Monitoring of Unfractionated Heparin Therapy in the Intensive Care Unit Using a Point-of-Care aPTT: A Comparative, Longitudinal Observational Study with Laboratory-Based aPTT and Anti-Xa Activity Measurement
Abstract
:1. Introduction
2. Methods
2.1. Patient Selection
2.2. UFH Management
2.3. POCT-APTT, Laboratory-Based APTT and Anti-Xa Activity
2.4. Confounding Factors
2.5. Time Tracking
2.6. Statistical Analysis
3. Results
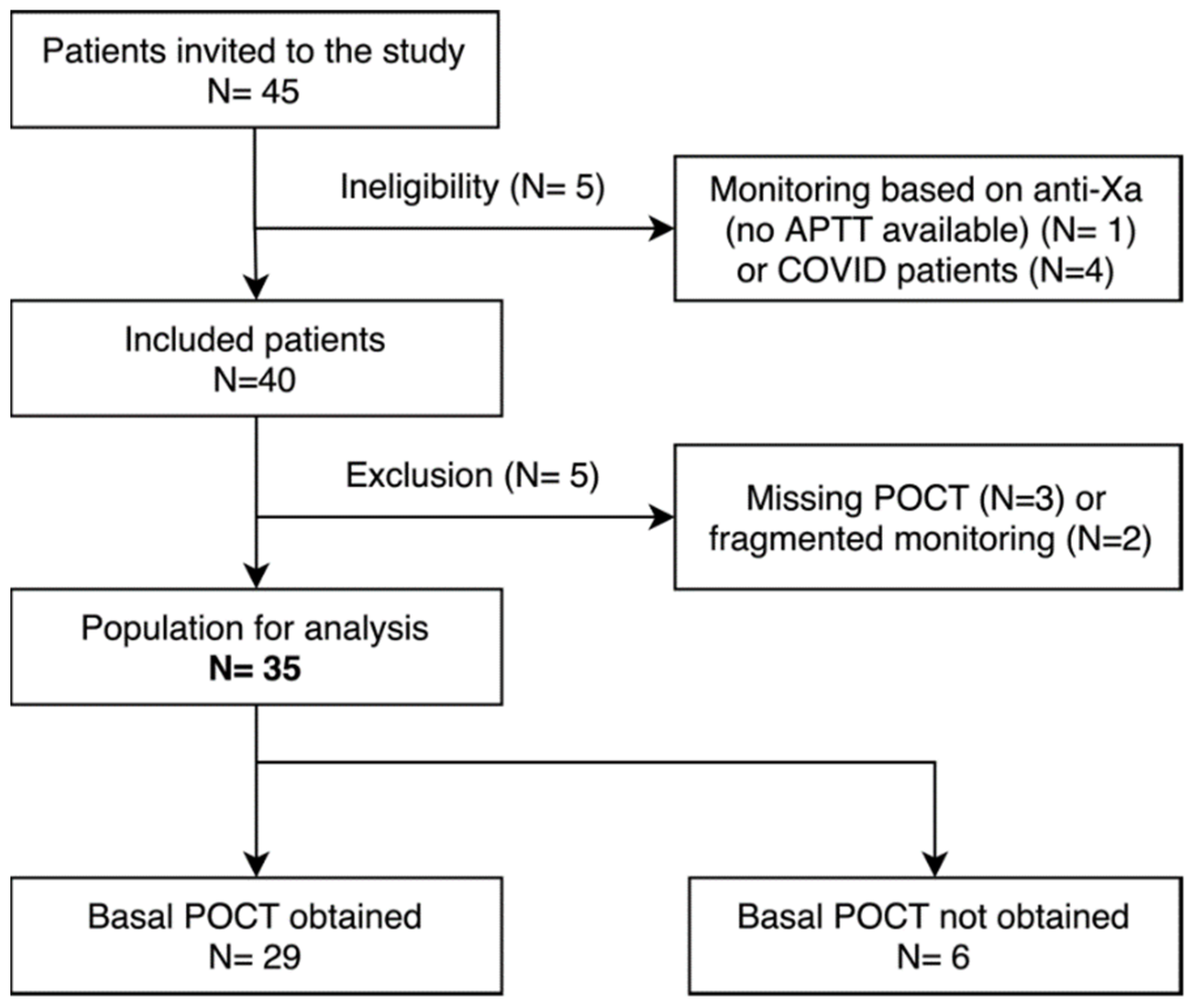
3.1. Study Population
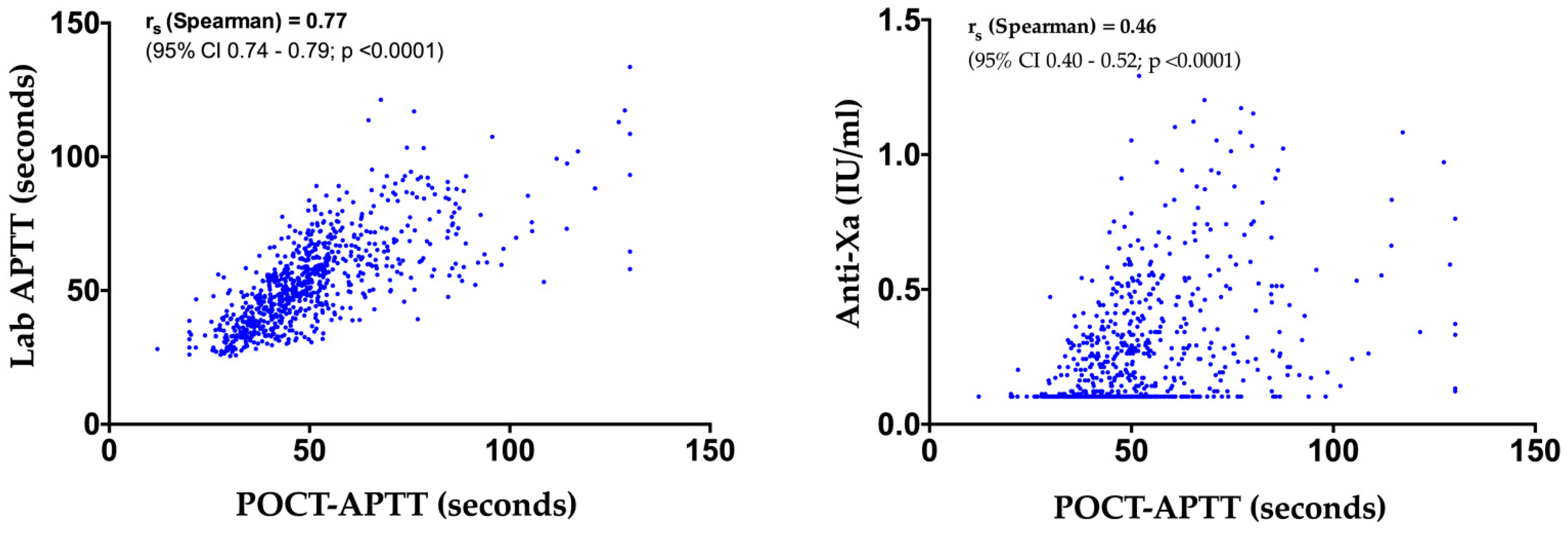
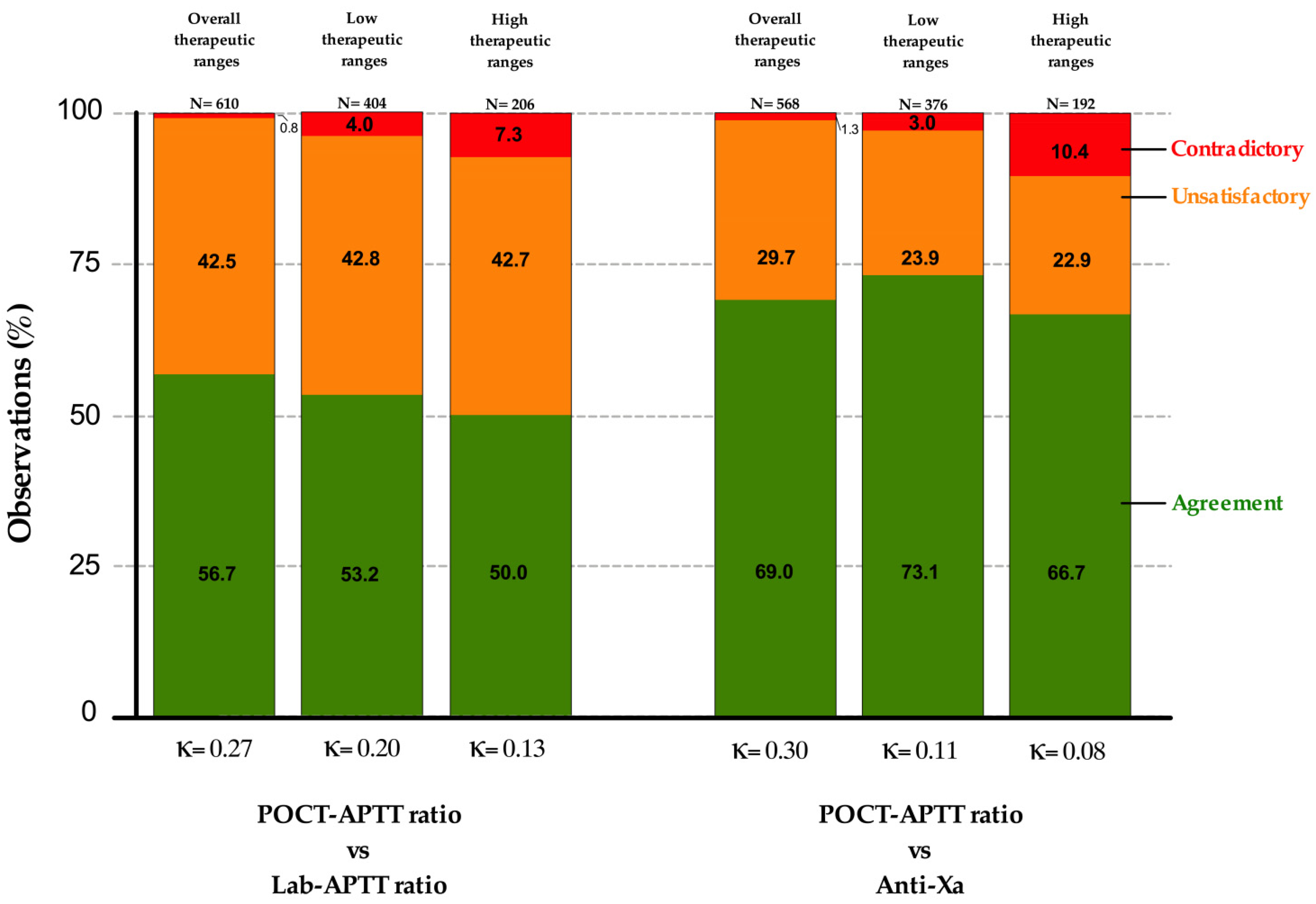
3.2. POCT-APTT, Laboratory-Based APTT and Anti-Xa Activity
3.3. Confounding Factors
3.4. Time Tracking
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Garcia, D.A.; Baglin, T.P.; Weitz, J.I.; Samama, M.M. Parenteral Anticoagulants. Chest 2012, 141, e24S–e43S. [Google Scholar] [CrossRef] [Green Version]
- The Extracorporeal Life Support Organization (ELSO). Extracorporeal Life Support Organization (ELSO) General Guidelines for All ECLS Cases. Available online: https://www.elso.org/Portals/0/ELSO%20Guidelines%20General%20All%20ECLS%20Version%201_4.pdf. (accessed on 14 January 2022).
