Development and Validation of a Scoring System for Assessment of Clinical Failure after Pediatric Robot-Assisted Laparoscopic Extravesical Ureteral Reimplantation: A Multi-Center Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Surgical Technique and Post-Operative Care
2.3. Development of Prediction Score and Statistical Analysis
3. Results
3.1. Prediction Model and Scoring System Development with Regression Analysis
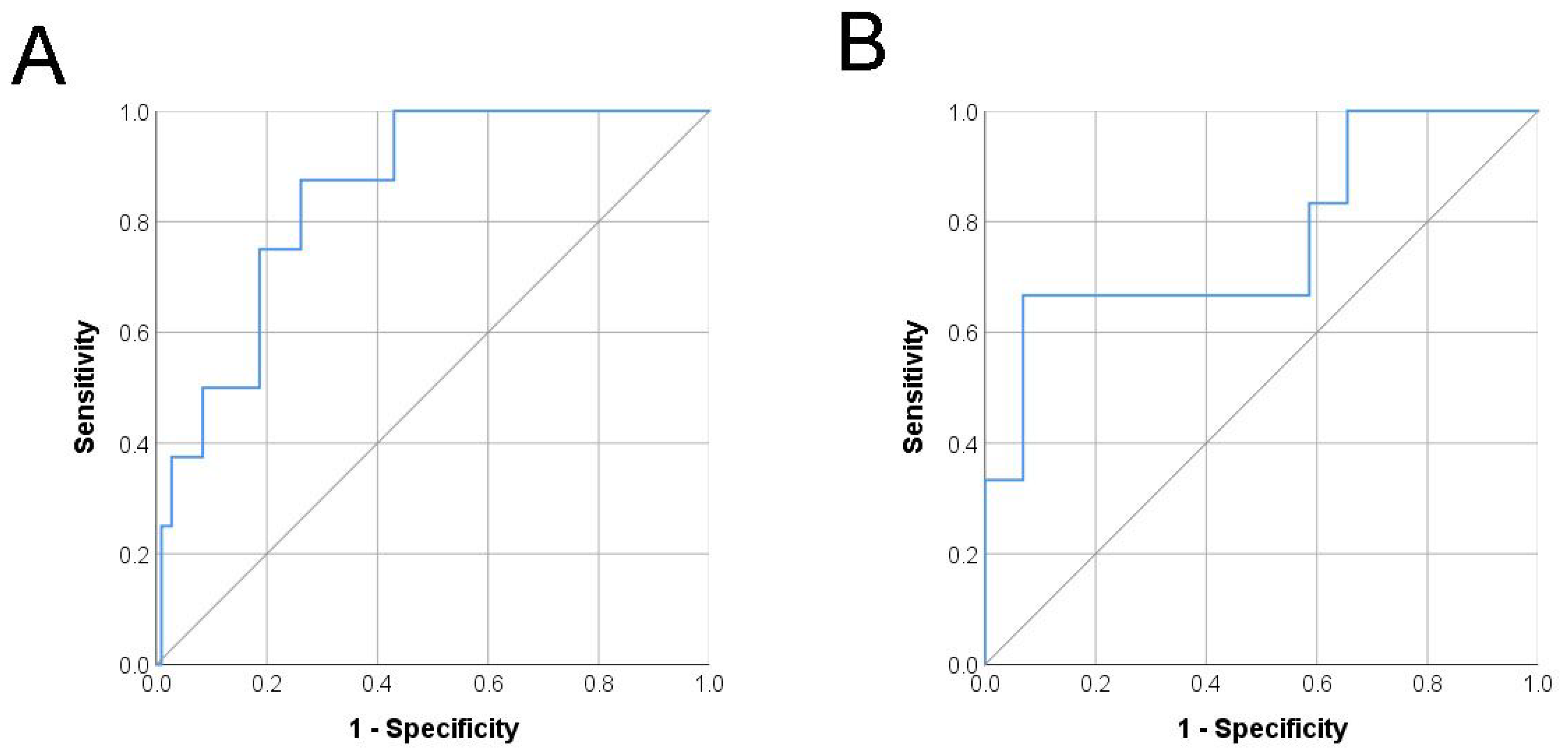
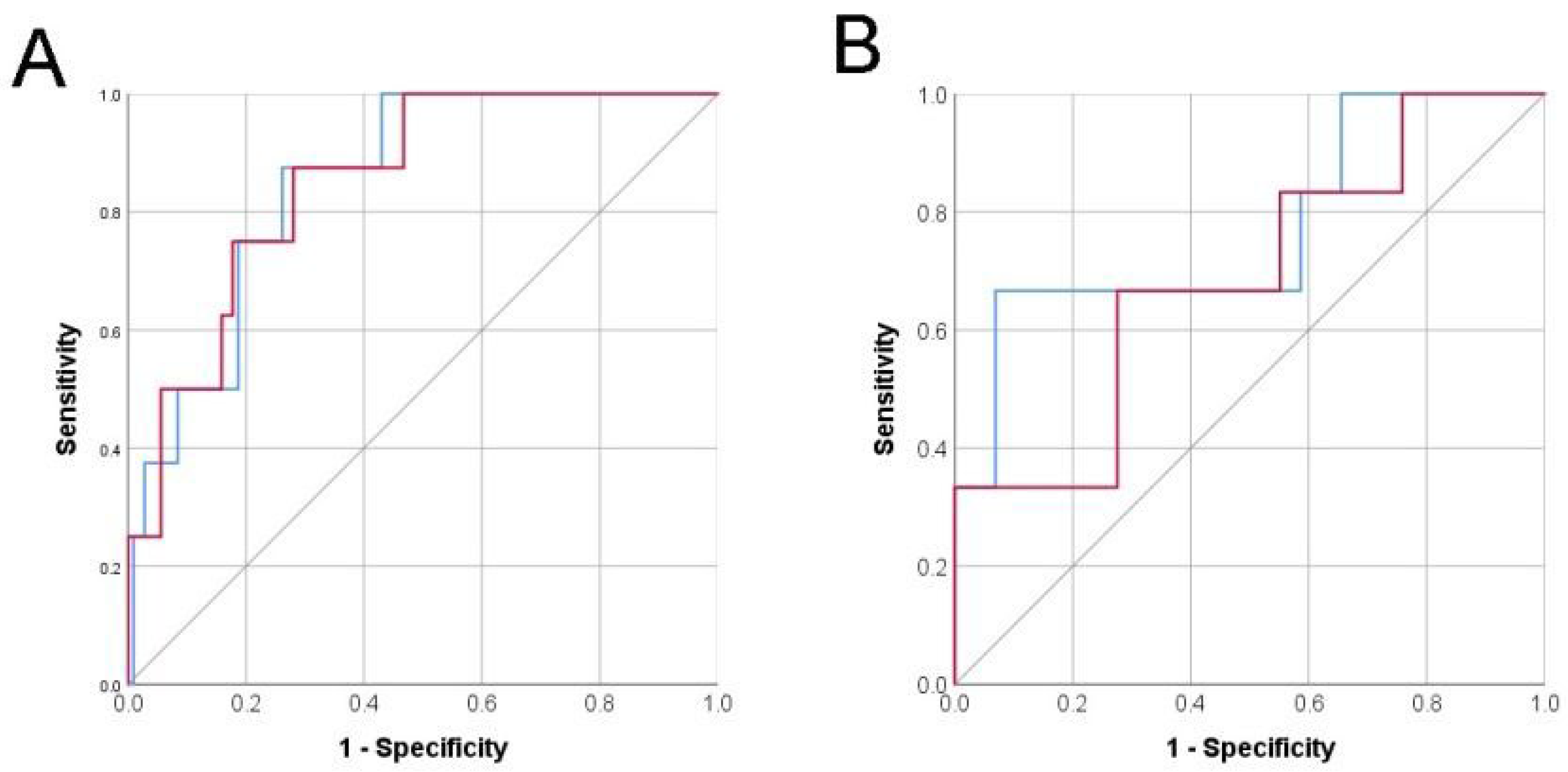
3.2. Risk Model Performance Validation
3.3. Risk Prediction with Only Pre-Operative Variables
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Williams, G.; Fletcher, J.T.; Alexander, S.I.; Craig, J.C. Vesicoureteral reflux. J. Am. Soc. Nephrol. 2008, 19, 847–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, C.A.; Skoog, S.J.; Arant, B.S., Jr.; Copp, H.L.; Elder, J.S.; Hudson, R.G.; Khoury, A.E.; Lorenzo, A.J.; Pohl, H.G.; Shapiro, E.; et al. Summary of the AUA Guideline on Management of Primary Vesicoureteral Reflux in Children. J. Urol. 2010, 184, 1134–1144. [Google Scholar] [CrossRef]
- Tekgul, S.; Riedmiller, H.; Hoebeke, P.; Kocvara, R.; Nijman, R.J.; Radmayr, C.; Stein, R.; Dogan, H.S. EAU guidelines on vesicoureteral reflux in children. Eur. Urol. 2012, 62, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Esposito, C.; Yamataka, A.; Varlet, F.; Castagnetti, M.; Scalabre, A.; Fourcade, L.; Ballouhey, Q.; Nappo, S.; Escolino, M. Current trends in 2021 in surgical management of vesico-ureteral reflux in pediatric patients: Results of a multicenter international survey on 552 patients. Minerva Urol. Nephrol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Zaontz, M.R.; Maizels, M.; Sugar, E.C.; Firlit, C.F. Detrusorrhaphy: Extravesical ureteral advancement to correct vesicoureteral reflux in children. J. Urol. 1987, 138, 947–949. [Google Scholar] [CrossRef]
- Boysen, W.R.; Akhavan, A.; Ko, J.; Ellison, J.S.; Lendvay, T.S.; Huang, J.; Garcia-Roig, M.; Kirsch, A.; Koh, C.J.; Schulte, M.; et al. Prospective multicenter study on robot-assisted laparoscopic extravesical ureteral reimplantation (RALUR-EV): Outcomes and complications. J. Pediatr. Urol. 2018, 14, 262.e1–262.e6. [Google Scholar] [CrossRef]
