Adverse Clinical Outcomes among Inflammatory Bowel Disease Patients Treated for Urinary Tract Infection
Abstract
:1. Introduction
2. Materials and Methods
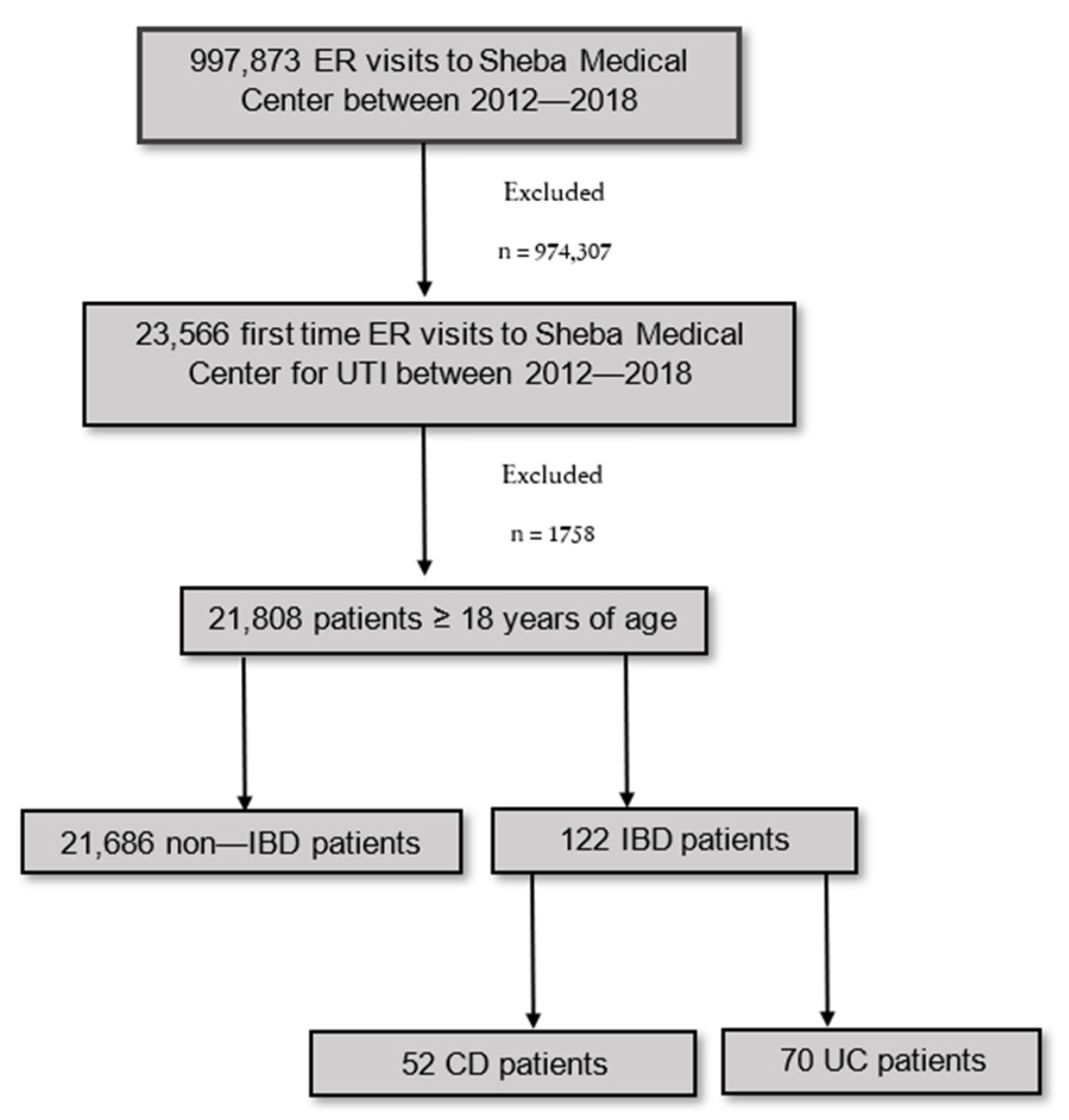
2.1. Study Design and Patient Selection
2.2. Data Extraction
2.3. Study Outcomes
2.4. Data Analyses and Statistical Methods
3. Results
3.1. Patient Characteristics
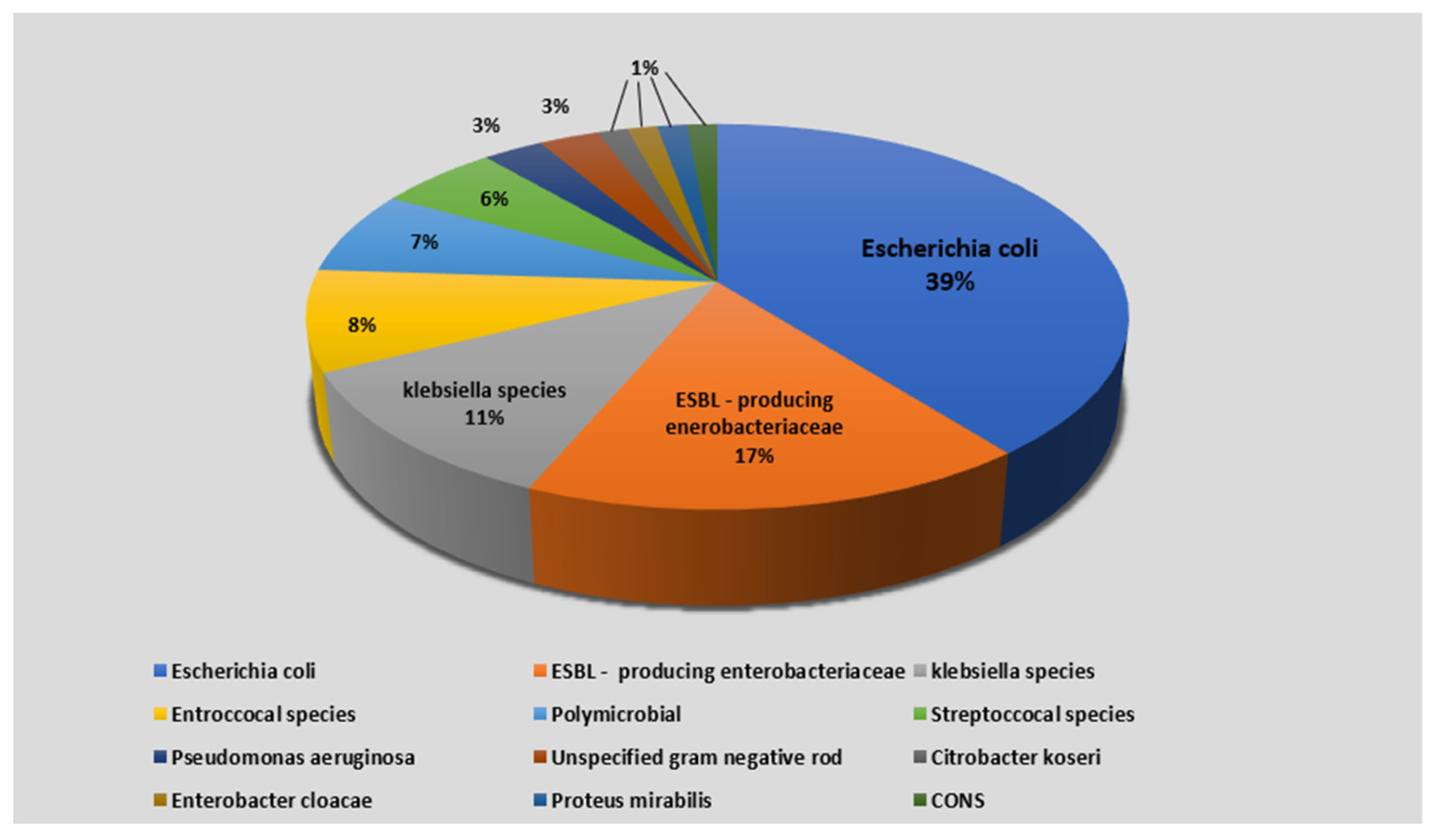
3.2. Microbiologic Characteristics
3.3. Outcomes and Predictors of Outcomes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sairenji, T.; Collins, K.L.; Evans, D.V. An Update on Inflammatory Bowel Disease. Prim. Care 2017, 44, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q. A Comprehensive Review and Update on the Pathogenesis of Inflammatory Bowel Disease. J. Immunol. Res. 2019, 2019, 7247238. [Google Scholar] [CrossRef] [Green Version]
- Ng, S.C.; Shi, H.Y.; Hamidi, N.; Underwood, F.E.; Tang, W.; Benchimol, E.I.; Panaccione, R.; Ghosh, S.; Wu, J.C.Y.; Chan, F.K.L. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: A systematic review of population-based studies. Lancet 2017, 390, 2769–2778. [Google Scholar] [CrossRef]
- Stulman, M.Y.; Asayag, N.; Focht, G.; Brufman, I.; Cahan, A.; Ledderman, N.; Matz, E.; Chowers, Y.; Eliakim, R.; Ben-Horin, S.; et al. Epidemiology of Inflammatory Bowel Diseases in Israel: A Nationwide Epi-Israeli IBD Research Nucleus Study. Inflamm. Bowel Dis. 2021, 11, 1784–1794. [Google Scholar] [CrossRef] [PubMed]
- Rahier, J.F.; Magro, F.; Abreu, C.; Armuzzi, A.; Ben-Horin, S.; Chowers, Y.; Cottone, M.; de Ridder, L.; Doherty, G.; Ehehalt, R.; et al. Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J. Crohns Colitis 2014, 8, 443–468. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B. Urinary tract infection syndromes. Occurrence, recurrence, bacteriology, risk factors, and disease burden. Infect. Dis. Clin. N. Am. 2014, 28, 1–13. [Google Scholar] [CrossRef]
- Ben-Ami, H.; Ginesin, Y.; Behar, D.M.; Fischer, D.; Edoute, Y.; Lavy, A. Diagnosis and treatment of urinary tract complications in Crohn’s disease: An experience over 15 years. Can. J. Gastroenterol. 2002, 16, 225–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kyle, J. Urinary complications of Crohn’s disease. World J. Surg. 1980, 4, 153–159. [Google Scholar] [CrossRef]