- Whitman-Purves, E.; Coons, J.C.; Miller, T.; DiNella, J.V.; Althouse, A.; Schmidhofer, M.; Smith, R.E. Performance of Anti-Factor Xa Versus Activated Partial Thromboplastin Time for Heparin Monitoring Using Multiple Nomograms. Clin. Appl. Thromb. Hemost. 2018, 24, 310–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arachchillage, D.R.J.; Vipond, L.; Laffan, M. Limitations on Point Care APTT for Monitoring of Unfractionated Heparin in Intensive Care Patients. Thromb. Res. 2019, 181, 124–126. [Google Scholar] [CrossRef]
- Basu, D.; Gallus, A.; Hirsh, J.; Cade, J. A Prospective Study of the Value of Monitoring Heparin Treatment with the Activated Partial Thromboplastin Time. N. Engl. J. Med. 1972, 287, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Kitchen, S.; Jennings, I.; Woods, T.A.; Preston, F.E. Wide Variability in the Sensitivity of APTT Reagents for Monitoring of Heparin Dosage. J. Clin. Pathol. 1996, 49, 10–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitchen, S.; Preston, F.E. The Therapeutic Range for Heparin Therapy: Relationship between Six Activated Partial Thromboplastin Time Reagents and Two Heparin Assays. Thromb. Haemost. 1996, 75, 734–739. [Google Scholar] [CrossRef] [PubMed]
- Ratano, D.; Alberio, L.; Delodder, F.; Faouzi, M.; Berger, M.M. Agreement between Activated Partial Thromboplastin Time and Anti-Xa Activity in Critically Ill Patients Receiving Therapeutic Unfractionated Heparin. Thromb. Res. 2019, 175, 53–58. [Google Scholar] [CrossRef]
- Chlebowski, M.M.; Baltagi, S.; Carlson, M.; Levy, J.H.; Spinella, P.C. Clinical Controversies in Anticoagulation Monitoring and Antithrombin Supplementation for ECMO. Crit. Care 2020, 24, 19. [Google Scholar] [CrossRef] [Green Version]
- Raschke, R.A. The Weight-Based Heparin Dosing Nomogram Compared with a Standard Care Nomogram: A Randomized Controlled Trial. Ann. Intern. Med. 1993, 119, 874. [Google Scholar] [CrossRef]
- Amanzadeh, J.; Reilly, R.F. Hematology: Issues in the dialysis patient: Anticoagulation and Continuous Renal Replacement Therapy: Anticoagulation and CRRT. Semin. Dial. 2006, 19, 311–316. [Google Scholar] [CrossRef]
- CoaguChek® Pro II Kit Insert (Roche Diagnostic). Available online: https://diagnostics.roche.com/global/en/products/instruments/coaguchek-pro-ii.html (accessed on 14 January 2022).
- Gouin-Thibaut, I.; Martin-Toutain, I.; Peynaud-Debayle, E.; Marion, S.; Napol, P.; Alhenc-Gelas, M. Monitoring Unfractionated Heparin with APTT: A French Collaborative Study Comparing Sensitivity to Heparin of 15 APTT Reagents. Thromb. Res. 2012, 129, 666–667. [Google Scholar] [CrossRef] [PubMed]
- Hirsh, J.; Warkentin, T.E.; Shaughnessy, S.G.; Anand, S.S.; Halperin, J.L.; Raschke, R.; Granger, C.; Ohman, E.M.; Dalen, J.E. Heparin and Low-Molecular-Weight Heparin Mechanisms of Action, Pharmacokinetics, Dosing, Monitoring, Efficacy, and Safety. Chest 2001, 119, 64S–94S. [Google Scholar] [CrossRef] [Green Version]
- Arachchillage, D.R.J.; Kamani, F.; Deplano, S.; Banya, W.; Laffan, M. Should We Abandon the APTT for Monitoring Unfractionated Heparin? Thromb. Res. 2017, 157, 157–161. [Google Scholar] [CrossRef]