- Deng, T.; Liu, B.; Luo, L.; Duan, X.; Cai, C.; Zhao, Z.; Zhu, W.; Wu, W.; Zeng, G. Robot-assisted laparoscopic versus open ureteral reimplantation for pediatric vesicoureteral reflux: A systematic review and meta-analysis. World J. Urol. 2018, 36, 819–828. [Google Scholar] [CrossRef]
- Zhang, Y.; Ouyang, W.; Xu, H.; Luan, Y.; Yang, J.; Lu, Y.; Hu, J.; Liu, Z.; Yu, X.; Guan, W.; et al. A Comparison of Robot-Assisted Laparoscopic Ureteral Reimplantation and Conventional Laparoscopic Ureteral Reimplantation for the Management of Benign Distal Ureteral Stricture. Urol. J. 2020, 17, 252–256. [Google Scholar] [CrossRef]
- Smith, R.P.; Oliver, J.L.; Peters, C.A. Pediatric robotic extravesical ureteral reimplantation: Comparison with open surgery. J. Urol. 2011, 185, 1876–1881. [Google Scholar] [CrossRef]
- Masieri, L.; Sforza, S.; Grosso, A.A.; Valastro, F.; Tellini, R.; Cini, C.; Landi, L.; Taverna, M.; Elia, A.; Mantovani, A.; et al. Robot-assisted laparoscopic pyeloplasty in children: A systematic review. Minerva Urol. Nefrol. 2020, 72, 673–690. [Google Scholar] [CrossRef]
- Hendren, W.H. Reoperation for the failed ureteral reimplantation. J. Urol. 1974, 111, 403–411. [Google Scholar] [CrossRef]
- Houle, A.M.; McLorie, G.A.; Heritz, D.M.; McKenna, P.H.; Churchill, B.M.; Khoury, A.E. Extravesical nondismembered ureteroplasty with detrusorrhaphy: A renewed technique to correct vesicoureteral reflux in children. J. Urol. 1992, 148, 704–707. [Google Scholar] [CrossRef]
- Silay, M.S.; Baek, M.; Koh, C.J. Robot-Assisted Laparoscopic Extravesical Ureteral Reimplantation in Children: Top-Down Suturing Technique without Stent Placement. J. Endourol. 2015, 29, 864–866. [Google Scholar] [CrossRef]
- Moons, K.G.; Altman, D.G.; Reitsma, J.B.; Ioannidis, J.P.; Macaskill, P.; Steyerberg, E.W.; Vickers, A.J.; Ransohoff, D.F.; Collins, G.S. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): Explanation and elaboration. Ann. Intern. Med. 2015, 162, W1–W73. [Google Scholar] [CrossRef] [Green Version]
