamade, E.; Alam, J.; Habib, A. Role of nitric oxide and CCAAT/enhancer-binding protein transcription factor in statin-dependent induction of heme oxygenase-1 in mouse macrophages. PLoS ONE 2013, 8, e64092. [Google Scholar] [CrossRef]
- Lee, T.-S.; Chang, C.-C.; Zhu, Y.; Shyy, J.Y.-J. Simvastatin induces heme oxygenase-1: A novel mechanism of vessel protection. Circulation 2004, 110, 1296–1302. [Google Scholar] [CrossRef]
- Pinho-Ribeiro, V.; Melo, A.C.; Kennedy-Feitosa, E.; Graca-Reis, A.; Barroso, M.V.; Cattani-Cavalieri, I.; Carvalho, G.M.C.; Zin, W.A.; Porto, L.C.; Gitirana, L.B. Atorvastatin and simvastatin promoted mouse lung repair after cigarette smoke-induced emphysema. Inflammation 2017, 40, 965–979. [Google Scholar] [CrossRef] [PubMed]
- El-Achkar, G.A.; Mrad, M.F.; Mouawad, C.A.; Badran, B.; Jaffa, A.A.; Motterlini, R.; Hamade, E.; Habib, A. Heme oxygenase-1—Dependent anti-inflammatory effects of atorvastatin in zymosan-injected subcutaneous air pouch in mice. PLoS ONE 2019, 14, e0216405. [Google Scholar]
- Lin, C.-C.; Lin, W.-N.; Cho, R.-L.; Yang, C.-C.; Yeh, Y.-C.; Hsiao, L.-D.; Tseng, H.-C.; Yang, C.-M. Induction of HO-1 by mevastatin mediated via a Nox/ROS-dependent c-Src/PDGFRα/PI3K/Akt/Nrf2/ARE cascade suppresses TNF-α-induced lung inflammation. J. Clin. Med. 2020, 9, 226. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.-H.; Ko, W.-J.; Hsu, J.-Y.; Chen, J.-S.; Lee, Y.-C.; Lai, I.-R.; Chen, C.-F. Simvastatin ameliorates established pulmonary hypertension through a heme oxygenase-1 dependent pathway in rats. Respir. Res. 2009, 10, 1–13. [Google Scholar] [CrossRef]
- Zhang, W.-h.; Zhang, Y.-j.; Liu, C.-p.; Yu, B.-x.; Lu, W.-x. Simvastatin protects against the development of monocrotaline-induced pulmonary hypertension in rats via a heme oxygenase-1–dependent pathway. Exp. Lung Res. 2011, 37, 492–499. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Li, Y.; Ji, Z. Atorvastatin attenuates inflammation and oxidative stress induced by ischemia/reperfusion in rat heart via the Nrf2 transcription factor. Int. J. Clin. Exp. Med. 2015, 8, 14837. [Google Scholar]
- Rodrigues, G.; Moreira, A.J.; Bona, S.; Schemitt, E.; Marroni, C.A.; Di Naso, F.C.; Dias, A.S.; Pires, T.R.; Picada, J.N.; Marroni, N.P. Simvastatin reduces hepatic oxidative stress and endoplasmic reticulum stress in nonalcoholic steatohepatitis experimental model. Oxidative Med. Cell. Longev. 2019, 2019, 3201873. [Google Scholar] [CrossRef]
- McGregor, G.H.; Campbell, A.D.; Fey, S.K.; Tumanov, S.; Sumpton, D.; Blanco, G.R.; Mackay, G.; Nixon, C.; Vazquez, A.; Sansom, O.J. Targeting the metabolic response to statin-mediated oxidative stress produces a synergistic antitumor response. Cancer Res. 2020, 80, 175–188. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Sun, C.-K.; Lu, T.-H.; Chen, Y.-C.; Lin, C.-J.; Wu, C.-J.; Rau, C.-S.; Jeng, S.-F. Simvastatin induces heme oxygenase-1 expression but fails to reduce inflammation in the capsule surrounding a silicone shell implant in rats. J. Surg. Res. 2011, 168, 272–280. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, K.S.; Kim, N.R.; Chin, H.S. Effects of simvastatin on the expression of heme oxygenase-1 in human RPE cells. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6456–6464. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.-C.; Huang, K.-C.; Lin, W.-W. HMG–CoA reductase inhibitors upregulate heme oxygenase-1 expression in murine RAW264. 7 macrophages via ERK, p38 MAPK and protein kinase G pathways. Cell. Signal. 2006, 18, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-M.; Lin, C.-C.; Yang, C.-C.; Cho, R.-L.; Hsiao, L.-D. Mevastatin-Induced AP-1-Dependent HO-1 Expression Suppresses Vascular Cell Adhesion Molecule-1 Expression and Monocyte Adhesion on Human Pulmonary Alveolar Epithelial Cells Challenged with TNF-α. Biomolecules 2020, 10, 381. [Google Scholar] [CrossRef] [PubMed]
- Ihoriya, C.; Satoh, M.; Komai, N.; Sasaki, T.; Kashihara, N. Nuclear factor erythroid 2-related factor 2 is activated by rosuvastatin via p21cip1 upregulation in endothelial cells. Biochem. Pharmacol. 2014, 4, 501–2167. [Google Scholar]
- Leung, P.-O.; Wang, S.-H.; Lu, S.-H.; Chou, W.-H.; Shiau, C.-Y.; Chou, T.-C. Simvastatin inhibits pro-inflammatory mediators through induction of heme oxygenase-1 expression in lipopolysaccharide-stimulated RAW264. 7 macrophages. Toxicol. Lett. 2011, 207, 159–166. [Google Scholar] [CrossRef]
- Hwang, A.-R.; Han, J.-H.; Lim, J.H.; Kang, Y.J.; Woo, C.-H. Fluvastatin inhibits AGE-induced cell proliferation and migration via an ERK5-dependent Nrf2 pathway in vascular smooth muscle cells. PLoS ONE 2017, 12, e0178278. [Google Scholar] [CrossRef]
- Makabe, S.; Takahashi, Y.; Watanabe, H.; Murakami, M.; Ohba, T.; Ito, H. Fluvastatin protects vascular smooth muscle cells against oxidative stress through the Nrf2-dependent antioxidant pathway. Atherosclerosis 2010, 213, 377–384. [Google Scholar] [CrossRef]
- Pantan, R.; Tocharus, J.; Suksamrarn, A.; Tocharus, C. Synergistic effect of atorvastatin and Cyanidin-3-glucoside on angiotensin II-induced inflammation in vascular smooth muscle cells. Exp. Cell Res. 2016, 342, 104–112. [Google Scholar] [CrossRef]
- Yeh, Y.-H.; Kuo, C.-T.; Chang, G.-J.; Chen, Y.-H.; Lai, Y.-J.; Cheng, M.-L.; Chen, W.-J. Rosuvastatin suppresses atrial tachycardia-induced cellular remodeling via Akt/Nrf2/heme oxygenase-1 pathway. J. Mol. Cell. Cardiol. 2015, 82, 84–92. [Google Scholar] [CrossRef]
- Kwok, S.C.; Samuel, S.P.; Handal, J. Atorvastatin activates heme oxygenase-1 at the stress response elements. J. Cell. Mol. Med. 2012, 16, 394–400. [Google Scholar] [CrossRef]
- Dulak, J.; Loboda, A.; Jazwa, A.; Zagorska, A.; Dörler, J.; Alber, H.; Dichtl, W.; Weidinger, F.; Frick, M.; Jozkowicz, A. Atorvastatin affects several angiogenic mediators in human endothelial cells. Endothelium 2005, 12, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Loboda, A.; Jazwa, A.; Jozkowicz, A.; Dorosz, J.; Balla, J.; Molema, G.; Dulak, J. Atorvastatin prevents hypoxia-induced inhibition of endothelial nitric oxide synthase expression but does not affect heme oxygenase-1 in human microvascular endothelial cells. Atherosclerosis 2006, 187, 26–30. [Google Scholar] [CrossRef] [PubMed]