- Gruner, J.S.; Sehon, J.K.; Johnson, L.W. Diagnosis and management of enterovesical fistulas in patients with Crohn’s disease. Am. Surg. 2002, 68, 714–719. [Google Scholar]
- Varda, B.K.; McNabb-Baltar, J.; Sood, A.; Ghani, K.R.; Kibel, A.S.; Letendre, J.; Menon, M.; Sammon, J.D.; Schmid, M.; Sun, M.; et al. Urolithiasis and Urinary Tract Infection among Patients with Inflammatory Bowel Disease: A Review of US Emergency Department Visits between 2006 and 2009. Urology 2015, 85, 764–770. [Google Scholar] [CrossRef] [PubMed]
- Peyrin-Biroulet, L.; Pillot, C.; Oussalah, A.; Billioud, V.; Aissa, N.; Balde, M.; Williet, N.; Germain, A.; Lozniewski, A.; Bresler, L.; et al. Urinary tract infections in hospitalized inflammatory bowel disease patients: A 10-year experience. Inflamm. Bowel Dis. 2012, 18, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Bendtzen, K.; Nielsen, O.H. Extraintestinal manifestations of inflammatory bowel disease: Epidemiology, diagnosis, and management. Ann. Med. 2010, 42, 97–114. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, C.G. Benign Prostatic Hyperplasia: An Overview. Rev. Urol. 2005, 7 (Suppl. S9), 3–14. [Google Scholar]
- Guillo, L.; D’Amico, F.; Serrero, M.; Angioi, K.; Loeuille, D.; Costanzo, A.; Danese, S.; Peyrin-Biroulet, L. Assessment of extraintestinal manifestations in inflammatory bowel diseases: A systematic review and a proposed guide for clinical trials. United Eur. Gastroenterol. J. 2020, 8, 1013–1030. [Google Scholar] [CrossRef] [PubMed]
- Yongzhi, L.; Shi, Y.; Jia, L.; Yili, L.; Xingwang, Z.; Xue, G. Risk factors for urinary tract infection in patients with urolithiasis—Primary report of a single center cohort. BMC Urol. 2018, 18, 45. [Google Scholar] [CrossRef]
- Sato, S.; Sasaki, I.; Naito, H.; Funayama, Y.; Fukushima, K.; Shibata, C.; Masuko, T.; Ogawa, H.; Ueno, T.; Hashimoto, A.; et al. Management of Urinary Complications in Crohn’s Disease. Jpn. J. Surg. 1999, 29, 713–717. [Google Scholar] [CrossRef]
- Hammami, M.B.; Mahadevan, U. Men with Inflammatory Bowel Disease: Sexual Function, Fertility, Medication Safety, and Prostate Cancer. Am. J. Gastroenterol. 2020, 115, 526–534. [Google Scholar] [CrossRef]