- Han, K.; Song, K.; Choi, B.W. How to Develop, Validate, and Compare Clinical Prediction Models Involving Radiological Parameters: Study Design and Statistical Methods. Korean J. Radiol. 2016, 17, 339–350. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, L.M.; Massaro, J.M.; D’Agostino, R.B., Sr. Presentation of multivariate data for clinical use: The Framingham Study risk score functions. Stat. Med. 2004, 23, 1631–1660. [Google Scholar] [CrossRef]
- Ozyuksel, G.; Arslan, U.E.; Boybeyi-Turer, O.; Tanyel, F.C.; Soyer, T. New scoring system to predict foreign body aspiration in children. J. Pediatr. Surg. 2020, 55, 1663–1666. [Google Scholar] [CrossRef]
- Citamak, B.; Dogan, H.S.; Ceylan, T.; Hazir, B.; Bilen, C.Y.; Sahin, A.; Tekgul, S. A new simple scoring system for prediction of success and complication rates in pediatric percutaneous nephrolithotomy: Stone-kidney size score. J. Pediatr. Urol. 2019, 15, 67.e1–67.e6. [Google Scholar] [CrossRef]
- Kuo, C.C.; Chang, C.M.; Liu, K.T.; Lin, W.K.; Chiang, H.Y.; Chung, C.W.; Ho, M.R.; Sun, P.R.; Yang, R.L.; Chen, K.T. Automation of the kidney function prediction and classification through ultrasound-based kidney imaging using deep learning. NPJ Digit. Med. 2019, 2, 29. [Google Scholar] [CrossRef]
- Herz, D.; Fuchs, M.; Todd, A.; McLeod, D.; Smith, J. Robot-assisted laparoscopic extravesical ureteral reimplant: A critical look at surgical outcomes. J. Pediatr. Urol. 2016, 12, e401–e402. [Google Scholar] [CrossRef]
- Boysen, W.R.; Ellison, J.S.; Kim, C.; Koh, C.J.; Noh, P.; Whittam, B.; Palmer, B.; Shukla, A.; Kirsch, A.; Gundeti, M.S. Multi-Institutional Review of Outcomes and Complications of Robot-Assisted Laparoscopic Extravesical Ureteral Reimplantation for Treatment of Primary Vesicoureteral Reflux in Children. J. Urol. 2017, 197, 1555–1561. [Google Scholar] [CrossRef]
- Akhavan, A.; Avery, D.; Lendvay, T.S. Robot-assisted extravesical ureteral reimplantation: Outcomes and conclusions from 78 ureters. J. Pediatr. Urol. 2014, 10, 864–868. [Google Scholar] [CrossRef]
- Gerber, J.A.; Koh, C.J. Robot-assisted laparoscopic ureteral reimplantation in children: A valuable alternative to open surgery. World J. Urol. 2020, 38, 1849–1854. [Google Scholar] [CrossRef]