- Barnett, M.; Hall, S.; Dixit, M.; Arany, I. Simvastatin attenuates oleic acid-induced oxidative stress through CREB-dependent induction of heme oxygenase-1 in renal proximal tubule cells. Pediatric Res. 2016, 79, 243–250. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Qiao, L.; Wu, J.; Fan, H.; Sun, J.; Zhang, Y. Simvastatin protects dopaminergic neurons against MPP+-induced oxidative stress and regulates the endogenous anti-oxidant system through ERK. Cell. Physiol. Biochem. 2018, 51, 1957–1968. [Google Scholar] [CrossRef]
- Grosser, N.; Hemmerle, A.; Berndt, G.; Erdmann, K.; Hinkelmann, U.; Schürger, S.; Wijayanti, N.; Immenschuh, S.; Schröder, H. The antioxidant defense protein heme oxygenase 1 is a novel target for statins in endothelial cells. Free. Radic. Biol. Med. 2004, 37, 2064–2071. [Google Scholar] [CrossRef]
- Hsieh, C.-H.; Rau, C.-S.; Hsieh, M.-W.; Chen, Y.-C.; Jeng, S.-F.; Lu, T.-H.; Chen, S.-S. Simvastatin-induced heme oxygenase-1 increases apoptosis of Neuro 2A cells in response to glucose deprivation. Toxicol. Sci. 2008, 101, 112–121. [Google Scholar] [CrossRef][Green Version]
- Benjamin, E.J.; Blaha, M.J.; Chiuve, S.E.; Cushman, M.; Das, S.R.; Deo, R.; De Ferranti, S.D.; Floyd, J.; Fornage, M.; Gillespie, C. Heart disease and stroke statistics—2017 update: A report from the American Heart Association. Circulation 2017, 135, e146–e603. [Google Scholar] [CrossRef]
- Wang, H.; Lai, Y.; Mathis, B.J.; Wang, W.; Li, S.; Qu, C.; Li, B.; Shao, L.; Song, H.; Janicki, J.S. Deubiquitinating enzyme CYLD mediates pressure overload-induced cardiac maladaptive remodeling and dysfunction via downregulating Nrf2. J. Mol. Cell. Cardiol. 2015, 84, 143–153. [Google Scholar] [CrossRef]
- Bata, I.R.; Gregor, R.D.; Wolf, H.K.; Brownell, B. Trends in five-year survival of patients discharged after acute myocardial infarction. Can. J. Cardiol. 2006, 22, 399–404. [Google Scholar] [CrossRef]
- Tyldum, G.A.; Schjerve, I.E.; Tjønna, A.E.; Kirkeby-Garstad, I.; Stølen, T.O.; Richardson, R.S.; Wisløff, U. Endothelial dysfunction induced by post-prandial lipemia: Complete protection afforded by high-intensity aerobic interval exercise. J. Am. Coll. Cardiol. 2009, 53, 200–206. [Google Scholar] [CrossRef]
- Johansson, S.; Rosengren, A.; Young, K.; Jennings, E. Mortality and morbidity trends after the first year in survivors of acute myocardial infarction: A systematic review. BMC Cardiovasc. Disord. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tjia, J.; Allison, J.; Saczynski, J.S.; Tisminetzky, M.; Givens, J.L.; Lapane, K.; Lessard, D.; Goldberg, R.J. Encouraging trends in acute myocardial infarction survival in the oldest old. Am. J. Med. 2013, 126, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Januzzi Jr, J. Troponin testing after cardiac surgery. HSR Proc. Intensive Care Cardiovasc. Anesth. 2009, 1, 22. [Google Scholar]
- Chen, J.; Liu, B.; Yuan, J.; Yang, J.; Zhang, J.; An, Y.; Tie, L.; Pan, Y.; Li, X. Atorvastatin reduces vascular endothelial growth factor (VEGF) expression in human non-small cell lung carcinomas (NSCLCs) via inhibition of reactive oxygen species (ROS) production. Mol. Oncol. 2012, 6, 62–72. [Google Scholar] [CrossRef]
- Bae, S.K.; Lee, S.J.; Kim, Y.G.; Kim, S.H.; Kim, J.W.; Kim, T.; Lee, M.G. Interspecies pharmacokinetic scaling of oltipraz in mice, rats, rabbits and dogs, and prediction of human pharmacokinetics. Biopharm. Drug Dispos. 2005, 26, 99–115. [Google Scholar] [CrossRef]
- Almeida, S.O.; Budoff, M. Effect of statins on atherosclerotic plaque. Trends Cardiovasc. Med. 2019, 29, 451–455. [Google Scholar] [CrossRef]
- Barnes, P.J. Future treatments for chronic obstructive pulmonary disease and its comorbidities. Proc. Am. Thorac. Soc. 2008, 5, 857–864. [Google Scholar] [CrossRef]
- Athanazio, R. Airway disease: Similarities and differences between asthma, COPD and bronchiectasis. Clinics 2012, 67, 1335–1343. [Google Scholar] [CrossRef]
- Marin, L.; Colombo, P.; Bebawy, M.; Young, P.M.; Traini, D. Chronic obstructive pulmonary disease: Patho-physiology, current methods of treatment and the potential for simvastatin in disease management. Expert Opin. Drug Deliv. 2011, 8, 1205–1220. [Google Scholar] [CrossRef]
- Mehrbod, P.; Omar, A.R.; Hair-Bejo, M.; Haghani, A.; Ideris, A. Mechanisms of action and efficacy of statins against influenza. BioMed Res. Int. 2014, 2014, 872370. [Google Scholar] [CrossRef]
- Liao, J.K.; Laufs, U. Pleiotropic effects of statins. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 89–118. [Google Scholar] [CrossRef] [PubMed]
- Momo, K.; Takagi, A.; Miyaji, A.; Koinuma, M. Assessment of statin-induced interstitial pneumonia in patients treated for hyperlipidemia using a health insurance claims database in Japan. Pulm. Pharmacol. Ther. 2018, 50, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Jo, T.; Michihata, N.; Yamana, H.; Morita, K.; Ishimaru, M.; Yamauchi, Y.; Hasegawa, W.; Urushiyama, H.; Uda, K.; Matsui, H. Risk of drug-induced interstitial lung disease in hospitalised patients: A nested case–control study. Thorax 2021, 76, 1193–1199. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.W.; Barrett, K.; Loke, Y.; Wilson, A.M. The effect of statin therapy on disease-related outcomes in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Respir. Med. Res. 2021, 80, 100792. [Google Scholar] [CrossRef]