- Ge, Y.; Shi, Q.; Yao, W.; Cheng, Y.; Ma, G. The association between in fl ammatory bowel disease and prostate cancer risk: A meta-analysis. Prostate Cancer Prostatic Dis. 2020, 23, 53–58. [Google Scholar] [CrossRef]
- Hsiao, C.-Y.; Chen, T.-H.; Lee, Y.-C.; Hsiao, M.-C.; Hung, P.-H.; Chen, Y.-Y.; Wang, M.-C. Urolithiasis Is a Risk Factor for Uroseptic Shock and Acute Kidney Injury in Patients With Urinary Tract Infection. Front. Med. 2019, 6, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, C.-Y.; Yang, H.-Y.; Hsiao, M.-C.; Hung, P.-H.; Wang, M.-C. Risk Factors for Development of Acute Kidney Injury in Patients with Urinary Tract Infection. PLoS ONE 2015, 10, e0133835. [Google Scholar] [CrossRef] [Green Version]
- Koza, Y. Acute kidney injury: Current concepts and new insights. J. INJ Violence Res. 2014, 8, 58–62. [Google Scholar] [CrossRef]
- Connell, A.; Laing, C. Acute kidney injury. Clin. Med. J. R. Coll. Phys. Lond. 2015, 15, 581–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagalingam, K. Acute Kidney Injury: The Hidden Killer in the Ward. J. Ren. Care 2020, 46, 72–73. [Google Scholar] [CrossRef] [PubMed]
- Wald, R.; Quinn, R.R.; Luo, J.; Li, P.; Scales, D.C.; Mamdani, M.M.; Ray, J.G.; the University of Toronto Acute Kidney Injury Research Group. Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA J. Am. Med. Assoc. 2009, 302, 1179–1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, L.J.; Go, A.S.; Chertow, G.M.; McCulloch, C.E.; Fan, D.; Ordoñez, J.D.; Hsu, C.Y. Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int. 2010, 76, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Hsu, C.-Y.; Chertow, G.M.; McCulloch, C.E.; Fan, D.; Ordoñez, J.D.; Go, A.S. Nonrecovery of kidney function and death after acute on chronic renal failure. Clin. J. Am. Soc. Nephrol. 2009, 4, 891–898. [Google Scholar] [CrossRef] [Green Version]
- Okumus, M.; Inci, M.F.; Ozkan, F.; Bozkurt, S.; Sucakli, M.H.; Altunoluk, B. Correlation of volume, position of stone, and hydronephrosis with microhematuria in patients with solitary urolithiasis. Med. Sci. Monit. 2013, 19, 295–299. [Google Scholar] [CrossRef] [Green Version]