- Devreese, K.M.J.; Groot, P.G.; Laat, B.; Erkan, D.; Favaloro, E.J.; Mackie, I.; Martinuzzo, M.; Ortel, T.L.; Pengo, V.; Rand, J.H.; et al. Guidance from the Scientific and Standardization Committee for Lupus Anticoagulant/Antiphospholipid Antibodies of the International Society on Thrombosis and Haemostasis: Update of the Guidelines for Lupus Anticoagulant Detection and Interpretation. J. Thromb. Haemost. 2020, 18, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Niederdöckl, J.; Dempfle, C.-E.; Schönherr, H.-R.; Bartsch, A.; Miles, G.; Laggner, A.; Pathil, A. Point-of-Care PT and APTT in Patients with Suspected Deficiencies of Coagulation Factors. Int. J. Lab. Hematol. 2016, 38, 426–434. [Google Scholar] [CrossRef]
- Colman, E.; Yin, E.B.; Laine, G.; Chatterjee, S.; Saatee, S.; Herlihy, J.P.; Reyes, M.A.; Bracey, A.W. Evaluation of a Heparin Monitoring Protocol for Extracorporeal Membrane Oxygenation and Review of the Literature. J. Thorac. Dis. 2019, 11, 3325–3335. [Google Scholar] [CrossRef] [PubMed]
- Lehman, C.M.; Frank, E.L. Laboratory Monitoring of Heparin Therapy: Partial Thromboplastin Time or Anti-Xa Assay? Lab. Med. 2009, 40, 47–51. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.P.; Phan, X.T.; Huynh, D.Q.; Viet Truong, H.T.; Hai Le, Y.N.; Nguyen, T.M.; Minh Du, Q.Q.; Le, T.P.; Truong, H.N.; Ho, T.T.; et al. Monitoring Unfractionated Heparin in Adult Patients Undergoing Extracorporeal Membrane Oxygenation (ECMO): ACT, APTT, or ANTI-XA? Crit. Care Res. Pract. 2021, 2021, 5579936. [Google Scholar] [CrossRef]
- Barton, R.; Ignjatovic, V.; Monagle, P. Anticoagulation during ECMO in Neonatal and Paediatric Patients. Thromb. Res. 2019, 173, 172–177. [Google Scholar] [CrossRef]
- Marlar, R.A.; Gausman, J.N. The Effect of Instrumentation and Laboratory Site on the Accuracy of the APTT-Based Heparin Therapeutic Range. Int. J. Lab. Hematol. 2012, 34, 614–620. [Google Scholar] [CrossRef]
- Favaloro, E.J.; Bonar, R.; Sioufi, J.; Wheeler, M.; Low, J.; Aboud, M.; Lloyd, J.; Street, A.; Marsden, K. An International Survey of Current Practice in the Laboratory Assessment of Anticoagulant Therapy with Heparin. Pathology 2005, 37, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Brigden, M.L.; Johnston, M.; ARTfor the Thrombosis Interest Group of Canada*. A Survey of APTT Reporting in Canadian Medical Laboratories. Am. J. Clin. Pathol. 2000, 114, 276–282. [Google Scholar] [CrossRef]
- Nankervis, C.A.; Preston, T.J.; Dysart, K.C.; Wilkinson, W.D.; Chicoine, L.G.; Welty, S.E.; Nelin, L.D. Assessing Heparin Dosing in Neonates on Venoarterial Extracorporeal Membrane Oxygenation. ASAIO J. 2007, 53, 111–114. [Google Scholar] [CrossRef] [PubMed]
- Coene, K.L.M.; van der Graaf, F.; van de Kerkhof, D. Protocolled Redefinition of the Therapeutic Range for Unfractionated Heparin: Lost in Translation? Clin. Appl. Thromb. Hemost. 2018, 24, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.N.; Hirsh, J.; Gent, M.; Turpie, A.G.; Cruickshank, M.; Weitz, J.; Anderson, D.; Johnson, M. A Randomized Trial Comparing Activated Thromboplastin Time with Heparin Assay in Patients with Acute Venous Thromboembolism Requiring Large Daily Doses of Heparin. Arch. Intern. Med. 1994, 154, 49–56. [Google Scholar] [CrossRef]
- Vandiver, J.W.; Vondracek, T.G. Antifactor Xa Levels versus Activated Partial Thromboplastin Time for Monitoring Unfractionated Heparin. Pharmacotherapy 2012, 32, 546–558. [Google Scholar] [CrossRef]
- Khan, J.; Chandler, W.L. Interference in the Anti-Xa Heparin Activity Assay Due to Hemolysis and Icterus during Pediatric Extracorporeal Life Support. Artif. Organs 2019, 43, 880–887. [Google Scholar] [CrossRef]