- Kang, J.; Jeong, S.-M.; Shin, D.W.; Cho, M.; Cho, J.H.; Kim, J. The associations of aspirin, statins, and metformin with lung cancer risk and related mortality: A time-dependent analysis of population-based nationally representative data. J. Thorac. Oncol. 2021, 16, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Coresh, J.; Astor, B.C.; Greene, T.; Eknoyan, G.; Levey, A.S. Prevalence of chronic kidney disease and decreased kidney function in the adult US population: Third National Health and Nutrition Examination Survey. Am. J. Kidney Dis. 2003, 41, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Levey, A.S.; Coresh, J.; Bolton, K.; Culleton, B.; Harvey, K.S.; Ikizler, T.A.; Johnson, C.A.; Kausz, A.; Kimmel, P.L.; Kusek, J. K/DOQI clinical practice guidelines for chronic kidney disease: Evaluation, classification, and stratification. Am. J. Kidney Dis. 2002, 39, S1–S266. [Google Scholar]
- Kopple, J.D. National kidney foundation K/DOQI clinical practice guidelines for nutrition in chronic renal failure. Am. J. Kidney Dis. 2001, 37, S66–S70. [Google Scholar] [CrossRef]
- Sarnak, M.J.; Levey, A.S.; Schoolwerth, A.C.; Coresh, J.; Culleton, B.; Hamm, L.L.; McCullough, P.A.; Kasiske, B.L.; Kelepouris, E.; Klag, M.J. Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 2003, 108, 2154–2169. [Google Scholar] [CrossRef]
- O’Donnell, M.P.; Kasiske, B.L.; Katz, S.A.; Schmitz, P.G.; Keane, W.F. Lovastatin but not enalapril reduces glomerular injury in Dahl salt-sensitive rats. Hypertension 1992, 20, 651–658. [Google Scholar] [CrossRef]
- Rubin, R.; Silbiger, S.; Sablay, L.; Neugarten, J. Combined antihypertensive and lipid-lowering therapy in experimental glomerulonephritis. Hypertension 1994, 23, 92–95. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Vázquez-Pérez, S.; Aragoncillo, P.; de las Heras, N.; Navarro-Cid, J.; Cediel, E.; Sanz-Rosa, D.; Ruilope, L.M.; Díaz, C.; Hernández, G.; Lahera, V. Atorvastatin prevents glomerulosclerosis and renal endothelial dysfunction in hypercholesterolaemic rabbits. Nephrol. Dial. Transplant. 2001, 16, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Epstein, M.; Campese, V.M. Pleiotropic effects of 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors on renal function. Am. J. Kidney Dis. 2005, 45, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Blanco-Colio, L.M.; Tuñón, J.; Martín-Ventura, J.L.; Egido, J. Anti-inflammatory and immunomodulatory effects of statins. Kidney Int. 2003, 63, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rong, S.; Feng, Y.; Zhao, L.; Hong, J.; Wang, R.; Yuan, W. Simvastatin attenuates renal ischemia/reperfusion injury from oxidative stress via targeting Nrf2/HO-1 pathway. Exp. Ther. Med. 2017, 14, 4460–4466. [Google Scholar] [CrossRef] [PubMed]
- Shlipak, M.G.; Fried, L.F.; Crump, C.; Bleyer, A.J.; Manolio, T.A.; Tracy, R.P.; Furberg, C.D.; Psaty, B.M. Elevations of inflammatory and procoagulant biomarkers in elderly persons with renal insufficiency. Circulation 2003, 107, 87–92. [Google Scholar] [CrossRef]
- Verma, A.; Ranganna, K.M.; Reddy, R.S.; Verma, M.; Gordon, N.F. Effect of rosuvastatin on C-reactive protein and renal function in patients with chronic kidney disease. Am. J. Cardiol. 2005, 96, 1290–1292. [Google Scholar] [CrossRef]
- Loria, P.; Lonardo, A.; Bellentani, S.; Day, C.; Marchesini, G.; Carulli, N. Non-alcoholic fatty liver disease (NAFLD) and cardiovascular disease: An open question. Nutr. Metab. Cardiovasc. Dis. 2007, 17, 684–698. [Google Scholar] [CrossRef]
- Targher, G.; Bertolini, L.; Padovani, R.; Rodella, S.; Arcaro, G.; Day, C. Differences and similarities in early atherosclerosis between patients with non-alcoholic steatohepatitis and chronic hepatitis B and C. J. Hepatol. 2007, 46, 1126–1132. [Google Scholar] [CrossRef]
- Longo, M.; Crosignani, A.; Battezzati, P.; Giussani, C.S.; Invernizzi, P.; Zuin, M.; Podda, M. Hyperlipidaemic state and cardiovascular risk in primary biliary cirrhosis. Gut 2002, 51, 265–269. [Google Scholar] [CrossRef]
- Dongiovanni, P.; Paolini, E.; Corsini, A.; Sirtori, C.; Ruscica, M. NAFLD or MAFLD diagnoses and cardiovascular diseases: From epidemiology to drug approaches. Eur. J. Clin. Investig. 2021, 51, e13519. [Google Scholar] [CrossRef] [PubMed]
- Jasiñska, M.; Owczarek, J.; Orszulak-Michalak, D. -Statins: A new insight into their mechanisms of action and consequent pleiotropic effects. Pharmacol. Rep. 2007, 59, 483. [Google Scholar] [PubMed]
- Ridker, P.M.; Rifai, N.; Clearfield, M.; Downs, J.R.; Weis, S.E.; Miles, J.S.; Gotto Jr, A.M. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. New Engl. J. Med. 2001, 344, 1959–1965. [Google Scholar] [CrossRef] [PubMed]
- Shitara, Y.; Sugiyama, Y. Pharmacokinetic and pharmacodynamic alterations of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors: Drug–drug interactions and interindividual differences in transporter and metabolic enzyme functions. Pharmacol. Ther. 2006, 112, 71–105. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Rahme, E.; Pilote, L. Are statins created equal? Evidence from randomized trials of pravastatin, simvastatin, and atorvastatin for cardiovascular disease prevention. Am. Heart J. 2006, 151, 273–281. [Google Scholar] [CrossRef]
- Moosmann, B.; Behl, C. Selenoproteins, cholesterol-lowering drugs, and the consequences revisiting of the mevalonate pathway. Trends Cardiovasc. Med. 2004, 14, 273–281. [Google Scholar] [CrossRef] [PubMed]
- WEISS, S.L.; SUNDE, R.A. Cis-acting elements are required for selenium regulation of glutathione peroxidase-1 mRNA levels. Rna 1998, 4, 816–827. [Google Scholar] [CrossRef]
- Athyros, V.G.; Tziomalos, K.; Gossios, T.D.; Griva, T.; Anagnostis, P.; Kargiotis, K.; Pagourelias, E.D.; Theocharidou, E.; Karagiannis, A.; Mikhailidis, D.P. Safety and efficacy of long-term statin treatment for cardiovascular events in patients with coronary heart disease and abnormal liver tests in the Greek Atorvastatin and Coronary Heart Disease Evaluation (GREACE) Study: A post-hoc analysis. Lancet 2010, 376, 1916–1922. [Google Scholar] [CrossRef]
- Fraunfelder, F. Ocular examination before initiation of lovastatin (Mevacor) therapy. Am. J. Ophthalmol. 1988, 105, 91–92. [Google Scholar] [CrossRef]
- Congdon, N.G.; Friedman, D.S.; Lietman, T. Important causes of visual impairment in the world today. JAMA 2003, 290, 2057–2060. [Google Scholar] [CrossRef] [PubMed]