- Sasmaz, M.I.; Kirpat, V. The relationship between the severity of pain and stone size, hydronephrosis and laboratory parameters in renal colic attack. Am. J. Emerg. Med. 2019, 37, 2107–2110. [Google Scholar] [CrossRef]
- Sacks, S.H.; Aparicio, S.A.; Bevan, A.; Oliver, D.O.; Will, E.J.; Davison, A.M. Late renal failure due to prostatic outflow obstruction: A preventable disease. BMJ 1989, 298, 156–159. [Google Scholar] [CrossRef] [Green Version]
- Gottlieb, R.H.; Voci, S.L.; Cholewinski, S.P.; Hartley, D.F.; Rubens, D.J.; Orloff, M.S.; Bronsther, O.L. Sonography: A Useful Tool to Detect the Transplant Dysfunction. J. Clin. Ultrasound. 1999, 27, 325–333. [Google Scholar] [CrossRef]
- Havard, J.D.J. Regular Review Ultrasonography in the diagnosis of renal obstruction. BMJ 1990, 301, 944–946. [Google Scholar] [CrossRef] [Green Version]
- Bellomo, R.; Kellum, J.A.; Ronco, C. Acute kidney injury. Lancet 2012, 380, 756–766. [Google Scholar] [CrossRef]
- Burisch, J.; Jess, T.; Martinato, M.; Lakatos, P.L. The burden of inflammatory bowel disease in Europe. J. Crohns Colitis 2013, 7, 322–337. [Google Scholar] [CrossRef] [Green Version]
- Ukashi, O.; Barash, Y.; Segel, M.J.; Ungar, B.; Soffer, S.; Ben-Horin, S.; Klang, E.; Kopylov, U. Predictors of mortality in inflammatory bowel disease patients treated for pneumonia. Ther. Adv. Gastroenterol. 2020, 13, 1756284820939453. [Google Scholar] [CrossRef] [PubMed]
- Kaye, K.S.; Anderson, D.J.; Cook, E.; Huang, S.S.; Siegel, J.D.; Zuckerman, J.M.; Talbot, T.R. Guidance for Infection Prevention and Healthcare Epidemiology Programs: Healthcare Epidemiologist Skills and Competencies. Infect. Control Hosp. Epidemiol. 2015, 36, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Kollef, M.H. Health Care—Associated Pneumonia: Perception versus Reality. Clin. Infect. Dis. 2009, 49, 1875–1877. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.E.; Muntner, P.; Chertow, G.M.; Warnock, D.G. Acute kidney injury and mortality in hospitalized patients. Am. J. Nephrol. 2012, 35, 349–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heit, J.A.; Melton, L.J.; Lohse, C.M.; Petterson, T.M.; Silverstein, M.D.; Mohr, D.N.; O’Fallon, W.M. Incidence of venous thromboembolism in hospitalized patients vs community residents. Mayo Clin. Proc. 2001, 76, 1102–1110. [Google Scholar] [CrossRef]