- Gundeti, M.S.; Boysen, W.R.; Shah, A. Robot-assisted Laparoscopic Extravesical Ureteral Reimplantation: Technique Modifications Contribute to Optimized Outcomes. Eur. Urol. 2016, 70, 818–823. [Google Scholar] [CrossRef]
- Casale, P.; Patel, R.P.; Kolon, T.F. Nerve sparing robotic extravesical ureteral reimplantation. J. Urol. 2008, 179, 1987–1989. [Google Scholar] [CrossRef]
- Loening-Baucke, V. Urinary incontinence and urinary tract infection and their resolution with treatment of chronic constipation of childhood. Pediatrics 1997, 100, 228–232. [Google Scholar] [CrossRef]
- Koff, S.A.; Wagner, T.T.; Jayanthi, V.R. The relationship among dysfunctional elimination syndromes, primary vesicoureteral reflux and urinary tract infections in children. J. Urol. 1998, 160, 1019–1022. [Google Scholar] [CrossRef]
- Upadhyay, J.; Bolduc, S.; Bagli, D.J.; McLorie, G.A.; Khoury, A.E.; Farhat, W. Use of the dysfunctional voiding symptom score to predict resolution of vesicoureteral reflux in children with voiding dysfunction. J. Urol. 2003, 169, 1842–1846. [Google Scholar] [CrossRef]
- Lee, H.E.; Farhat, W.; Park, K. Translation and linguistic validation of the korean version of the dysfunctional voiding symptom score. J. Korean Med. Sci. 2014, 29, 400–404. [Google Scholar] [CrossRef] [Green Version]
- Davies, G.J.; Crowder, M.; Reid, B.; Dickerson, J.W. Bowel function measurements of individuals with different eating patterns. Gut 1986, 27, 164–169. [Google Scholar] [CrossRef] [Green Version]
- Afshar, K.; Mirbagheri, A.; Scott, H.; MacNeily, A.E. Development of a symptom score for dysfunctional elimination syndrome. J. Urol. 2009, 182, 1939–1943. [Google Scholar] [CrossRef] [PubMed]
- Vergouwe, Y.; Steyerberg, E.W.; Eijkemans, M.J.; Habbema, J.D. Substantial effective sample sizes were required for external validation studies of predictive logistic regression models. J. Clin. Epidemiol. 2005, 58, 475–483. [Google Scholar] [CrossRef] [PubMed]
- Hubert, K.C.; Kokorowski, P.J.; Huang, L.; Prasad, M.M.; Rosoklija, I.; Retik, A.B.; Nelson, C.P. Clinical outcomes and long-term resolution in patients with persistent vesicoureteral reflux after open ureteral reimplantation. J. Urol. 2012, 188, 1474–1479. [Google Scholar] [CrossRef] [Green Version]