- VanderBeek, B.L.; Zacks, D.N.; Talwar, N.; Nan, B.; Stein, J.D. Role of statins in the development and progression of age-related macular degeneration. Retina 2013, 33, 414–422. [Google Scholar] [CrossRef]
- Stein, J.D.; Newman-Casey, P.A.; Talwar, N.; Nan, B.; Richards, J.E.; Musch, D.C. The relationship between statin use and open-angle glaucoma. Ophthalmology 2012, 119, 2074–2081. [Google Scholar] [CrossRef] [PubMed]
- Marcus, M.W.; Müskens, R.P.; Ramdas, W.D.; Wolfs, R.C.; De Jong, P.T.; Vingerling, J.R.; Hofman, A.; Stricker, B.H.; Jansonius, N.M. Cholesterol-lowering drugs and incident open-angle glaucoma: A population-based cohort study. PLoS ONE 2012, 7, e29724. [Google Scholar] [CrossRef] [PubMed]
- Vajaranant, T.S.; Wu, S.; Torres, M.; Varma, R. The changing face of primary open-angle glaucoma in the United States: Demographic and geographic changes from 2011 to 2050. Am. J. Ophthalmol. 2012, 154, 303–314.e303. [Google Scholar] [CrossRef] [PubMed]
- Hui, Q.; Karlstetter, M.; Xu, Z.; Yang, J.; Zhou, L.; Eilken, H.M.; Terjung, C.; Cho, H.; Gong, J.; Lai, M.J. Inhibition of the Keap1-Nrf2 protein-protein interaction protects retinal cells and ameliorates retinal ischemia-reperfusion injury. Free. Radic. Biol. Med. 2020, 146, 181–188. [Google Scholar] [CrossRef]
- Nagaoka, T.; Takahashi, A.; Sato, E.; Izumi, N.; Hein, T.W.; Kuo, L.; Yoshida, A. Effect of systemic administration of simvastatin on retinal circulation. Arch. Ophthalmol. 2006, 124, 665–670. [Google Scholar] [CrossRef]
- Schmeer, C.; Kretz, A.; Isenmann, S. Statin-mediated protective effects in the central nervous system: General mechanisms and putative role of stress proteins. Restor. Neurol. Neurosci. 2006, 24, 79–95. [Google Scholar] [PubMed]
- Wong, W.W.; Dimitroulakos, J.; Minden, M.; Penn, L. HMG-CoA reductase inhibitors and the malignant cell: The statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 2002, 16, 508–519. [Google Scholar] [CrossRef]
- Chan, K.K.; Oza, A.M.; Siu, L.L. The statins as anticancer agents. Clin. Cancer Res. 2003, 9, 10–19. [Google Scholar]
- Mannello, F.; Tonti, G.A. Statins and breast cancer: May matrix metalloproteinase be the missing link. Cancer Investig. 2009, 27, 466–470. [Google Scholar] [CrossRef]
- Denoyelle, C.; Albanese, P.; Uzan, G.; Hong, L.; Vannier, J.-P.; Soria, J.; Soria, C. Molecular mechanism of the anti-cancer activity of cerivastatin, an inhibitor of HMG-CoA reductase, on aggressive human breast cancer cells. Cell. Signal. 2003, 15, 327–338. [Google Scholar] [CrossRef]
- Seeger, H.; Wallwiener, D.; Mueck, A. Statins can inhibit proliferation of human breast cancer cells in vitro. Exp. Clin. Endocrinol. Diabetes 2003, 111, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Padayatty, S.; Marcelli, M.; Shao, T.; Cunningham, G. Lovastatin-induced apoptosis in prostate stromal cells. J. Clin. Endocrinol. Metab. 1997, 82, 1434–1439. [Google Scholar] [CrossRef] [PubMed]
- Wächtershäuser, A.; Akoglu, B.; Stein, J. HMG-CoA reductase inhibitor mevastatin enhances the growth inhibitory effect of butyrate in the colorectal carcinoma cell line Caco-2. Carcinogenesis 2001, 22, 1061–1067. [Google Scholar] [CrossRef] [PubMed]
- Hawk, M.A.; Cesen, K.T.; Siglin, J.C.; Stoner, G.D.; Ruch, R.J. Inhibition of lung tumor cell growth in vitro and mouse lung tumor formation by lovastatin. Cancer Lett. 1996, 109, 217–222. [Google Scholar] [CrossRef]
- Blais, L.; Desgagné, A.; LeLorier, J. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors and the risk of cancer: A nested case-control study. Arch. Intern. Med. 2000, 160, 2363–2368. [Google Scholar] [CrossRef]
- Haukka, J.; Sankila, R.; Klaukka, T.; Lonnqvist, J.; Niskanen, L.; Tanskanen, A.; Wahlbeck, K.; Tiihonen, J. Incidence of cancer and statin usage—record linkage study. Int. J. Cancer 2010, 126, 279–284. [Google Scholar] [CrossRef]
- Desai, P.; Chlebowski, R.; Cauley, J.A.; Manson, J.E.; Wu, C.; Martin, L.W.; Jay, A.; Bock, C.; Cote, M.; Petrucelli, N. Prospective analysis of association between statin use and breast cancer risk in the women’s health initiative. Cancer Epidemiol. Prev. Biomark. 2013, 22, 1868–1876. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ren, Q.-W.; Yu, S.-Y.; Teng, T.-H.K.; Li, X.; Cheung, K.-S.; Wu, M.-Z.; Li, H.-L.; Wong, P.-F.; Tse, H.-F.; Lam, C.S. Statin associated lower cancer risk and related mortality in patients with heart failure. Eur. Heart J. 2021, 42, 3049–3059. [Google Scholar] [CrossRef] [PubMed]
- Eliassen, A.H.; Colditz, G.A.; Rosner, B.; Willett, W.C.; Hankinson, S.E. Serum lipids, lipid-lowering drugs, and the risk of breast cancer. Arch. Intern. Med. 2005, 165, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Boudreau, D.M.; Yu, O.; Miglioretti, D.L.; Buist, D.S.; Heckbert, S.R.; Daling, J.R. Statin use and breast cancer risk in a large population-based setting. Cancer Epidemiol. Prev. Biomark. 2007, 16, 416–421. [Google Scholar] [CrossRef]
- Friis, S.; Poulsen, A.H.; Johnsen, S.P.; McLaughlin, J.K.; Fryzek, J.P.; Dalton, S.O.; Sørensen, H.T.; Olsen, J.H. Cancer risk among statin users: A population-based cohort study. Int. J. Cancer 2005, 114, 643–647. [Google Scholar] [CrossRef]
- Friedman, G.D.; Flick, E.D.; Udaltsova, N.; Chan Pharm, D.J.; Quesenberry, C.P., Jr.; Habel, L.A. Screening statins for possible carcinogenic risk: Up to 9 years of follow-up of 361 859 recipients. Pharmacoepidemiol. Drug Saf. 2008, 17, 27–36. [Google Scholar] [CrossRef]
- Graaf, M.R.; Beiderbeck, A.B.; Egberts, A.C.; Richel, D.J.; Guchelaar, H.-J. The risk of cancer in users of statins. J. Clin. Oncol. 2004, 22, 2388–2394. [Google Scholar] [CrossRef] [PubMed]
- Coogan, P.F.; Rosenberg, L.; Strom, B.L. Statin use and the risk of 10 cancers. Epidemiology 2007, 18, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Zielinski, S.L. Following positive epidemiologic studies, statins to enter clinical trials for cancer prevention. J. Natl. Cancer Inst. 2005, 97, 1172–1173. [Google Scholar] [CrossRef][Green Version]