- Grace, R.; Bownik, H.; Scott, F.; Lichtenstein, G. Infectious Complications in IBD Patients on Immunomodulators, Corticosteroids, and Vedolizumab: Is Older Age a Predictor of Higher Complication Rates or Worsened Response?: 1940. Am. J. Gastroenterol. 2015, 110, S823. [Google Scholar] [CrossRef]
- Waljee, A.K.; Wiitala, W.L.; Govani, S.; Stidham, R.; Saini, S.; Hou, J.; Feagins, L.A.; Khan, N.; Good, C.B.; Vijan, S.; et al. Corticosteroid use and complications in a US inflammatory bowel disease cohort. PLoS ONE 2016, 11, e0158017. [Google Scholar] [CrossRef] [Green Version]
| IBD Patients (n = 122) | Non-IBD Patients (n = 21,686) | p-Value | |
|---|---|---|---|
| Age(y)—median (IQR) | 72.00 (49.75–83.00) | 70.00 (51.00–82.00) | 0.351 |
| Male (%) | 52 (43%) | 9996 (46%) | 0.443 |
| Comorbidity (%) | |||
| Diabetes (%) | 20 (16%) | 4353 (20%) | 0.311 |
| Benign prostate hyperplasia (%) $ | 11 (21%) | 1043 (10%) | 0.010 |
| Urolithiasis (%) | 14 (11.5%) | 623 (3%) | <0.001 |
| Urologic tumor (%) | 2 (2%) | 588 (3%) | 0.467 |
| IBD medications (%) | |||
| Amino salicylic acid and similar agents (%) | 52 (42%) | 12 (<1%) | <0.001 |
| Corticosteroids (%) | 21 (17%) | 998 (5%) | <0.001 |
| Azathioprine (%) | 10 (8%) | 1 (<1%) | <0.001 |
| Methotrexate (%) | 3 (3%) | 113 (<1%) | 0.003 |
| Tumor necrosis factor alpha inhibitors (%) | 14 (12%) | 3 (<1%) | <0.001 |
| Miscellaneous (%) | |||
| Recent hospitalization (<3 months) (%) | 26 (21%) | 1790 (8%) | <0.001 |
| CD Patients (n = 52) | UC Patients (n = 70) | p-Value | |
|---|---|---|---|
| Age(y)—median (IQR) | 61.50 (37.50–79.50) | 75.00 (65.75–84.25) | 0.001 |
| Male (%) | 24 (40%) | 28 (46%) | 0.497 |
| Comorbidity (%) | |||
| Diabetes (%) | 5 (10%) | 15 (21%) | 0.081 |
| Benign prostate hyperplasia (%) $ | 5 (21%) | 6 (21%) | 0.950 |
| Urolithiasis (%) | 6 (12%) | 8 (11%) | 0.985 |
| Urologic tumor (%) | 0 (0%) | 2 (3%) | 0.219 |
| IBD medications (%) | |||
| Amino salicylic acid and similar agents (%) | 13 (25%) | 39 (56%) | 0.001 |
| Corticosteroids (%) | 11 (21%) | 10 (14%) | 0.320 |