- Heinze, G.; Dunkler, D. Five myths about variable selection. Transpl. Int. 2017, 30, 6–10. [Google Scholar] [CrossRef]
- Grossklaus, D.J.; Pope, J.C.; Adams, M.C.; Brock, J.W. Is postoperative cystography necessary after ureteral reimplantation? Urology 2001, 58, 1041–1045. [Google Scholar] [CrossRef]
| Parameters | Development Cohort | Validation Cohort | p-Value |
|---|---|---|---|
| No. Patients | 77 | 28 | |
| Total Ureter Units | 115 | 46 | |
| Gender (%) | |||
| Male | 20 (26.0) | 11 (39.3) | 0.122 |
| Female | 57 (74.0) | 17 (60.7) | |
| Median age in years (range) | 5.5 (1–16) | 6.5 (0.3–46) | 0.179 |
| Laterality (%) | |||
| Left | 20 (26.0) | 5 (17.9) | 0.054 |
| Right | 19 (24.7) | 5 (17.9) | |
| Bilateral | 38 (49.4) | 18 (64.3) | |
| BBD | 53 (46.1%) | 5 (10.9%) | <0.001 |
| VUR Grade (%) | |||
| I | 12 (10.4) | 1 (2.2) | 0.048 |
| II | 16 (13.9) | 8 (17.4) | |
| III | 51 (44.3) | 13 (28.3) | |
| IV | 29 (25.2) | 18 (39.1) | |
| V | 7 (6.1) | 6 (13.0) | |
| Median total operative time (min) | 196 (98–273) | 195 (120–360) | 0.927 |
| Median console time (min) | 148 (75–240) | 105 (75–225) | <0.001 |
| Median length of stay in days (range) | 1.0 (1–6) | 2 (2–7) | <0.001 |
| Median follow-up in months (range) | 4.3 (1–19) | 10 (1–41) | <0.001 |
| Follow-up VCUG 1 or RNC 2 conducted (%) | 66 (57.4) | 37 (80.4) | <0.001 |
| Clinical success (%) | 107 (93.0) | 40 (87.0) | 0.227 |
| Variable | Development Cohort | ||
|---|---|---|---|
| Success (n = 107) | Failure (n = 8) | p-Value | |
| Age | 4.9 (1–16.2) | 8.75 (6–13) | 0.007 |
| Gender | 1.000 | ||
| Female | 81 (75.7%) | 6 (75.0%) | |
| Male | 26 (24.3%) | 2 (25.0%) | |
| BMI 1 | 17.5 (13.4–41.4) | 20.5 (15.2–24.3) | 0.141 |
| BBD 2 | 0.141 | ||
| No | 60 (56.1%) | 2 (25.0%) | |
| Yes | 47 (43.9%) | 6 (75.0%) | |
| Laterality | 0.453 | ||
| Unilateral | 37 (34.6%) | 4 (50.0%) | |
| Bilateral | 70 (94.6%) | 4 (50.0%) | |
| VUR 3 Grade | 0.028 | ||
| I | 12 (11.2%) | 0 (0%) | |
| II | 16 (15.0%) | 0 (0%) | |
| III | 48 (44.9%) | 3 (37.5%) | |
| IV | 27 (25.2%) | 2 (25.0%) | |
| V | 4 (3.7%) | 3 (37.5%) | |
| Console time | 146.0 (75–270) | 188.5 (171–221) | <0.001 |
| No. of detrusorrhaphy stitches | 6 (6–7) | 6 (6–6) | 0.647 |