- Brookhart, M.A.; Patrick, A.R.; Dormuth, C.; Avorn, J.; Shrank, W.; Cadarette, S.M.; Solomon, D.H. Adherence to lipid-lowering therapy and the use of preventive health services: An investigation of the healthy user effect. Am. J. Epidemiol. 2007, 166, 348–354. [Google Scholar] [CrossRef] [PubMed]
- Group, C.f.t.A.C.R. The antihypertensive and lipid-lowering treatment to prevent heart attack trial. Major outcomes in moderately hypercholesterolemic, hypertensive patients randomized to pravastatin vs usual care: The antihypertensive and lipid-lowering treatment to prevent heart attack trial (ALLHAT-LLT). JAMA 2002, 288, 2981–2997. [Google Scholar]
- Ford, I.; Murray, H.; Packard, C.J.; Shepherd, J.; Macfarlane, P.W.; Cobbe, S.M. Long-term follow-up of the West of Scotland Coronary Prevention Study. New Engl. J. Med. 2007, 357, 1477–1486. [Google Scholar] [CrossRef]
- Sivaprasad, U.; Abbas, T.; Dutta, A. Differential efficacy of 3-hydroxy-3-methylglutaryl CoA reductase inhibitors on the cell cycle of prostate cancer cells. Mol. Cancer Ther. 2006, 5, 2310–2316. [Google Scholar] [CrossRef]
- Duncan, R.E.; El-Sohemy, A.; Archer, M.C. Statins and cancer development. Cancer Epidemiol. Prev. Biomark. 2005, 14, 1897–1898. [Google Scholar] [CrossRef]
- Hamilton, R.J.; Goldberg, K.C.; Platz, E.A.; Freedland, S.J. The influence of statin medications on prostate-specific antigen levels. JNCI J. Natl. Cancer Inst. 2008, 100, 1511–1518. [Google Scholar] [CrossRef]
- Platz, E.A.; Leitzmann, M.F.; Visvanathan, K.; Rimm, E.B.; Stampfer, M.J.; Willett, W.C.; Giovannucci, E. Statin drugs and risk of advanced prostate cancer. J. Natl. Cancer Inst. 2006, 98, 1819–1825. [Google Scholar] [CrossRef]
- Flick, E.D.; Habel, L.A.; Chan, K.A.; Van Den Eeden, S.K.; Quinn, V.P.; Haque, R.; Orav, E.J.; Seeger, J.D.; Sadler, M.C.; Quesenberry, C.P. Statin use and risk of prostate cancer in the California Men’s Health Study cohort. Cancer Epidemiol. Prev. Biomark. 2007, 16, 2218–2225. [Google Scholar] [CrossRef]
- Tenhunen, R.; Marver, H.S.; Schmid, R. The enzymatic conversion of heme to bilirubin by microsomal heme oxygenase. Proc. Natl. Acad. Sci. USA 1968, 61, 748. [Google Scholar] [CrossRef]
- Itoh, K.; Wakabayashi, N.; Katoh, Y.; Ishii, T.; Igarashi, K.; Engel, J.D.; Yamamoto, M. Keap1 represses nuclear activation of antioxidant responsive elements by Nrf2 through binding to the amino-terminal Neh2 domain. Genes Dev. 1999, 13, 76–86. [Google Scholar] [CrossRef]
- Rock, R.B.; Gekker, G.; Hu, S.; Sheng, W.S.; Cheeran, M.; Lokensgard, J.R.; Peterson, P.K. Role of microglia in central nervous system infections. Clin. Microbiol. Rev. 2004, 17, 942. [Google Scholar] [CrossRef]
- Garzón, D.; Cabezas, R.; Vega, N.; Ávila-Rodriguez, M.; Gonzalez, J.; Gómez, R.M.; Echeverria, V.; Aliev, G.; Barreto, G.E. Novel approaches in astrocyte protection: From experimental methods to computational approaches. J. Mol. Neurosci. 2016, 58, 483–492. [Google Scholar] [CrossRef]
- Kipnis, J. Multifaceted interactions between adaptive immunity and the central nervous system. Science 2016, 353, 766–771. [Google Scholar] [CrossRef]
- Greter, M.; Merad, M. Regulation of microglia development and homeostasis. Glia 2013, 61, 121–127. [Google Scholar] [CrossRef]
- Wu, T.; Tan, L.; Cheng, N.; Yan, Q.; Zhang, Y.-F.; Liu, C.-J.; Shi, B. PNIPAAM modified mesoporous hydroxyapatite for sustained osteogenic drug release and promoting cell attachment. Mater. Sci. Eng. C 2016, 62, 888–896. [Google Scholar] [CrossRef]
- Thameem Dheen, S.; Kaur, C.; Ling, E.-A. Microglial activation and its implications in the brain diseases. Curr. Med. Chem. 2007, 14, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Zhang, Y.; Chen, Y.; Zhu, J.; Yang, Y.; Zhang, H.-L. Role of microglia in neurological disorders and their potentials as a therapeutic target. Mol. Neurobiol. 2017, 54, 7567–7584. [Google Scholar] [CrossRef] [PubMed]
- Bachiller, S.; Jiménez-Ferrer, I.; Paulus, A.; Yang, Y.; Swanberg, M.; Deierborg, T.; Boza-Serrano, A. Microglia in neurological diseases: A road map to brain-disease dependent-inflammatory response. Front. Cell. Neurosci. 2018, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Parizadeh, S.M.; Azarpazhooh, M.R.; Moohebati, M.; Nematy, M.; Ghayour-Mobarhan, M.; Tavallaie, S.; Rahsepar, A.A.; Amini, M.; Sahebkar, A.; Mohammadi, M. Simvastatin therapy reduces prooxidant-antioxidant balance: Results of a placebo-controlled cross-over trial. Lipids 2011, 46, 333–340. [Google Scholar] [CrossRef]
- Sahebkar, A.; Serban, C.; Ursoniu, S.; Mikhailidis, D.P.; Undas, A.; Lip, G.Y.; Bittner, V.; Ray, K.K.; Watts, G.F.; Kees Hovingh, G. The impact of statin therapy on plasma levels of von Willebrand factor antigen: Systematic review and meta-analysis of randomised placebo-controlled trials. Thromb. Haemost. 2016, 115, 520–532. [Google Scholar] [CrossRef]
- van der Most, P.J.; Dolga, A.M.; Nijholt, I.M.; Luiten, P.G.; Eisel, U.L. Statins: Mechanisms of neuroprotection. Prog. Neurobiol. 2009, 88, 64–75. [Google Scholar] [CrossRef]
- McFarland, A.; Davey, A.; Anoopkumar-Dukie, S. Statins reduce lipopolysaccharide-induced cytokine and inflammatory mediator release in an in vitro model of microglial-like cells. Mediat. Inflamm. 2017, 2017, 2582745. [Google Scholar] [CrossRef]
- Chen, X.; Yan, L.; Guo, Z.; Chen, Z.; Chen, Y.; Li, M.; Huang, C.; Zhang, X.; Chen, L. Adipose-derived mesenchymal stem cells promote the survival of fat grafts via crosstalk between the Nrf2 and TLR4 pathways. Cell Death Dis. 2016, 7, e2369. [Google Scholar] [CrossRef]
- Huang, J.; Shen, X.-D.; Yue, S.; Zhu, J.; Gao, F.; Zhai, Y.; Busuttil, R.W.; Ke, B.; Kupiec-Weglinski, J.W. Adoptive transfer of heme oxygenase-1 (HO-1)-modified macrophages rescues the nuclear factor erythroid 2-related factor (Nrf2) antiinflammatory phenotype in liver ischemia/reperfusion injury. Mol. Med. 2014, 20, 448–455. [Google Scholar] [CrossRef]