| Azathioprine (%) | 8 (15%) | 2 (3%) | 0.013 |
| Methotrexate (%) | 2 (4%) | 1(1%) | 0.394 |
| Tumor necrosis factor alpha inhibitors (%) | 11 (21%) | 3 (4%) | 0.004 |
| Miscellaneous (%) | |||
| Recent hospitalization (<3 months) (%) | 9 (17%) | 10 (14%) | 0.682 |
| IBD Patients | Non-IBD Patients | p-Value | CD Patients | UC Patients | p-Value | |
|---|---|---|---|---|---|---|
| 30-day mortality (%) | 7 (5.7%) | 1106 (5.1%) | 0.750 | 3 (5.8%) | 4 (5.7%) | 0.990 |
| Hospitalization (%) | 84 (68.9%) | 12,863 (59.3%) | 0.032 | 37 (71.2%) | 47 (67.1%) | 0.636 |
| Hospitalization LOS median (d) median (IQR) | 3.00 (1.00–5.75) | 3.00 (1.00–5.00) | 0.990 | 3.0 (1.0–5.5) | 3.0 (2.0–6.0) | 0.251 |
| 30-day recurrent hospitalization (%) | 19 (15.6%) | 1591 (7.3%) | 0.001 | 9 (17.3%) | 10 (14.3%) | 0.649 |
| Acute kidney injury (%) | 17 (13.9%) | 998 (4.6%) | <0.001 | 10 (19.2%) | 7 (10.0%) | 0.145 |
| Outcome | 30-Day Mortality | Hospitalization | ||||||
|---|---|---|---|---|---|---|---|---|
| Analysis | Univariable | Multivariable $ | Univariable | Multivariable $ | ||||
| p-Value | AOR | 95% CI | p-Value | p-Value | AOR | 95% CI | p-Value | |
| Age | 0.126 | 0.034 | 1.044 | 1.013–1.076 | 0.005 ^ | |||
| Sex | 0.990 | 0.268 | ||||||
| IBD disease | 0.990 | 0.636 | ||||||
| Previous SHx | 0.615 | 0.534 | ||||||
| 5-ASA | 0.990 | 0.938 | ||||||
| Corticosteroids | 0.412 | 0.779 | ||||||
| Azathioprine | 0.545 | 0.427 | ||||||
| Methotrexate | 0.665 | 0.934 | ||||||
| TNF-alpha inhibitors | 0.327 | 0.315 | ||||||
| Diabetes | 0.228 | 0.239 | ||||||
| BPH | 0.616 | 0.283 | ||||||
| Urolithiasis | 0.327 | 0.404 | ||||||
| Urologic tumors | 0.725 | 0.338 | ||||||
| Positive blood culture | 0.060 | 5.90 | 0.75–46.43 | 0.091 | 0.049 | |||
| Hydronephrosis | 0.470 | 0.698 | ||||||
| Recent hospitalization * | 0.152 | 0.050 | 11.067 | 1.161–105.471 | 0.037 ^ | |||
| Hospitalization | 0.067 | - | ||||||
| Acute kidney injury | 0.978 | 0.063 | ||||||
| Hospitalization LOS | 0.225 | - | ||||||
| Outcome | Acute Kidney Injury | 30-Day Recurrent Hospitalization | ||||||
|---|---|---|---|---|---|---|---|---|
| Analysis | Univariable | Multivariable $ | Univariable | Multivariable $ | ||||