- Kostousov, V.; Nguyen, K.; Hundalani, S.G.; Teruya, J. The Influence of Free Hemoglobin and Bilirubin on Heparin Monitoring by Activated Partial Thromboplastin Time and Anti-Xa Assay. Arch. Pathol. Lab. Med. 2014, 138, 1503–1506. [Google Scholar] [CrossRef] [Green Version]
- Ranucci, M.; Cotza, M.; Isgrò, G.; Carboni, G.; Ballotta, A.; Baryshnikova, E.; For the Surgical Clinical Outcome REsearch (SCORE) Group. Anti-Factor Xa–Based Anticoagulation during Extracorporeal Membrane Oxygenation: Potential Problems and Possible Solutions. Semin. Thromb. Hemost 2020, 46, 419–427. [Google Scholar] [CrossRef]
- Bouman, C.S.; de Pont, A.-C.J.; Meijers, J.C.; Bakhtiari, K.; Roem, D.; Zeerleder, S.; Wolbink, G.; Korevaar, J.C.; Levi, M.; de Jonge, E. The Effects of Continuous Venovenous Hemofiltration on Coagulation Activation. Crit. Care 2006, 10, R150. [Google Scholar] [CrossRef] [Green Version]
- Takemoto, C.M.; Streiff, M.B.; Shermock, K.M.; Kraus, P.S.; Chen, J.; Jani, J.; Kickler, T. Activated Partial Thromboplastin Time and Anti-Xa Measurements in Heparin Monitoring. Am. J. Clin. Pathol. 2013, 139, 450–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Connell, N.T.; Sylvester, K.W. To APTT or Not to APTT: Evaluating the Optimal Monitoring Strategy for Unfractionated Heparin. Thromb. Res. 2021, S004938482100520X. [Google Scholar] [CrossRef] [PubMed]
- Samuel, S.; Allison, T.A.; Sharaf, S.; Yau, G.; Ranjbar, G.; Mckaig, N.; Nguyen, A.; Escobar, M.; Choi, H.A. Antifactor Xa Levels vs. Activated Partial Thromboplastin Time for Monitoring Unfractionated Heparin. A Pilot Study. J. Clin. Pharm. Ther. 2016, 41, 499–502. [Google Scholar] [CrossRef] [PubMed]
- Zaki, H.V.; Elbeialy, M.A.K.; Soliman, A.M. The Use of Activated Partial Thromboplastin Time versus Antifactor Xa Assay in Monitoring Continuous Unfractionated Heparin Infusion Therapy in Obstetric Intensive Care Unit. Ain-Shams J. Anesthesiol. 2019, 11, 3. [Google Scholar] [CrossRef] [Green Version]
- van Roessel, S.; Middeldorp, S.; Cheung, Y.W.; Zwinderman, A.H. Accuracy of APTT Monitoring in Critically Ill Patients Treated with Unfractionated Heparin. Neth. J. Med. 2014, 72, 6. [Google Scholar]
- Aarab, R.; van Es, J.; de Pont, A.C.J.M.; Vroom, M.B.; Middeldorp, S. Monitoring of Unfractionated Heparin in Critically Ill Patients. Neth. J. Med. 2013, 71, 466–471. [Google Scholar]
- Moerer, O.; Huber-Petersen, J.F.; Schaeper, J.; Binder, C.; Wand, S. Factor XIII Activity Might Already Be Impaired before Veno-Venous ECMO in ARDS Patients: A Prospective, Observational Single-Center Cohort Study. J. Clin. Med. 2021, 10, 1203. [Google Scholar] [CrossRef]
- Buchtele, N.; Schwameis, M.; Schellongowski, P.; Quehenberger, P.; Knöbl, P.; Traby, L.; Schmid, M.; Schoergenhofer, C.; Herkner, H.; Jilma, B.; et al. Prevalence and Clinical Impact of Reduced Coagulation Factor XII Activity in Patients Receiving Extracorporeal Membrane Oxygenation. Crit. Care Med. 2021, 49, e1206–e1211. [Google Scholar] [CrossRef]
- Wallisch, M.; Lorentz, C.U.; Lakshmanan, H.H.S.; Johnson, J.; Carris, M.R.; Puy, C.; Gailani, D.; Hinds, M.T.; McCarty, O.J.T.; Gruber, A.; et al. Antibody Inhibition of Contact Factor XII Reduces Platelet Deposition in a Model of Extracorporeal Membrane Oxygenator Perfusion in Nonhuman Primates. Res. Pract. Thromb. Haemost. 2020, 4, 205–216. [Google Scholar] [CrossRef] [Green Version]