- Kang, K.; Kim, Y.; Kim, Y.; Roh, J.; Nam, J.; Kim, P.; Ryu, W.; Lee, S.; Yoon, B. Lithium pretreatment reduces brain injury after intracerebral hemorrhage in rats. Neurol. Res. 2012, 34, 447–454. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, H.; Ghasemi, F.; Barreto, G.E.; Sathyapalan, T.; Jamialahmadi, T.; Sahebkar, A. The effects of statins on microglial cells to protect against neurodegenerative disorders: A mechanistic review. BioFactors 2020, 46, 309–325. [Google Scholar] [CrossRef] [PubMed]
- King, G.L.; Buzney, S.M.; Kahn, C.R.; Hetu, N.; Buchwald, S.; Macdonald, S.; Rand, L. Differential responsiveness to insulin of endothelial and support cells from micro-and macrovessels. J. Clin. Investig. 1983, 71, 974–979. [Google Scholar] [CrossRef]
- He, X.; Kan, H.; Cai, L.; Ma, Q. Nrf2 is critical in defense against high glucose-induced oxidative damage in cardiomyocytes. J. Mol. Cell. Cardiol. 2009, 46, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chapple, S.J.; Patel, B.; Puszyk, W.; Sugden, D.; Yin, X.; Mayr, M.; Siow, R.C.; Mann, G.E. Gestational diabetes mellitus impairs Nrf2-mediated adaptive antioxidant defenses and redox signaling in fetal endothelial cells in utero. Diabetes 2013, 62, 4088–4097. [Google Scholar] [CrossRef]
- Meigs, J.B.; O’Donnell, C.J.; Tofler, G.H.; Benjamin, E.J.; Fox, C.S.; Lipinska, I.; Nathan, D.M.; Sullivan, L.M.; D’Agostino, R.B.; Wilson, P.W. Hemostatic markers of endothelial dysfunction and risk of incident type 2 diabetes: The Framingham Offspring Study. Diabetes 2006, 55, 530–537. [Google Scholar] [CrossRef]
- Sakamoto, K.; Murata, T.; Chuma, H.; Hori, M.; Ozaki, H. Fluvastatin prevents vascular hyperplasia by inhibiting phenotype modulation and proliferation through extracellular signal-regulated kinase 1 and 2 and p38 mitogen-activated protein kinase inactivation in organ-cultured artery. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 327–333. [Google Scholar] [CrossRef]
- Group, H.P.S.C. Cost-effectiveness of simvastatin in people at different levels of vascular disease risk: Economic analysis of a randomised trial in 20 536 individuals. Lancet 2005, 365, 1779–1785. [Google Scholar]
- Christ, M.; Bauersachs, J.; Liebetrau, C.; Heck, M.; Günther, A.; Wehling, M. Glucose increases endothelial-dependent superoxide formation in coronary arteries by NAD (P) H oxidase activation: Attenuation by the 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitor atorvastatin. Diabetes 2002, 51, 2648–2652. [Google Scholar] [CrossRef]
- Rajpathak, S.N.; Kumbhani, D.J.; Crandall, J.; Barzilai, N.; Alderman, M.; Ridker, P.M. Statin therapy and risk of developing type 2 diabetes: A meta-analysis. Diabetes Care 2009, 32, 1924–1929. [Google Scholar] [CrossRef] [PubMed]
- Sattar, N.; Preiss, D.; Murray, H.M.; Welsh, P.; Buckley, B.M.; de Craen, A.J.; Seshasai, S.R.K.; McMurray, J.J.; Freeman, D.J.; Jukema, J.W. Statins and risk of incident diabetes: A collaborative meta-analysis of randomised statin trials. Lancet 2010, 375, 735–742. [Google Scholar] [CrossRef]
- Ridker, P.M.; Pradhan, A.; MacFadyen, J.G.; Libby, P.; Glynn, R.J. Cardiovascular benefits and diabetes risks of statin therapy in primary prevention: An analysis from the JUPITER trial. Lancet 2012, 380, 565–571. [Google Scholar] [CrossRef]
- Xia, F.; Xie, L.; Mihic, A.; Gao, X.; Chen, Y.; Gaisano, H.Y.; Tsushima, R.G. Inhibition of cholesterol biosynthesis impairs insulin secretion and voltage-gated calcium channel function in pancreatic β-cells. Endocrinology 2008, 149, 5136–5145. [Google Scholar] [CrossRef] [PubMed]
- Magni, P.; Macchi, C.; Morlotti, B.; Sirtori, C.R.; Ruscica, M. Risk identification and possible countermeasures for muscle adverse effects during statin therapy. Eur. J. Intern. Med. 2015, 26, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Ward, N.C.; Watts, G.F.; Eckel, R.H. Statin Toxicity. Circ. Res. 2019, 124, 328–350. [Google Scholar] [CrossRef] [PubMed]
- Bytyci, I.; Bajraktari, G.; Sahebkar, A.; Penson, P.E.; Rysz, R.; Banach, M.Y. The prevalence of statin intolerance worldwide: A systematic review and meta-analysis with 4,143,517 patients. Eur. Heart J. 2021, 42, ehab724.2943. [Google Scholar] [CrossRef]
- Mach, F.; Ray, K.K.; Wiklund, O.; Corsini, A.; Catapano, A.L.; Bruckert, E.; De Backer, G.; Hegele, R.A.; Hovingh, G.K.; Jacobson, T.A.; et al. Adverse effects of statin therapy: Perception vs. the evidence—Focus on glucose homeostasis, cognitive, renal and hepatic function, haemorrhagic stroke and cataract. Eur. Heart J. 2018, 39, 2526–2539. [Google Scholar] [CrossRef] [PubMed]
- Bouitbir, J.; Charles, A.-L.; Rasseneur, L.; Dufour, S.; Piquard, F.; Geny, B.; Zoll, J. Atorvastatin treatment reduces exercise capacities in rats: Involvement of mitochondrial impairments and oxidative stress. J. Appl. Physiol. 2011, 111, 1477–1483. [Google Scholar] [CrossRef]
- Liu, A.; Wu, Q.; Guo, J.; Ares, I.; Rodríguez, J.-L.; Martínez-Larrañaga, M.-R.; Yuan, Z.; Anadón, A.; Wang, X.; Martínez, M.-A. Statins: Adverse reactions, oxidative stress and metabolic interactions. Pharmacol. Ther. 2019, 195, 54–84. [Google Scholar] [CrossRef]
- Mollazadeh, H.; Tavana, E.; Fanni, G.; Bo, S.; Banach, M.; Pirro, M.; Von Haehling, S.; Jamialahmadi, T.; Sahebkar, A. Effects of statins on mitochondrial pathways. J. Cachexia Sarcopenia Muscle 2021, 12, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Ghosh, M.; Ghosh, S.; Bhattacharyya, S.; Sil, P.C. Atorvastatin induced hepatic oxidative stress and apoptotic damage via MAPKs, mitochondria, calpain and caspase12 dependent pathways. Food Chem. Toxicol. 2015, 83, 36–47. [Google Scholar] [CrossRef] [PubMed]
- Bouitbir, J.; Charles, A.-L.; Echaniz-Laguna, A.; Kindo, M.; Daussin, F.; Auwerx, J.; Piquard, F.; Geny, B.; Zoll, J. Opposite effects of statins on mitochondria of cardiac and skeletal muscles: A ‘mitohormesis’ mechanism involving reactive oxygen species and PGC-1. Eur. Heart J. 2012, 33, 1397–1407. [Google Scholar] [CrossRef] [PubMed]