| p-Value | AOR | 95% CI | p-Value | p-Value | AOR | 95% CI | p-Value | |
| Age | 0.634 | 0.096 | ||||||
| Sex | 0.354 | 0.579 | ||||||
| IBD disease | 0.145 | 0.649 | ||||||
| Previous SHx | 0.238 | 0.108 | ||||||
| 5-ASA | 0.025 | 0.271 | 0.072–1.013 | 0.052 | 0.118 | |||
| Corticosteroids | 0.151 | 0.629 | ||||||
| Azathioprine | 0.184 | 0.687 | ||||||
| Methotrexate | 0.326 | 0.390 | ||||||
| TNF-alpha inhibitors | 0.389 | 0.154 | ||||||
| Diabetes | 0.578 | 0.452 | ||||||
| BPH | 0.627 | 0.534 | ||||||
| Urolithiasis | 0.093 | 0.154 | ||||||
| Urologic tumors | 0.566 | 0.540 | ||||||
| Positive blood culture | 0.289 | 0.264 | ||||||
| Hydronephrosis | 0.904 | 0.005 | 10.383 | 2.039–52.865 | 0.005 ^ | |||
| Recent hospitalization * | 0.810 | 0.016 | 4.494 | 1.420–14.221 | 0.011 ^ | |||
| Hospitalization | 0.063 | - | ||||||
| Acute kidney injury | - | 0.016 | 4.683 | 1.325–16.548 | 0.017 ^ | |||
| Hospitalization LOS | 0.007 | 1.089 | 0.993–1.193 | 0.070 | 0.224 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ukashi, O.; Barash, Y.; Klang, E.; Zilberman, T.; Ungar, B.; Kopylov, U.; Ben-Horin, S.; Veisman, I. Adverse Clinical Outcomes among Inflammatory Bowel Disease Patients Treated for Urinary Tract Infection. J. Clin. Med. 2022, 11, 1359. https://doi.org/10.3390/jcm11051359
Ukashi O, Barash Y, Klang E, Zilberman T, Ungar B, Kopylov U, Ben-Horin S, Veisman I. Adverse Clinical Outcomes among Inflammatory Bowel Disease Patients Treated for Urinary Tract Infection. Journal of Clinical Medicine. 2022; 11(5):1359. https://doi.org/10.3390/jcm11051359
Chicago/Turabian StyleUkashi, Offir, Yiftach Barash, Eyal Klang, Tal Zilberman, Bella Ungar, Uri Kopylov, Shomron Ben-Horin, and Ido Veisman. 2022. "Adverse Clinical Outcomes among Inflammatory Bowel Disease Patients Treated for Urinary Tract Infection" Journal of Clinical Medicine 11, no. 5: 1359. https://doi.org/10.3390/jcm11051359
APA StyleUkashi, O., Barash, Y., Klang, E., Zilberman, T., Ungar, B., Kopylov, U., Ben-Horin, S., & Veisman, I. (2022). Adverse Clinical Outcomes among Inflammatory Bowel Disease Patients Treated for Urinary Tract Infection. Journal of Clinical Medicine, 11(5), 1359. https://doi.org/10.3390/jcm11051359