| Heparin Therapeutic Target Range | Bleeding Risk (Examples) | Initiation Phase | Adjustment Phase (aPTT Ratio) | Comments |
|---|---|---|---|---|
| 1.5–2.0 times baseline APTT | Low, intermediate or High (e.g., CVVH, ECMO) | Bolus of 25 U/kg and initial flow rate at 5 U/kg/h * | <1.2: bolus of 1000 U and flow rate increased by 200 U/h; check 6 h later 1.2–1.5: flow rate increased by 100 U/h; check 4 h later 1.5–2: the same dose is kept; check 8 h later 2–2.5: flow rate stopped for 30 min, then decreased by 100 U/h; check 4 h later >2.5: flow rate stopped for 1 h and decreased by 200 U/h; check 4 h later | In order to reduce the samples collected at steady-states, coagulation checks are spaced out if the aPTT ratio is within 1.5–2.0; the next check is thus planned 8 h later if the flow rate is unchanged and 12 h later if the flow rate is unchanged on two occasions. The doses are rounded to 250 IU for boluses; to 50 units for flow rate adjustments. |
| 1.5–2.0 times baseline APTT | Very high (e.g., CVVH or ECMO with a recent bleeding event) | Initial flow rate at 5 U/kg/h, except if CVVH is initiated where the initial rate is automatically 500 U/h | <1.2: flow rate increased by 200 U/h; check 4 h later 1.2–1.5: flow rate increased by 100 U/h; check 4 h later 1.5–2: the same dose is kept; check 8 h later 2–2.5: flow rate stopped for 30 min, then decreased by 100 U/h; check 4 h later >2.5: flow rate stopped for 1 h and decreased by 200 U/h; check 4 h later | |
| 2.0–2.5 times baseline APTT | Low (e.g., fresh pulmonary embolism or DVT) | Bolus of 80 U/kg (with a maximum of 10,000 U) and initial flow rate of 18 U/kg/h. | <1.5: bolus of 80 U/kg and flow rate increased by 4 U/kg/h 1.5–2: bolus of 40 U/kg and flow rate increased by 2 U/kg/h 2–2.5: the same dose is kept 2.5–3.0: dose reduction of 2 U/kg/h >3: dose reduction of 3 U/kg/h, and flow rate stopped for one hour | Coagulation checks are normally done every 6 h, until equilibrium is reached. If equilibrium is reached, the next control is requested 8 h later. If two successive checks are within the aPTT ratio of 2.0–2.5, the next control is requested 12 h later. |
| 2.0–2.5 times baseline APTT | Intermediate (e.g., ACS, peripheral vascular ischemia, ICU patients) | Bolus of 60 U/kg (with a maximum of 5000 U) and initial flow rate of 12 U/kg/h | <1.5: bolus of 60 U/kg and flow rate increased by 4 U/kg/h. 1.5–2: bolus of 30 U/kg and flow rate increased by 2 U/kg/h 2–2.5: the same dose is kept 2.5–3: dose reduction of 2 U/kg/h >3: dose reduction of 3 U/kg/h, and flow rate stopped for one hour | |
| 2.0–2.5 times baseline APTT | High (e.g., recent postoperative patient (≤5 days) and/or drains) | Bolus of 40 U/kg (with a maximum of 5000 U) and initial flow rate of 12 U/kg/h. | <1.5: bolus of 40 U/kg and flow rate increased by 4 U/kg/h 1.5–2: bolus of 20 U/kg and flow rate increased by 2 U/kg/h 2–2.5: the same dose is kept 2.5–3: dose reduction of 2 U/kg/h >3: dose reduction of 3 U/kg/h, and flow rate stopped for one hour | |