- Pastori, D.; Pani, A.; Di Rocco, A.; Menichelli, D.; Gazzaniga, G.; Farcomeni, A.; D’Erasmo, L.; Angelico, F.; Del Ben, M.; Baratta, F. Statin liver safety in non-alcoholic fatty liver disease: A systematic review and metanalysis. Br. J. Clin. Pharmacol. 2022, 88, 441–451. [Google Scholar] [CrossRef]
- Vahedian-Azimi, A.; Shojaie, S.; Banach, M.; Heidari, F.; Cicero, A.F.; Khoshfetrat, M.; Jamialahmadi, T.; Sahebkar, A. Statin therapy in chronic viral hepatitis: A systematic review and meta-analysis of nine studies with 195,602 participants. Ann. Med. 2021, 53, 1228–1243. [Google Scholar] [CrossRef]
- Xu, J.; Mukherjee, S. State laws that authorize pharmacists to prescribe naloxone are associated with increased naloxone dispensing in retail pharmacies. Drug Alcohol Depend. 2021, 227, 109012. [Google Scholar] [CrossRef]
- Zhao, L.; Li, S.; Gao, Y. Efficacy of statins on renal function in patients with chronic kidney disease: A systematic review and meta-analysis. Ren. Fail. 2021, 43, 718–728. [Google Scholar] [CrossRef]
- Simic, I.; Reiner, Z. Adverse effects of statins-myths and reality. Curr. Pharm. Des. 2015, 21, 1220–1226. [Google Scholar] [CrossRef]
- Ruscica, M.; Macchi, C.; Pavanello, C.; Corsini, A.; Sahebkar, A.; Sirtori, C.R. Appropriateness of statin prescription in the elderly. Eur. J. Intern. Med. 2018, 50, 33–40. [Google Scholar] [CrossRef] [PubMed]
| Animal | Model | Diseases | Statin Type | Dose | Duration | Main Effect | Ref |
|---|---|---|---|---|---|---|---|
| Mice | Foxn1nu mice | Diabetes mellitus, cardiovascular diseases | Atorvastatin | 30 μmol/L | 2 weeks | Atorvastatin-treated precursors of myeloid angiogenic cells (PAC) — no effect on angiogenesis | [40] |
| Mice | BALB/c or C57BL/6 mice | Inflammatory diseases | Simvastatin and fluvastatin | 5 mM simvastatin and 2 mM fluvastatin; | 6, 12 or 24 h | Statins have anti-inflammatory effects and induce HO-1 in primary macrophages | [41] |
| Mice | male C57BL/6J mice | Coronary heart disease | Simvastatin | (0.75 to 5 mg/kg) | 3, 6, 12, or 24 h | Simvastatin activates HO-1, HO-1 have cytoprotective effects, HO-1 are delivered to hearts and vessels of animal models in order to study myocardial protection—statins cause cardiovascular damage | [42] |
| Mice | male C57BL/6 mice | Chronic obstructive pulmonary disease (COPD) | Atorvastatin and simvastatin | 1 mg/mL | 60 days | Atorvastatin and simvastatin improve the repair of lung damage in mice exposed to cigarettes | [43] |
| C57BL/6J female mice | Inflammatory lung diseases | Atorvastatin | 5 mg/kg | 10 days | Statins have anti-inflammatory effect by influencing HO-1 pathway in vivo | [44] | |
| Mice | Inflammatory lung diseases | Mevastatin | 0.1 mg/kg body weight | 24 h | Mevastatin reduces TNF-α induced ICAM-1 expression via p47phox/Nox/ROS/c-Src/PDGFR_/PI3K/Akt/Nrf2/ARE/HO-1 - statins have beneficial effects in inflammatory lung diseases | [45] | |
| Rats | (MCT-PH) and (CH-PH) rats | Pulmonary hypertension | Simvastatin | (10 mg/kgw | days 21~23) | Simvastatin decreases the severity of PH in two rat models (MCT- and CH-PH) by influencing HO-1 activity | [46] |
| Rat | Sprague-Dawley rats | Pulmonary hypertension | Simvastatin | 2 mg/kg/day | 4 weeks | Simvastatin therapy was useful in early phase of the pulmonary hypertension and in severe inflammation | [47] |
| Rat | Sprague-Dawley rats | Myocardial ischemia/reperfusion injury. | Atorvastatin | 10 mg/kg, i.v | Atorvastatin has protective effects in myocardial ischemia reperfusion injury by activating Nrf2/ARE pathway | [48] | |
| Mice | male C57BL/6 mice | Non-alcoholic steatohepatitis | Simvastatin | mg/kg | 4 weeks | Simvastatin reduces liver damage caused by oxidative and endoplasmic reticulum stress effect in mice with experimental non-alcoholic steatohepatitis | [49] |
| Mice | C57BL/6J background | Cancers | Simvastatin | 50 mg/kg | 7 days | Due to their antioxidant effects statins might be effective in cancer treatment by influencing several specific metabolic pathways and increased oxidative stress which causes cancer effective in cure of cancer | [50] |
| Mice | C57BL/6J female mice | Inflammatory diseases | Atorvastatin | 5 mg/kg | 10 days | Statins have anti-inflammatory effect in inflammatory diseases due to their effect on HO-1 which is an anti-inflammatory substance | [44] |
| Mice | Pulmonary inflammation disease | Mevastatin | 0.1 mg/kg | 24 h | Mevastatin induces HO-1via c-Src/PDGFR_/PI3K/Akt-regulated Nrf2/ARE pathway—TNF-α is suppressed which finally improves inflammatory pulmonary disease | [45] | |
| Mice | male C57BL/6J mice | Cardiovascular disease | Simvastatin | 0.75 to 5 mg/kg | 3, 6, 12, or 24 h | Simvastatin have anti-inflammatory and anti-proliferative effects in diseases by their influence on HO-1 | [42] |
| Rat | adult male Sprague-Dawley rats | Heart disease | Simvastatin | 2 mg/kg | 1, 2 wk, 1, 2, and 3 mo | Simvastatin could not reduce inflammation and could not up-regulate HO-1 | [51] |
| Cell Culture | Diseases | Statin Type | Main Effect | Ref |
|---|---|---|---|---|
| Human RPE cells (ARPE-19; ATCC No.CRL-2302) | Age-related macular degeneration (ARMD) | Simvastatin | Simvastatin may have some clinical benefits in preventing ARMDs due to oxidative stress | [52] |
| Human HAoEC cells peripheral blood CD34+ cells | Diabetes mellitus, cardiovascular diseases | Atorvastatin | High concentrations of atorvastatin could improve the paracrine angiogenic activity | [40] |
| Murine RAW264.7 macrophages RAW264.7 | Inflammatory diseases | Lovastatin, fluvastatin, atorvastatin, simvastatin, mevastatin, and pravastatin | Statins induce HO-1 gene expression and therefore have anti-inflammatory effects | [53] |
| Human pulmonary alveolar epithelial cells (HPAEpiC) | Inflammatory diseases | Mevastatin | Mevastatin induced HO-1 expression so it has an important role in inflammatory diseases via up-regulation of AP-1/HO-1 system | [54] |