| Hospital stay | 1 (1–6) | 1.5 (1–3) | 0.595 |
| Variable | Without Intra-and Post-Operative Variable Model | With Intra-and Post-Operative Variable Model | ||||||
|---|---|---|---|---|---|---|---|---|
| β Coefficient | OR | 95% CI | p-Value | β Coefficient | OR | 95% CI | p-Value | |
| Age | 0.229 | 1.258 | 0.995–1.591 | 0.056 | 0.43 | 1.54 | 1.03–2.29 | 0.033 |
| BMI 1 | 0.059 | 1.061 | 0.865–1.301 | 0.570 | 0.02 | 1.02 | 0.70–1.51 | 0.883 |
| BBD 2 | 1.512 | 4.538 | 0.699–29.448 | 0.113 | 2.35 | 10.58 | 0.72–182.15 | 0.067 |
| VUR 3 Grade | 1.288 | 3.627 | 1.283–10.252 | 0.015 | 1.96 | 7.12 | 1.19–42.56 | 0.031 |
| Console time | 0.06 | 1.06 | 1.01–1.11 | 0.010 | ||||
| Hospital stay | 1.4 | 4.05 | 0.10–1.33 | 0.130 | ||||
| Risk Group | Development (n = 115) * | Validation (n = 46) † | ||
|---|---|---|---|---|
| n (%) | Resolution (%) | n (%) | Resolution | |
| Low-risk group (<52 points) | 23 (20.0) | 100 | 4 (8.7) | 100 |
| Intermediate-risk group (52–70 points) | 62 (53.9) | 96.8 | 24 (52.2) | 91.7 |
| High-risk group (≥71 points) | 30 (26.1) | 80 | 18 (39.1) | 77.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koh, C.J.; Kim, K.S.; Gerber, J.A.; Bhatia, V.; Zhu, H.; Baek, M.; Song, S.H. Development and Validation of a Scoring System for Assessment of Clinical Failure after Pediatric Robot-Assisted Laparoscopic Extravesical Ureteral Reimplantation: A Multi-Center Study. J. Clin. Med. 2022, 11, 1327. https://doi.org/10.3390/jcm11051327
Koh CJ, Kim KS, Gerber JA, Bhatia V, Zhu H, Baek M, Song SH. Development and Validation of a Scoring System for Assessment of Clinical Failure after Pediatric Robot-Assisted Laparoscopic Extravesical Ureteral Reimplantation: A Multi-Center Study. Journal of Clinical Medicine. 2022; 11(5):1327. https://doi.org/10.3390/jcm11051327
Chicago/Turabian StyleKoh, Chester J., Kun Suk Kim, Jonathan A. Gerber, Vinaya Bhatia, Huirong Zhu, Minki Baek, and Sang Hoon Song. 2022. "Development and Validation of a Scoring System for Assessment of Clinical Failure after Pediatric Robot-Assisted Laparoscopic Extravesical Ureteral Reimplantation: A Multi-Center Study" Journal of Clinical Medicine 11, no. 5: 1327. https://doi.org/10.3390/jcm11051327