| 2.0–2.5 times baseline APTT | Very high (e.g., recent bleeding events, very recent surgery, postoperative MHV) | No bolus.Initial flow rate of 12 U/kg/h. | <1.5: flow rate increased by 4 U/kg/h 1.5–2: flow rate increased by 2 U/kg/h 2–2.5: the same dose is kept 2.5–3: dose reduction of 2 U/kg/h >3: dose reduction of 3U/kg/h, and flow rate stopped for one hour | Algorithm similar to the two previous ones except that no bolus will ever be administered neither at initiation, nor during adjustments. It is expected that the target is obtained later, but with a lower risk of exceeding it. Coagulation checks are more frequent (every 4 h instead of every 6 h) until the target is reached. |
| Lab-APTT ratio or Anti-Xa activity | Supra- | Disagreement Contradictory | Disagreement Unsatisfactory | Agreement POCT = |
| Therapeutic range a,b | Disagreement Unsatisfactory | Agreement POCT = | Disagreement Unsatisfactory | |
| Infra- | Agreement POCT = | Disagreement Unsatisfactory | Disagreement Contradictory | |
| Infra- | Therapeutic range b | Supra- | ||
| POCT-APTT Ratio | ||||
| Variables | N/Median | %/IQR |
|---|---|---|
| Demographics | ||
| Gender, females (F) | 13 | 37.1 |
| Age (years) | 64.7 | 56.9–70.7 |
| Weight (kg) | 76.5 | 65.0–94.5 |
| Duration of inclusion (days) | 6.0 | 5.0–11.2 |
| Heparin therapeutic ranges | ||
| Low | 23 | 65.7 |
| High | 8 | 22.9 |
| From low to high or conversely | 4 | 11.4 |
| Time to desired range (hours) | 29.1 | 15.4–37.6 |
| Clinical indications for UFH | ||
| CVVH | 14 | 40.0 |
| Mechanical valve | 6 | 17.0 |
| DVT | 5 | 14.3 |
| AF | 3 | 8.6 |
| AKI | 1 | 2.9 |
| ECMO | 3 | 8.6 |
| PE | 3 | 8.6 |
| Estimated risk of bleeding | ||
| Low risk | 2 | 5.7 |
| Medium risk | 16 | 45.7 |
| High risk | 4 | 11.5 |
| Very high risk | 13 | 37.1 |
| Outcome | ||
| Deaths in ICU | 7 | 20.0 |
| Major bleedings | 6 | 17.1 |
| Minor bleedings | 6 | 17.1 |
| No bleeding | 23 | 62.9 |
| Thrombosis | 0 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lardinois, B.; Hardy, M.; Michaux, I.; Horlait, G.; Rotens, T.; Jacqmin, H.; Lessire, S.; Bulpa, P.; Dive, A.; Mullier, F. Monitoring of Unfractionated Heparin Therapy in the Intensive Care Unit Using a Point-of-Care aPTT: A Comparative, Longitudinal Observational Study with Laboratory-Based aPTT and Anti-Xa Activity Measurement. J. Clin. Med. 2022, 11, 1338. https://doi.org/10.3390/jcm11051338
Lardinois B, Hardy M, Michaux I, Horlait G, Rotens T, Jacqmin H, Lessire S, Bulpa P, Dive A, Mullier F. Monitoring of Unfractionated Heparin Therapy in the Intensive Care Unit Using a Point-of-Care aPTT: A Comparative, Longitudinal Observational Study with Laboratory-Based aPTT and Anti-Xa Activity Measurement. Journal of Clinical Medicine. 2022; 11(5):1338. https://doi.org/10.3390/jcm11051338
Chicago/Turabian StyleLardinois, Benjamin, Michaël Hardy, Isabelle Michaux, Geoffrey Horlait, Thomas Rotens, Hugues Jacqmin, Sarah Lessire, Pierre Bulpa, Alain Dive, and François Mullier. 2022. "Monitoring of Unfractionated Heparin Therapy in the Intensive Care Unit Using a Point-of-Care aPTT: A Comparative, Longitudinal Observational Study with Laboratory-Based aPTT and Anti-Xa Activity Measurement" Journal of Clinical Medicine 11, no. 5: 1338. https://doi.org/10.3390/jcm11051338
APA StyleLardinois, B., Hardy, M., Michaux, I., Horlait, G., Rotens, T., Jacqmin, H., Lessire, S., Bulpa, P., Dive, A., & Mullier, F. (2022). Monitoring of Unfractionated Heparin Therapy in the Intensive Care Unit Using a Point-of-Care aPTT: A Comparative, Longitudinal Observational Study with Laboratory-Based aPTT and Anti-Xa Activity Measurement. Journal of Clinical Medicine, 11(5), 1338. https://doi.org/10.3390/jcm11051338