| Primary human umbilical vein endothelial cells (HUVECs) | Cardiovascular diseases, chronic kidney disease | Rosuvastatin | Rosuvastatin has antioxidant effects by activating Nrf2 via p21Cip1 up-regulation - statins might be effective in improving antioxidative capacity | [55] |
| RAW 264.7 and J774A.1 cells | Inflammatory diseases | Simvastatin and fluvastatin | Statins have anti-inflammatory effects and induce HO-1 in macrophage cell lines | [41] |
| Rat aortic VSMCs (RASMCs) were isolated from thoracic aortas of Sprague-Dawley rats; human aortic VSMCs (HASMCs) | Coronary heart disease | Simvastatin | Simvastatin activates HO-1 which has cytoprotective effects - statins have anti-inflammatory and anti-proliferative effects | [42] |
| Mouse RAW264.7 macrophages | Hypercholesterolemia | Simvastatin | Simvastatin has anti-inflammatory effects due to induction of HO-1 | [56] |
| HPAEpiCs | Inflammatory lung diseases | Mevastatin | Mevastatin reduces TNF-α induced ICAM-1 expression via p47phox/Nox/ROS/c-Src/PDGFR_/PI3K/Akt/Nrf2/ARE/HO-1—statins have anti-inflammatory effects in HPAEpiCs | [45] |
| Neuro-2A mouse neuroblastoma cells | Inflammatory diseases | Statins | Statins induce HO-1 by binding with p38 | [51] |
| HT-29 cells HCT-116 | Coloncancer | Simvastatin | Simvastatin improves colon cancer by activation of Nrf2 and expression of several antioxidant enzymes in pathways including ERK and PI3K/Akt | [47] |
| Vascular smooth muscle cells (VSMCs) | Diabetic vasculopathy | Fluvastatin | Statins improve diabetes complications by activating Nrf2 pathway reducing VSMC proliferation and migration and inducing AGEs and the ERK5-Nrf2 signal | [57] |
| Human coronary artery smooth muscle cells (hCASMCs) | Cardiovascular disease | Fluvastatin | Statins have a protective role in oxidative injury inducing antioxidant enzymes in Nrf2/ARE pathway | [58] |
| Human aortic smooth muscle cells (HASMCs) | Atherosclerosis | Atorvastatin | Simultaneous use of atorvastatin and C3G could activate Nrf2 pathway and increase antioxidative effects including GCLC, NQO-1, and HO-1 and SOD activity removing superoxide radicals and finally improving atherosclerosis | [59] |
| HL-1 cells | Atrial fibrillation (AF) | Rosuvastatin | Statins improve AF by activation of Akt/Nrf2/HO-1 signaling and inducing antioxidant HO-1 | [60] |
| Human prostate adenocarcinoma (PC-3) and breast adenocarcinoma MCF-7 cell lines | Adenocarcinoma of the prostate and breast adenocarcinoma | Atorvastatin | Significant up-regulation of HO-1 (all six ARE-like elements are present in the HO-1 promotor activated by atorvastatin) and apoptosis was induced in both PC-3(at a concentration of 1 µM) and MCF-7(at a concentration of 50 µM) cell lines | [61] |
| Human umbilical endothelial cells HUVEC | Cardiovascular disease | Atorvastatin | Atorvastatin moderately increased eNOS and HO-1 mRNA expression but HO-1 protein levels did not change significantly | [62] |
| Human microvascular endothelial cells (HMEC-1) | Cardiovascular diseases | Atorvastatin | Atorvastatin at concentration 0.1 μM enhanced the expression of eNOS and was ineffective in modulation of HO-1 protein level | [63] |
| (NRK52E) cells | Lipotoxic injury in kidney | Simvastatin | Simvastatin transcriptionally activates HO-1 that protect renal cells from lipotoxic injury | [64] |
| SH-SY5Y cells | Parkinson’s disease | Simvastatin | Simvastatin has antioxidant activity via ERK1/2-mediated modulation of the antioxidant system | [65] |
| The rat renal proximal tubule cell line (NRK52E) | Kidney disease | Simvastatin | Antioxidant effect of simvastatin via HO-1 may protect the kidney | [64] |
| SH-SY5Y cells | Parkinson’s disease | Simvastatin | Simvastatin has antioxidant effect via ERK1/2-mediated reduction – this may decrease the incidence of Parkinson’s disease | [65] |
| Human endothelial cells cell line (ECV304) | Cardiovascular disease | Statins | Statins have antioxidant and anti-inflammatory effects via HMG-CoA reductase by activating HO-1 promoter | [66] |
| Murine RAW264.7 | Inflammatory disease | Statins | Statins activate protein kinase A and after affecting ERK and p38 MAPK pathways finally activate HO-1 gene expression and acting on this pathway are beneficial in anti-inflammatory diseases | [53] |
| Rat aortic VSMCs (RASMCs) | Cardiovascular disease | Simvastatin | Simvastatin has anti-inflammatory and anti-proliferative effects by affecting HO-1 | [42] |
| HCT116 and HT-29 cells | Colon cancer | Simvastatin | Simvastatin had beneficial effects in colon cancer cells by influencing Nrf2 and ERK and PI3K/Akt pathways | [51] |
| A mouse neuroblastoma cell line | Degenerative neurological diseases | Simvastatin | Simvastatin induced HO-1 expression in Neuro 2A cells by having effect on Nrf2 protein | [67] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansouri, A.; Reiner, Ž.; Ruscica, M.; Tedeschi-Reiner, E.; Radbakhsh, S.; Bagheri Ekta, M.; Sahebkar, A. Antioxidant Effects of Statins by Modulating Nrf2 and Nrf2/HO-1 Signaling in Different Diseases. J. Clin. Med. 2022, 11, 1313. https://doi.org/10.3390/jcm11051313
Mansouri A, Reiner Ž, Ruscica M, Tedeschi-Reiner E, Radbakhsh S, Bagheri Ekta M, Sahebkar A. Antioxidant Effects of Statins by Modulating Nrf2 and Nrf2/HO-1 Signaling in Different Diseases. Journal of Clinical Medicine. 2022; 11(5):1313. https://doi.org/10.3390/jcm11051313
Chicago/Turabian StyleMansouri, Atena, Željko Reiner, Massimiliano Ruscica, Eugenia Tedeschi-Reiner, Shabnam Radbakhsh, Mariam Bagheri Ekta, and Amirhossein Sahebkar. 2022. "Antioxidant Effects of Statins by Modulating Nrf2 and Nrf2/HO-1 Signaling in Different Diseases" Journal of Clinical Medicine 11, no. 5: 1313. https://doi.org/10.3390/jcm11051313
APA StyleMansouri, A., Reiner, Ž., Ruscica, M., Tedeschi-Reiner, E., Radbakhsh, S., Bagheri Ekta, M., & Sahebkar, A. (2022). Antioxidant Effects of Statins by Modulating Nrf2 and Nrf2/HO-1 Signaling in Different Diseases. Journal of Clinical Medicine, 11(5), 1313. https://doi.org/10.3390/jcm11051313






